A Bibliometric Analysis of Research on the Sources and Formation Processes of Forest Soil Organic Matter Under Climate Change
Abstract
1. Introduction
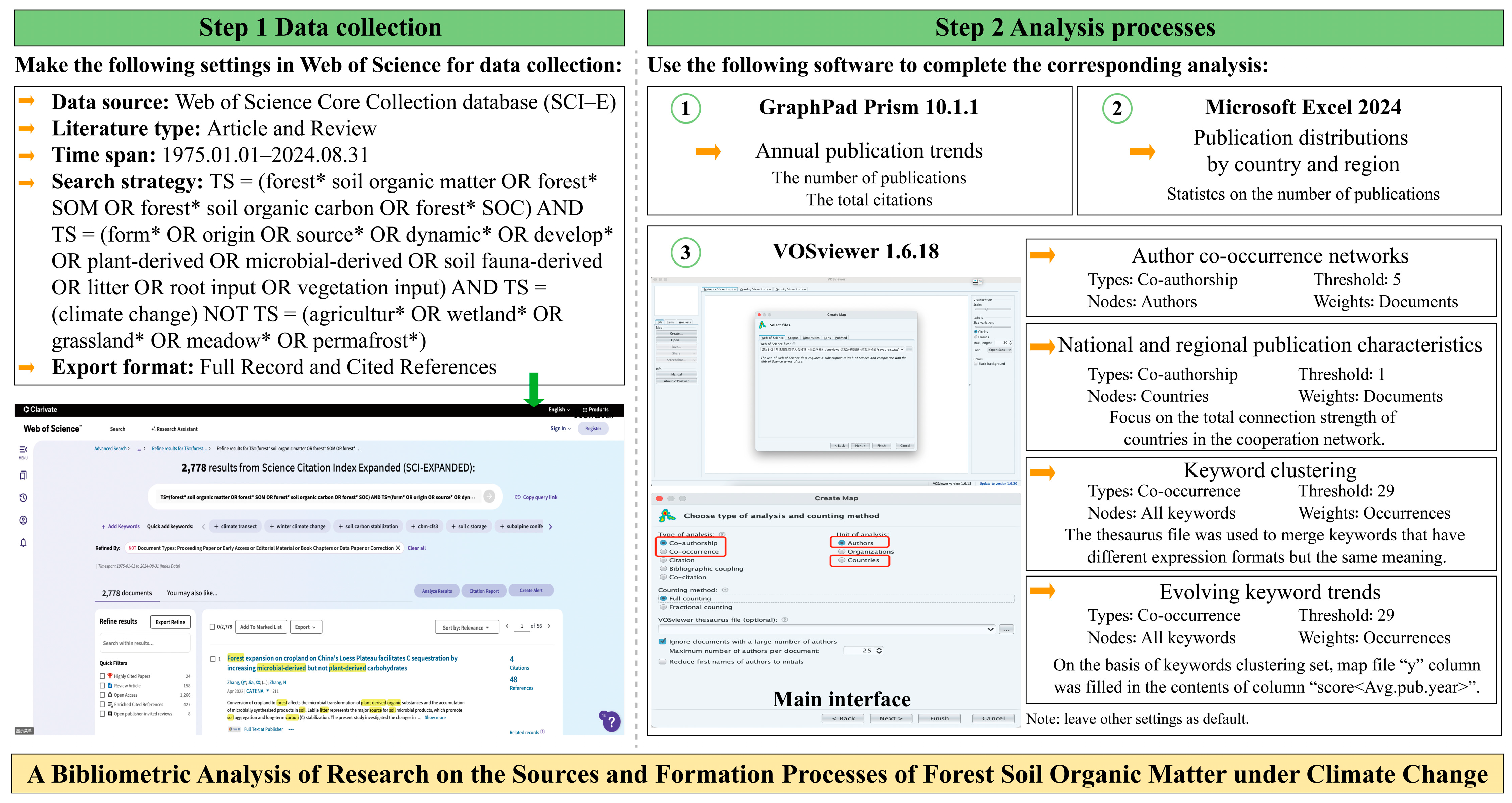
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis Methods
3. Results
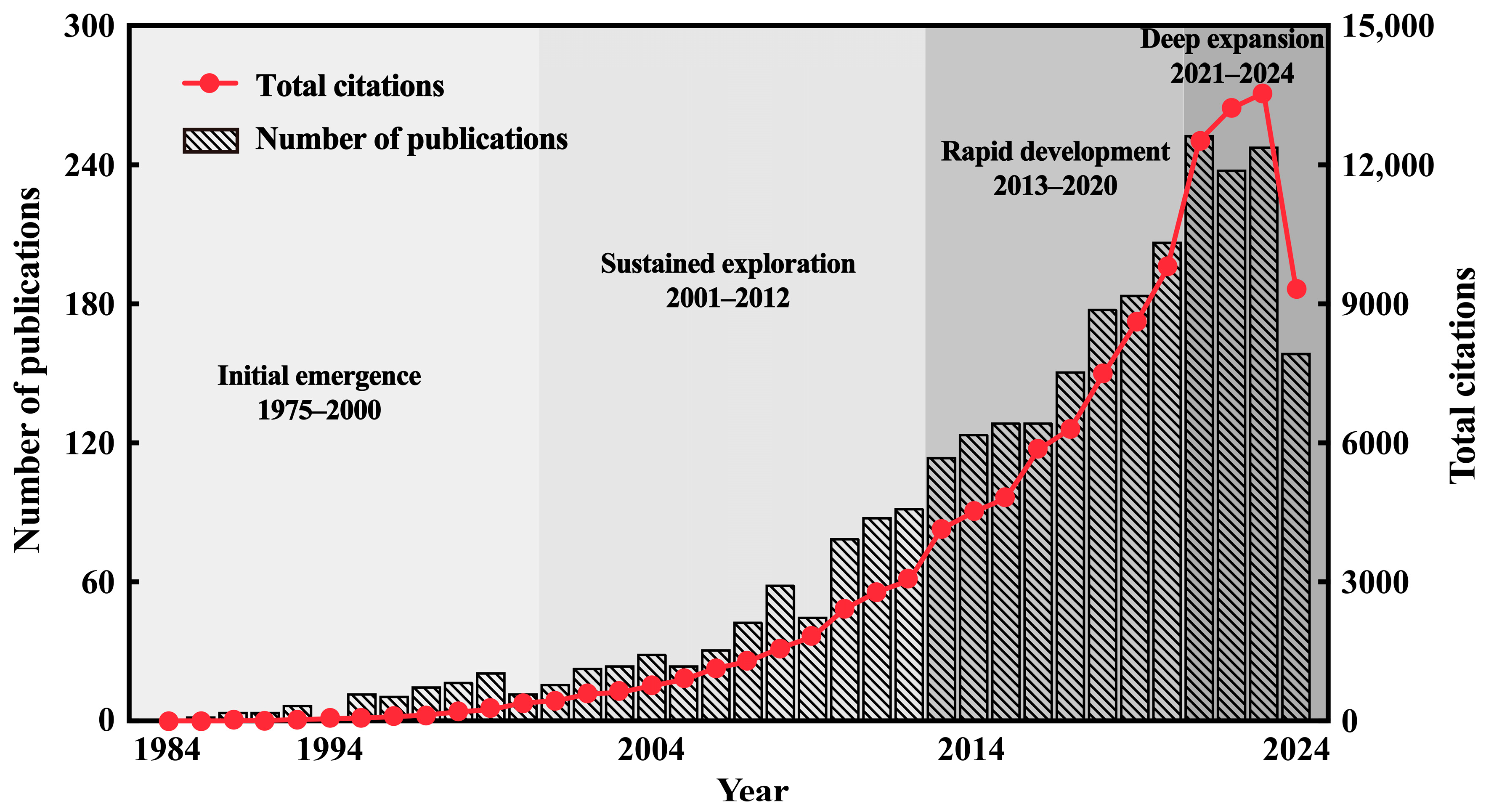
3.1. Annual Publication Trends
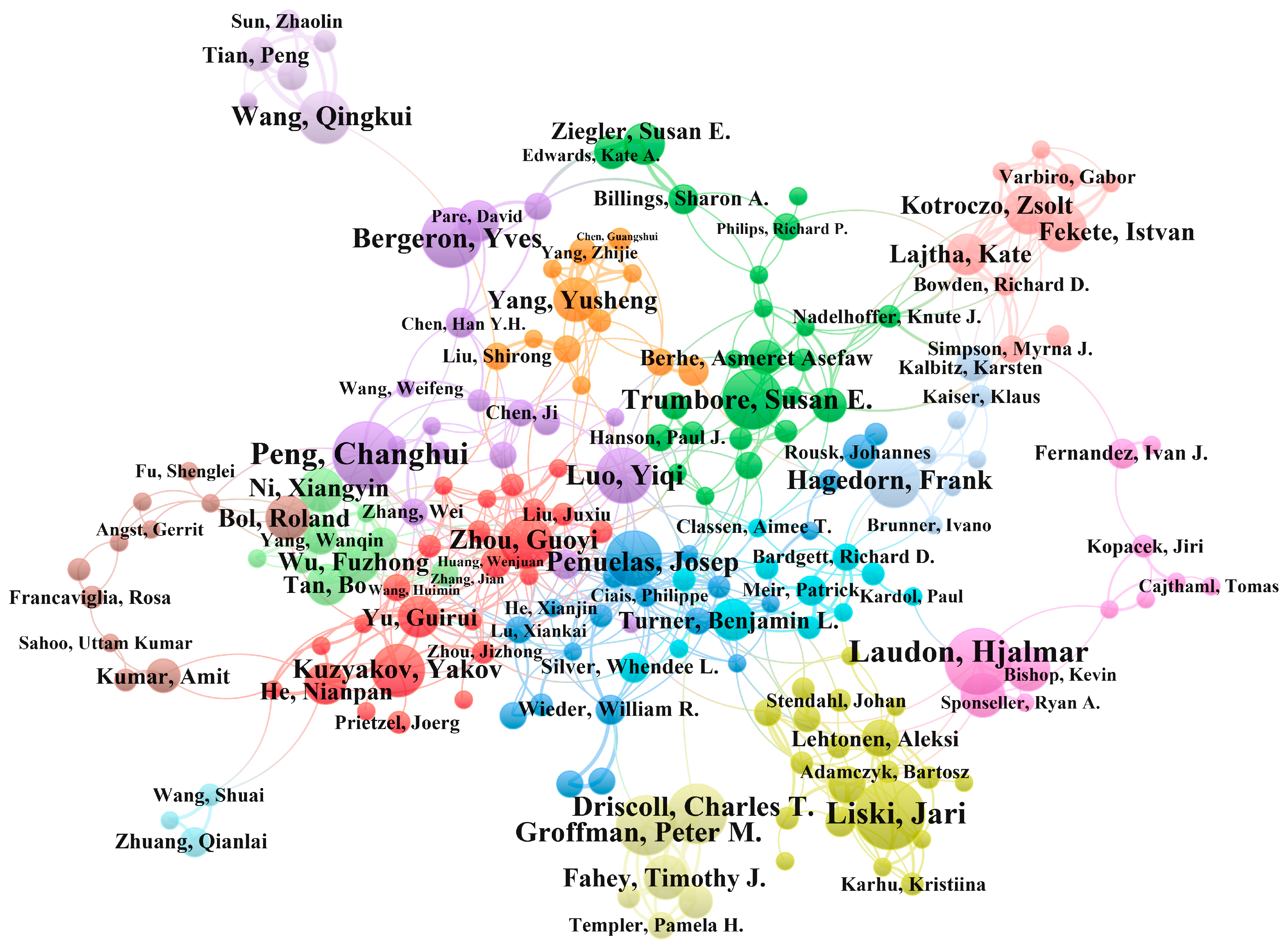
3.2. Author Co-Occurrence Network Analysis
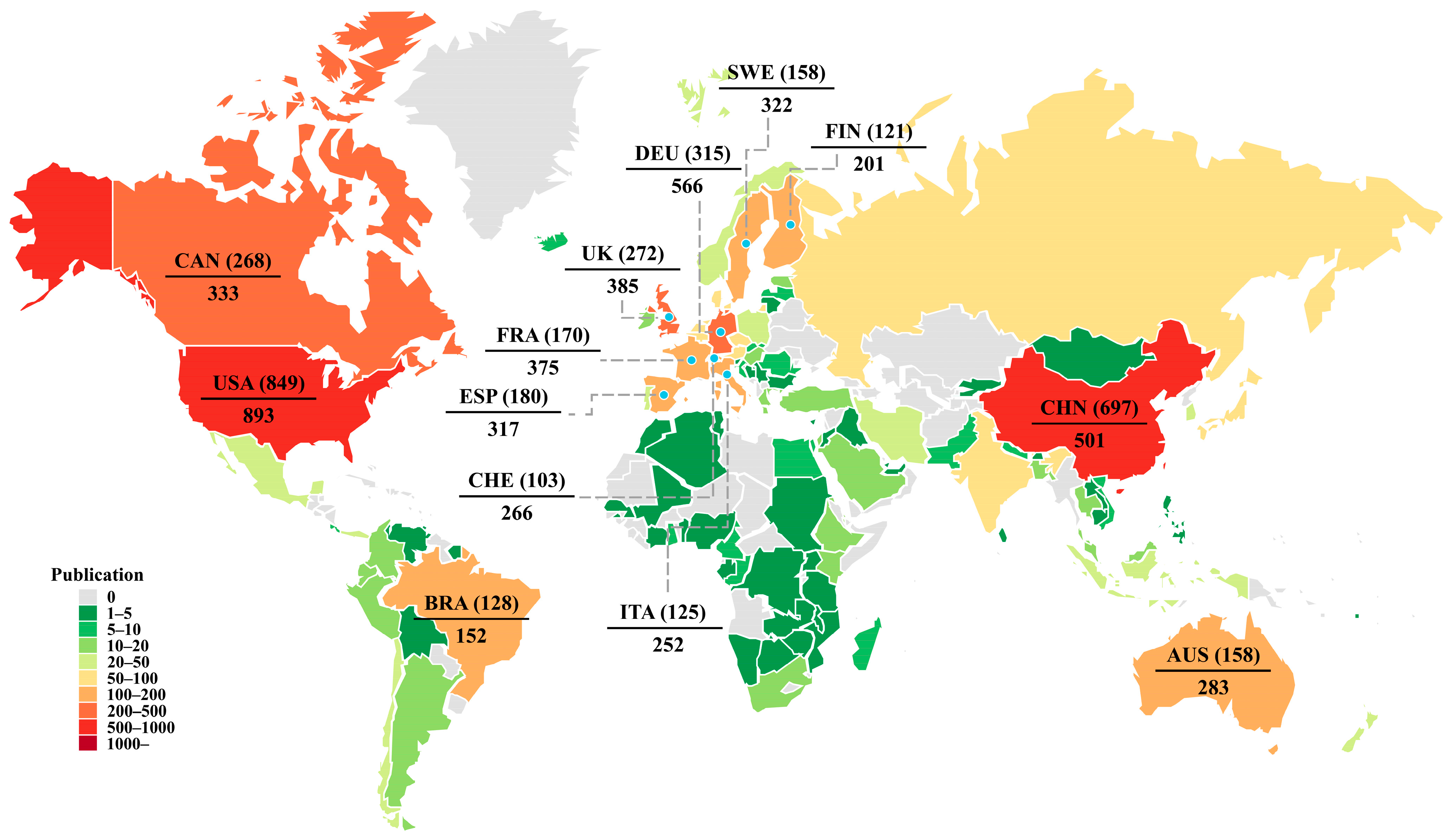
3.3. National and Regional Publication Characteristics
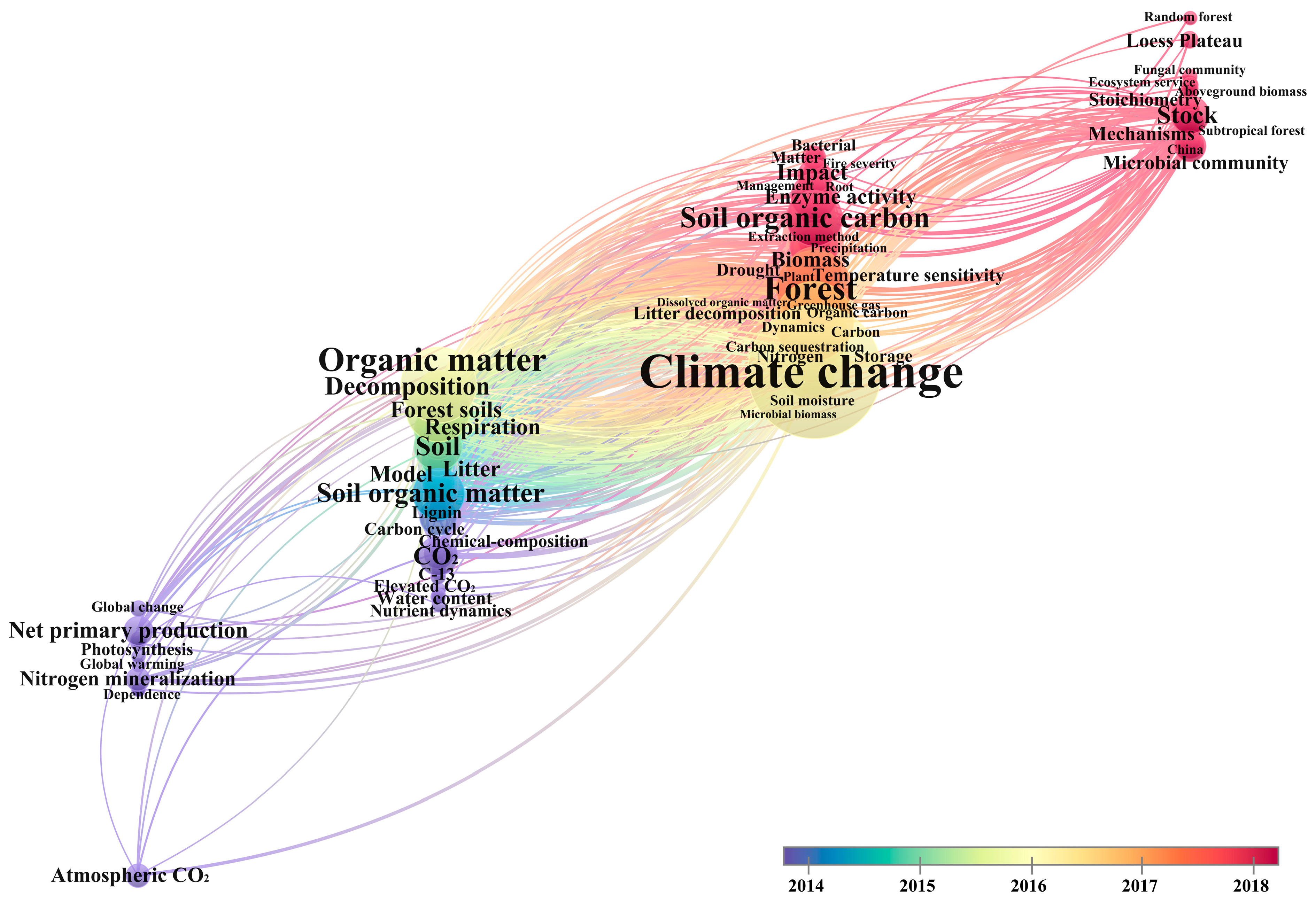
3.4. High-Frequency Keyword Analysis
3.5. Evolution of Research Hotspots
4. Discussion
4.1. Research Foundation
4.2. Sources of Forest SOM
4.3. Formation Processes of Forest SOM
4.3.1. Formation Processes of Plant-Derived SOM
4.3.2. Formation Processes of Microbial-Derived SOM
4.3.3. Formation Processes of Soil Fauna-Derived SOM
4.4. Impacts of Climate Change on the Sources and Formation Processes of Forest SOM
4.4.1. Impacts of Climate Change on Plant-Derived SOM
4.4.2. Impacts of Climate Change on Microbial-Derived SOM
4.4.3. Impacts of Climate Change on Soil Fauna-Derived SOM
4.5. Measurement Methods and Model-Based Analysis Techniques
4.5.1. Biomarker and Indicator Analysis
4.5.2. Organic Matter Component and Quantitative Analysis
4.5.3. In Situ Monitoring Analysis
4.5.4. Mathematical Model-Based Dynamic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kohl, L.; Philben, M.; Edwards, K.A.; Podrebarac, F.A.; Warren, J.; Ziegler, S.E. The origin of soil organic matter controls its composition and bioreactivity across a mesic boreal forest latitudinal gradient. Glob. Change Biol. 2018, 24, E458–E473. [Google Scholar] [CrossRef]
- Farrar, J.; Hawes, M.; Jones, D.; Lindow, S. How roots control the flux of carbon to the rhizosphere. Ecology 2003, 84, 827–837. [Google Scholar] [CrossRef]
- Fahey, T.J.; Yavitt, J.B.; Goebel, M.; Pipes, G. Incorporation of fine root detritus into forest soil organic matter. Biogeochemistry 2023, 165, 151–163. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, P.; Sun, Z.; Zhao, X. Research on soil organic matter in forest ecosystems: Status and challenge. Chin. J. Ecol. 2020, 39, 3829–3843. [Google Scholar] [CrossRef]
- Schmidt, M.; Torn, M.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Trumbore, S.E.; Davidson, E.A.; de Camargo, P.B.; Nepstad, D.C.; Martinelli, L.A. Belowground cycling of carbon in forests and pastures of Eastern Amazonia. Glob. Biogeochem. Cycles 1995, 9, 515–528. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; De la Rosa, J.M.; Jiménez-Morillo, N.T.; Almendros, G.; González-Pérez, J.A.; Knicker, H. Post-fire recovery of soil organic matter in a Cambisol from typical Mediterranean forest in Southwestern Spain. Sci. Total Environ. 2016, 572, 1414–1421. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.M.; Strakoyá, P.; Prommer, J.; Wild, B.; Hagner, M.; Pihlatie, M.; Fritze, H.; Richter, A.; Heinonsalo, J. Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat. Commun. 2019, 10, 3982. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, A.T.; Meir, P.; Velasquez, E.; Turner, B.L. Soil carbon loss by experimental warming in a tropical forest. Nature 2020, 584, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Qafoku, O.; Andersen, A.; Zhao, Q.; Mergelsberg, S.T.; Kew, W.R.; Eder, E.K.; Resch, C.T.; Graham, E.B.; Qafoku, N.P. Synergetic effects of soil organic matter components during interactions with minerals. Environ. Sci. Technol. 2024, 58, 23018–23030. [Google Scholar] [CrossRef]
- He, H.; Ge, R.; Ren, X.; Zhang, L.; Chang, Q.; Xu, Q.; Zhou, G.; Xie, Z.; Wang, S.; Wang, H.; et al. Reference carbon cycle dataset for typical Chinese forests via colocated observations and data assimilation. Sci. Data 2021, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Soldatova, E.; Krasilnikov, S.; Kuzyakov, Y. Soil organic matter turnover: Global implications from δ13C and δ15N signatures. Sci. Total Environ. 2024, 912, 169423. [Google Scholar] [CrossRef] [PubMed]
- Gundale, M.J.; Axelsson, E.P.; Buness, V.; Callebaut, T.; Deluca, T.H.; Hupperts, S.F.; Ibáñez, T.S.; Metcalfe, D.B.; Nilsson, M.C.; Peichl, M.; et al. The biological controls of soil carbon accumulation following wildfire and harvest in boreal forests: A review. Glob. Change Biol. 2024, 30, e17276. [Google Scholar] [CrossRef]
- Price, D.T.; Alfaro, R.I.; Brown, K.J.; Flannigan, M.D.; Fleming, R.A.; Hogg, E.H.; Girardin, M.P.; Lakusta, T.; Johnston, M.; McKenney, D.W.; et al. Anticipating the consequences of climate change for Canada’s boreal forest ecosystems. Environ. Rev. 2013, 365, 322–365. [Google Scholar] [CrossRef]
- Rodell, M.; Li, B. Changing intensity of hydroclimatic extreme events revealed by GRACE and GRACE-FO. Nat. Water 2023, 1, 241–248. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Meinshausen, M.; Arora, V.K.; Jones, C.D.; Anav, A.; Liddicoat, S.K.; Knutti, R. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 2014, 27, 511–526. [Google Scholar] [CrossRef]
- Melillo, J.; Steudler, P.; Aber, J.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Pisani, O.; Frey, S.D.; Simpson, A.J.; Simpson, M.J. Soil warming and nitrogen deposition alter soil organic matter composition at the molecular-level. Biogeochemistry 2015, 123, 391–409. [Google Scholar] [CrossRef]
- Streit, K.; Hagedorn, F.; Hiltbrunner, D.; Portmann, M.; Saurer, M.; Buchmann, N.; Siegwolf, R.T.W. Soil warming alters microbial substrate use in alpine soils. Glob. Change Biol. 2014, 20, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lv, J.; Peng, Y.; Yao, Y.; Wei, X.; Wang, X. Mechanisms controlling the stability and sequestration of mineral associated organic carbon upon erosion and deposition. Catena 2024, 242, 108119. [Google Scholar] [CrossRef]
- Cheng, X.; Xing, W.; Liu, J. Litter chemical traits, microbial and soil stoichiometry regulate organic carbon accrual of particulate and mineral-associated organic matter. Biol. Fertil. Soils 2023, 59, 777–790. [Google Scholar] [CrossRef]
- Mekonnen, Z.A.; Riley, W.J. Climate change will increase biomass proportion of global forest carbon stocks under an SSP5-8.5 climate trajectory. Geophys. Res. Lett. 2023, 50, e2023GL104612. [Google Scholar] [CrossRef]
- Islam, M.R.; Jönsson, A.M.; Bergkvist, J.; Lagergren, F.; Lindeskog, M.; Mölder, M.; Scholze, M.; Kljun, N. Projected effects of climate change and forest management on carbon fluxes and biomass of a boreal forest. Agric. For. Meteorol. 2024, 349, 109959. [Google Scholar] [CrossRef]
- Sun, F.; Fan, L.; Deng, G.; Kuzyakov, Y.; Zhang, Y.; Wang, J.; Li, Y.; Wang, F.; Li, Z.; Tariq, A.; et al. Responses of tropical forest soil organic matter pools to shifts in precipitation patterns. Soil Biol. Biochem. 2024, 197, 109530. [Google Scholar] [CrossRef]
- Knoepp, J.D.; See, C.R.; Vose, J.M.; Miniat, C.F.; Clark, J.S. Total C and N pools and fluxes vary with time, soil temperature, and moisture along an elevation, precipitation, and vegetation gradient in southern appalachian forests. Ecosystems 2018, 21, 1623–1638. [Google Scholar] [CrossRef]
- Holland, E.A.; Neff, J.C.; Townsend, A.R.; McKeown, B. Uncertainties in the temperature sensitivity of decomposition in tropical and subtropical ecosystems: Implications for models. Glob. Biogeochem. Cycles 2000, 14, 1137–1151. [Google Scholar] [CrossRef]
- Karavanova, E.I. Dissolved organic matter: Fractional composition and sorbability by the soil solid phase (Review of literature). Eurasian Soil Sci. 2013, 46, 833–844. [Google Scholar] [CrossRef]
- Liu, J.; Gao, W.; Liu, T.; Dai, L.; Wu, L.; Miao, H.; Yang, C. A bibliometric analysis of the impact of ecological restoration on carbon sequestration in ecosystems. Forests 2023, 14, 1442. [Google Scholar] [CrossRef]
- Whalen, E.D.; Grandy, A.S.; Sokol, N.W.; Keiluweit, M.; Ernakovich, J.; Smith, R.G.; Frey, S.D. Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding. Glob. Change Biol. 2022, 28, 7167–7185. [Google Scholar] [CrossRef]
- Li, J.; Goerlandt, F.; Reniers, G. An overview of scientometric mapping for the safety science community: Methods, tools, and framework. Saf. Sci. 2021, 134, 105093. [Google Scholar] [CrossRef]
- Gao, K. Research on the application of bibliometric analysis software VOSviewer. J. Libr. Inf. Sci. 2015, 25, 95–98. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, F.; Yang, M. Research hotspots and progress of soil organic carbon mineralization based on bibliometrics method. Acta Pedol. Sin. 2022, 59, 381–392. [Google Scholar] [CrossRef]
- Smith, K.A. After the Kyoto Protocol: Can soil scientists make a useful contribution? Soil Use Manag. 2006, 15, 71–75. [Google Scholar] [CrossRef]
- Delire, C.; Ngomanda, A.; Jolly, D. Possible impacts of 21st century climate on vegetation in Central and West Africa. Global Planet. Change 2008, 64, 3–15. [Google Scholar] [CrossRef]
- Aas, E.R.; de Wit, H.A.; Berntsen, T.K. Modeling boreal forest soil dynamics with the microbially explicit soil model MIMICS+ (v1.0). Geosci. Model Dev. 2024, 17, 2929–2959. [Google Scholar] [CrossRef]
- DellaSala, D.A.; Keith, H.; Sheehan, T.; Strittholt, J.; Mackey, B.; Connolly, M.; Werner, J.R.; Fredeen, A.L. Estimating carbon stocks and stock changes in Interior Wetbelt forests of British Columbia, Canada. Ecosphere 2022, 13, e4020. [Google Scholar] [CrossRef]
- Myers-Pigg, A.N.; Kaiser, K.; Benner, R.; Ziegler, S.E. Soil organic matter diagenetic state informs boreal forest ecosystem feedbacks to climate change. Biogeosciences 2023, 20, 489–503. [Google Scholar] [CrossRef]
- Plante, A.F.; Sanderman, J.; Asanopoulos, C.H.; Bell, S.; Baldock, J. Interpreting ramped combustion thermograms using 13C NMR spectroscopy to characterize soil organic matter composition. Geoderma 2023, 432, 116415. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Ouimette, A.P. Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 2009, 95, 355–371. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Yao, X.; Fan, A.; Wang, W.; Guo, J.; Yang, Z.; Yang, Y.; Chen, G. Functional type mediates the responses of root litter-driven priming effect and new carbon formation to warming. Sci. Total Environ. 2024, 934, 173203. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Li, Z.; Zhang, D.; Tang, X.; Zhou, C.; Yan, J.; Mo, J. Old-growth forests can accumulate carbon in soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Schindlbacher, A.; Liu, S.; Yang, Y.; Tian, H.; Chen, L.; Ming, A.; Wang, J.; Li, J.; et al. Soil respiration related to the molecular composition of soil organic matter in subtropical and temperate forests under soil warming. Soil Biol. Biochem. 2025, 201, 109661. [Google Scholar] [CrossRef]
- Nagel, R.; Meyer, P.; Blaschke, M.; Feldmann, E. Strict forest protection: A meaningful contribution to Climate-Smart Forestry? An evaluation of temporal trends in the carbon balance of unmanaged forests in Germany. Front. For. Glob. Change 2023, 6, 1099558. [Google Scholar] [CrossRef]
- Lindroth, A.; Lagergren, F.; Grelle, A.; Klemedtsson, L.; Langvall, O.; Weslien, P.; Tuulik, J. Storms can cause Europe-wide reduction in forest carbon sink. Glob. Change Biol. 2009, 15, 346–355. [Google Scholar] [CrossRef]
- Araujo, J.K.S.; de Souza Júnior, V.S.; Marques, F.A.; Voroney, P.; da Silva Souza, R.A. Assessment of carbon storage under rainforests in Humic Hapludox along a climosequence extending from the Atlantic coast to the highlands of northeastern Brazil. Sci. Total Environ. 2016, 568, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Muqaddas, B.; Zhou, X.; Lewis, T.; Wild, C.; Chen, C. Long-term frequent prescribed fire decreases surface soil carbon and nitrogen pools in a wet sclerophyll forest of Southeast Queensland, Australia. Sci. Total Environ. 2015, 536, 39–47. [Google Scholar] [CrossRef]
- Saiz, G.; Bird, M.I.; Domingues, T.; Schrodt, F.; Schwarz, M.; Feldpausch, T.R.; Veenendaal, E.; Djagbletey, G.; Hien, F.; Compaore, H.; et al. Variation in soil carbon stocks and their determinants across a precipitation gradient in West Africa. Glob. Change Biol. 2012, 18, 1670–1683. [Google Scholar] [CrossRef]
- Holtvoeth, J.; Kolonic, S.; Wagner, T. Soil organic matter as an important contributor to late Quaternary sediments of the tropical West African continental margin. Geochim. Cosmochim. Acta 2005, 69, 2031–2041. [Google Scholar] [CrossRef]
- Irina, K.K.; Ekaterina, N.T.; Ruzalia, V.U.; Ekaterina, V.M.; Marina, V.S. Effect of temperature on litter decomposition, soil microbial community structure and biomass in a mixed-wood forest in European Russia. Curr. Sci. 2019, 116, 765–772. [Google Scholar] [CrossRef]
- Leal, F.; Aguilera, D.; Echeverría, C.; Gatica-Saavedra, P.; Aburto, F. Forest degradation hinders soil fauna’s contribution to litter decomposition and community dynamics in Andean temperate Nothofagus forests. Appl. Soil Ecol. 2024, 200, 105450. [Google Scholar] [CrossRef]
- Kaal, J.; Cortizas, A.M.; Angst, G.; Kallenbach, C.; Vázquez, C.F.; Criado-Boado, F. Soil organic matter persistence in hyperhumic colluvial soils caused by palaeofires, root inputs and mineral binding. Org. Geochem. 2024, 195, 104848. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, J.; Cao, P.; Wang, Y.; Chen, H.; Wang, W.; Ruan, H. Cellulose dominantly affects soil fauna in the decomposition of forest litter: A meta-analysis. Geoderma 2020, 378, 114620. [Google Scholar] [CrossRef]
- Bonfanti, J.; Potapov, A.M.; Angst, G.; Gananlt, P.; Briones, M.J.I.; Calderón-Sanou, I.; Chen, T.; Conti, E.; Degrune, F.; Eisenhauer, N.; et al. Linking effect traits of soil fauna to processes of organic matter transformation. Funct. Ecol. 2024, 39, 446–461. [Google Scholar] [CrossRef]
- Bardgett, R.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef]
- Filser, J.; Faber, J.H.; Tiunov, A.V.; Brussaard, L.; Frouz, J.; De Deyn, G.; Uvarov, A.V.; Berg, M.P.; Lavelle, P.; Loreau, M.; et al. Soil fauna: Key to new carbon models. Soil 2016, 2, 565–582. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Ding, F.; Gao, X.; Li, S.; Sun, L.; An, T.; Pei, J.; Li, M.; Wang, Y.; et al. Process of plant residue transforming into soil organic matter and mechanism of its stabilization: A review. Acta Pedol. Sin. 2019, 56, 528–540. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X. An overview of the soil microbial carbon pump mechanism for carbon sequestration. Sci. Sin. Terrae 2021, 51, 680–695. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.-C.; Vadeboncoeur, M.A.; Yang, Z.; Chen, S.; Xiong, D.; Xu, C.; Li, Y.; Yang, Y. Root litter inputs exert greater influence over soil C than does aboveground litter in a subtropical natural forest. Plant Soil 2019, 444, 489–499. [Google Scholar] [CrossRef]
- Grandy, A.S.; Wieder, W.R.; Wickings, K.; Kyker-Snowman, E. Beyond microbes: Are fauna the next frontier in soil biogeochemical models? Soil Biol. Biochem. 2016, 102, 40–44. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Dou, Y.; Xue, Z.; Sun, H.; Wang, Y.; Liang, C.; An, S. Advances in the research of transformation and stabilization of soil organic carbon from plant and microbe. Chin. J. Appl. Ecol. 2024, 35, 111–123. [Google Scholar] [CrossRef]
- Rumpel, C.; Eusterhues, K.; Kögel-Knabner, I. Non-cellulosic neutral sugar contribution to mineral associated organic matter in top- and subsoil horizons of two acid forest soils. Soil Biol. Biochem. 2010, 42, 379–382. [Google Scholar] [CrossRef]
- Angst, G.; Potapov, A.; Joly, F.-X.; Angst, Š.; Frouz, J.; Ganault, P.; Eisenhauer, N. Conceptualizing soil fauna effects on labile and stabilized soil organic matter. Nat. Commun. 2024, 15, 5005. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Simpson, A.J.; Wilson, K.P.; Dudley Williams, D.; Simpson, M.J. Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat. Geosci. 2008, 1, 836–839. [Google Scholar] [CrossRef]
- Schnecker, J.; Borken, W.; Schindlbacher, A.; Wanek, W. Little effects on soil organic matter chemistry of density fractions after seven years of forest soil warming. Soil Biol. Biochem. 2016, 103, 300–307. [Google Scholar] [CrossRef]
- Alekseev, I.; Abakumov, E. Soil organic carbon stocks and stability of organic matter in permafrost-affected soils of Yamal region, Russian Arctic. Geoderma Reg. 2022, 28, e00454. [Google Scholar] [CrossRef]
- Lai, L.; Li, Y.; Tian, Y.; Jiang, L.; Zhao, X.; Zhu, L.; Chen, X.; Gao, Y.; Wang, S.; Zheng, Y.; et al. Effects of added organic matter and water on soil carbon sequestration in an arid region. PLoS ONE 2013, 8, e70224. [Google Scholar] [CrossRef]
- Sulman, B.N.; Phillips, R.P.; Oishi, A.C.; Shevliakova, E.; Pacala, S.W. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Change 2014, 4, 1099–1102. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Hafner, B.D.; Schweizer, S.A.; Höschen, C.; Possinger, A.; Lehmann, J.; Bauerle, T. Root exudates simultaneously form and disrupt soil organo-mineral associations. Commun. Earth Environ. 2024, 5, 699. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Zhang, W.; Mao, Q.; Liu, R.; Wang, C.; Wang, S.; Zheng, M.; Taiki, M.; Mao, J.; et al. Effects of simulated atmospheric nitrogen deposition on forest ecosystems in China: An overview. J. Trop. Subtrop. Bot. 2019, 27, 500–522. Available online: http://jtsb.ijournals.cn/html/2019/5/4113.htm (accessed on 8 February 2025).
- Feldman, A.F.; Feng, X.; Felton, A.J.; Konings, A.G.; Knapp, A.K.; Biederman, J.A.; Poulter, B. Plant responses to changing rainfall frequency and intensity. Nat. Rev. Earth Environ. 2024, 5, 276–294. [Google Scholar] [CrossRef]
- Nie, M.; Pendall, E.; Bell, C.; Wallenstein, M.D. Soil aggregate size distribution mediates microbial climate change feedbacks. Soil Biol. Biochem. 2014, 68, 357–365. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Li, Y.; Liu, J.; Wang, G.; Liu, X.; Xie, Z.; Herbert, S.J.; Jin, J. Carbon flow in the plant-soil-microbe continuum in response to atmospheric elevated CO2. Soil Crop 2018, 7, 22–30. Available online: http://sc.iga.ac.cn/article/doi/10.11689/j.issn.2095-2961.2018.01.003 (accessed on 8 February 2025).
- Zhu, Y.; Li, Y.; Han, J.; Yao, H. Effects of changes in water status on soil microbes and their response mechanism: A review. Chin. J. Appl. Ecol. 2019, 30, 4323–4332. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Dong, W.; Li, X.; Song, Y. Role of soil fauna on soil organic matter formation. Soils 2016, 48, 211–218. [Google Scholar] [CrossRef]
- Tan, B.; Wu, F.; Yang, W.; Wang, A.; Yang, Y. Soil fauna community dynamics during soil thawing period in the subalpine and alpine forests of western China. Pol. J. Ecol. 2012, 60, 750–766. Available online: https://www.nstl.gov.cn/paper_detail.html?id=10c77cde89513dce7d8acfc69cfe379d (accessed on 8 February 2025).
- Guidi, C.; Frey, B.; Brunner, I.; Meusburger, K.; Vogel, M.E.; Chen, X.; Stucky, T.; Gwiazdowicz, D.J.; Skubala, P.; Bose, A.K.; et al. Soil fauna drives vertical redistribution of soil organic carbon in a long-term irrigated dry pine forest. Glob. Change Biol. 2022, 28, 3145–3160. [Google Scholar] [CrossRef]
- Niu, G.; Liu, T.; Zhao, Z.; Zhang, X.; Guan, H.; He, X.; Lu, X. Subtropical forest macro-decomposers rapidly transfer litter carbon and nitrogen into soil mineral-associated organic matter. For. Ecosyst. 2024, 11, 100172. [Google Scholar] [CrossRef]
- Ott, D.; Rall, B.C.; Brose, U. Climate change effects on macrofaunal litter decomposition: The interplay of temperature, body masses and stoichiometry. Phil. Trans. R. Soc. B 2012, 367, 3025–3032. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Y.; Zhu, X.; Liu, H.; Liu, J.; Chen, S.; Chen, J.; Zhang, A. Integrated analysis of soil organic matter molecular composition changes under different land uses. Environ. Sci. 2024, 45, 2848–2858. [Google Scholar] [CrossRef]
- Wan, X.; Huang, Z. Application of plant biomarkers to studying carbon cycling in forest soil systems. Acta Pedol. Sin. 2013, 50, 1207–1215. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.; Liu, T.; Jia, J.; Dai, G.; Ma, T.; Liu, Z. Biomarkers and their applications in ecosystem research. Chin. J. Plant Ecol. 2020, 44, 384–394. [Google Scholar] [CrossRef]
- Ma, S.; Chen, G.; Du, E.; Tian, D.; Xing, A.; Shen, H.; Ji, C.; Zheng, C.; Zhu, J.; Zhu, J.; et al. Effects of nitrogen addition on microbial residues and their contribution to soil organic carbon in China’s forests from tropical to boreal zone. Environ. Pollut. 2021, 268, 115941. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.H.; Grandy, A.S.; Sparks, J.P. Elevated atmospheric CO2 drives decreases in stable soil organic carbon in arid ecosystems: Evidence from a physical fractionation and organic compound analysis. Glob. Change Biol. 2024, 30, e17175. [Google Scholar] [CrossRef]
- Becker, J.N.; Dippold, M.A.; Hemp, A.; Kuzyakov, Y. Ashes to ashes: Characterization of organic matter in Andosols along a 3400 m elevation transect at Mount Kilimanjaro using analytical pyrolysis. Catena 2019, 180, 271–281. [Google Scholar] [CrossRef]
- Merino, A.; Fonturbel, M.T.; Fernández, C.; Chávez-Vergera, B.; García-Oliva, F.; Vega, J.A. Inferring changes in soil organic matter in post-wildfire soil burn severity levels in a temperate climate. Sci. Total Environ. 2018, 627, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Startsev, V.; Gorbach, N.; Mazur, A.; Prokushkin, A.; Karpenko, L.; Dymov, A. Macrocharcoal signals in histosols reveal wildfire history of vast western siberian forest-peatland complexes. Plants 2022, 11, 3478. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ma, H.; Luo, C.; Hu, T. Forest soil organic carbon fraction and its measure methods. Chin. J. Soil Sci. 2010, 41, 1018–1024. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, Z.; Xiao, W.; Wang, N.; Liu, J. Organic carbon, organic matter and bulk density regression models for forest soils in Lanlingxi watershed, Three Gorges Reservoir area. J. South China Agric. Univ. 2016, 37, 89–95. [Google Scholar] [CrossRef]
- Blonska, E.; Lasota, J.; Prazuch, W.; Ilek, A. Stabilization of soil organic matter following the impact of selected tree species in temperate climate. Catena 2024, 243, 108185. [Google Scholar] [CrossRef]
- Pan, J.; Wang, C.; Wang, X. Seven-year decomposition rates of roots with different diameters for three temperate broadleaf tree species. Acta Ecol. Sin. 2021, 41, 5166–5174. [Google Scholar] [CrossRef]
- Meyer, N.; Xu, Y.; Karjalainen, K.; Adamczyk, S.; Biasi, C.; van Delden, L.; Martin, A.; Mganga, K.; Myller, K.; Sietiö, O.-M.; et al. Living, dead, and absent trees-How do moth outbreaks shape small-scale patterns of soil organic matter stocks and dynamics at the Subarctic mountain birch treeline? Glob. Change Biol. 2022, 28, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Marty, C.; Piquette, J.; Morin, H.; Bussières, D.; Thiffault, N.; Houle, D.; Bradley, R.L.; Simpson, M.J.; Ouimet, R.; Paré, M.C. Nine years of in situ soil warming and topography impact the temperature sensitivity and basal respiration rate of the forest floor in a Canadian boreal forest. PLoS ONE 2019, 14, 0226909. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Yue, S.; Li, F.; Dong, L.; Bi, M. Retrieval of forest topsoil organic matter’s spatial pattern based on LiDAR data. Chin. J. Appl. Ecol. 2012, 23, 2451–2458. [Google Scholar] [CrossRef]
- Bispo, D.F.A.; Guimaraes, D.V.; Marques, J.J.G.D.E.M.; Beniaich, A.; Acuña-Guzman, S.F.; Silva, M.L.N.; Curi, N. Soil organic carbon as response to reforestation age and land use changes: A qualitative approach to ecosystem services. Sustainability 2023, 15, 6863. [Google Scholar] [CrossRef]
- Jupin, J.L.J.; Ruiz-Fernández, A.C.; Sifeddine, A.; Mendez-Millan, M.; Sanchez-Cabeza, J.A.; Pérez-Bernal, L.H.; Cardoso-Mohedano, J.G.; Gómez-Ponce, M.A.; Flores-Trujillo, J.G. Terrestrial inputs boost organic carbon accumulation in Mexican mangroves. Sci. Total Environ. 2024, 940, 173440. [Google Scholar] [CrossRef] [PubMed]
- Poeplau, C.; Don, A.; Dondini, M.; Leifeld, J.; Nemo, R.; Schumacher, J.; Senapati, N.; Wiesmeier, M. Reproducibility of a soil organic carbon fractionation method to derive RothC carbon pools. Eur. J. Soil Sci. 2013, 64, 735–746. [Google Scholar] [CrossRef]
- Wang, T.; Dong, L.; Liu, Z. Factors driving carbon accumulation in forest biomass and soil organic carbon across natural forests and planted forests in China. Front. For. Glob. Change 2024, 6, 1333868. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Yang, Q.; Ding, X.; Sun, D.; Wei, S. Prediction of soil organic matter content based on artificial neural network model and GF-1 remote sensing data. Soils 2022, 54, 191–197. [Google Scholar] [CrossRef]


| Keyword | Frequency | Total Link Strength | Keyword | Frequency | Total Link Strength |
|---|---|---|---|---|---|
| Climate change | 1401 | 8582 | Vegetation | 177 | 1093 |
| Dynamics | 583 | 3864 | Land use change | 171 | 1110 |
| Organic matter | 537 | 3181 | Stock | 170 | 1138 |
| Forest | 530 | 3371 | Carbon sequestration | 165 | 1142 |
| Nitrogen | 499 | 3410 | Soil respiration | 165 | 1223 |
| Carbon | 440 | 2840 | Temperature sensitivity | 159 | 1165 |
| Decomposition | 407 | 2948 | Boreal forest | 148 | 969 |
| Soil organic carbon | 328 | 2135 | Dissolved organic carbon | 142 | 805 |
| Organic carbon | 311 | 1965 | Growth | 135 | 852 |
| Biomass | 308 | 2119 | Impact | 135 | 823 |
| Soil organic matter | 277 | 1727 | Responses | 135 | 964 |
| Litter decomposition | 256 | 1752 | Litter | 129 | 874 |
| Storage | 255 | 1788 | Leaf litter | 128 | 895 |
| Soil | 247 | 1497 | Model | 120 | 775 |
| Respiration | 246 | 1819 | Management | 119 | 805 |
| Sequestration | 246 | 1680 | Microbial community | 119 | 836 |
| Matter | 235 | 1574 | Pattern | 115 | 762 |
| Forest soils | 218 | 1376 | Net primary production | 109 | 741 |
| Ecosystems | 216 | 1449 | Terrestrial ecosystems | 109 | 755 |
| Temperature | 211 | 1522 | Turnover | 108 | 774 |
| Land use | 189 | 1219 | Elevated CO2 | 106 | 694 |
| Microbial biomass | 188 | 1310 | Mineralization | 105 | 712 |
| Soil carbon | 180 | 1304 | Diversity | 103 | 659 |
| CO2 | 177 | 1198 | Carbon cycle | 102 | 749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Yue, K.; Peng, Y.; Zhang, H.; Li, C.; Li, Y. A Bibliometric Analysis of Research on the Sources and Formation Processes of Forest Soil Organic Matter Under Climate Change. Forests 2025, 16, 336. https://doi.org/10.3390/f16020336
Shen Z, Yue K, Peng Y, Zhang H, Li C, Li Y. A Bibliometric Analysis of Research on the Sources and Formation Processes of Forest Soil Organic Matter Under Climate Change. Forests. 2025; 16(2):336. https://doi.org/10.3390/f16020336
Chicago/Turabian StyleShen, Zhentao, Kai Yue, Yan Peng, Hui Zhang, Cuihuan Li, and Yan Li. 2025. "A Bibliometric Analysis of Research on the Sources and Formation Processes of Forest Soil Organic Matter Under Climate Change" Forests 16, no. 2: 336. https://doi.org/10.3390/f16020336
APA StyleShen, Z., Yue, K., Peng, Y., Zhang, H., Li, C., & Li, Y. (2025). A Bibliometric Analysis of Research on the Sources and Formation Processes of Forest Soil Organic Matter Under Climate Change. Forests, 16(2), 336. https://doi.org/10.3390/f16020336






