Short-Term Effects of Three Tree Species on Soil Physicochemical Properties and Microbial Communities During Land-Use Change from Farmland to Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sample Collection
2.3. Physiochemical Characteristics and Enzymatic Activities
2.4. DNA Extraction, Sequencing, and Quantification
2.5. Statistical Analysis
3. Results
3.1. Effects of Tree Species on Soil Properties
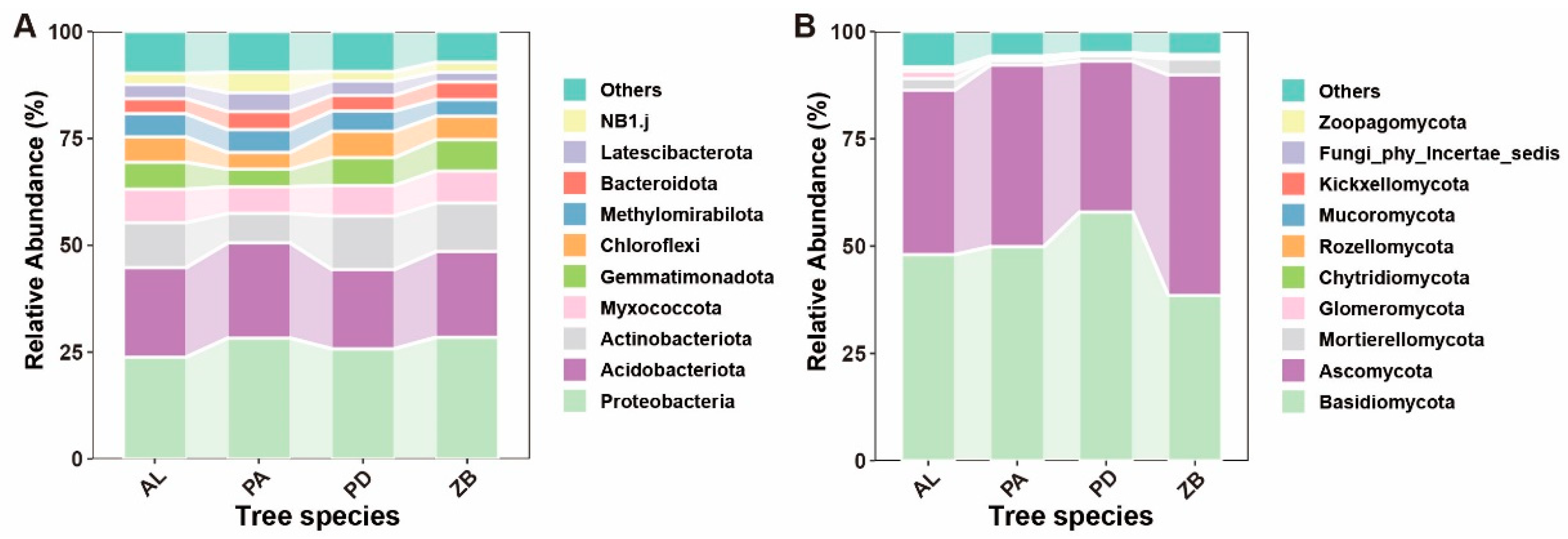
3.2. Effects of Afforestation Tree Species on Microbial Community Composition
3.3. Effects of Afforestation Tree Species on Microbial Community Diversity
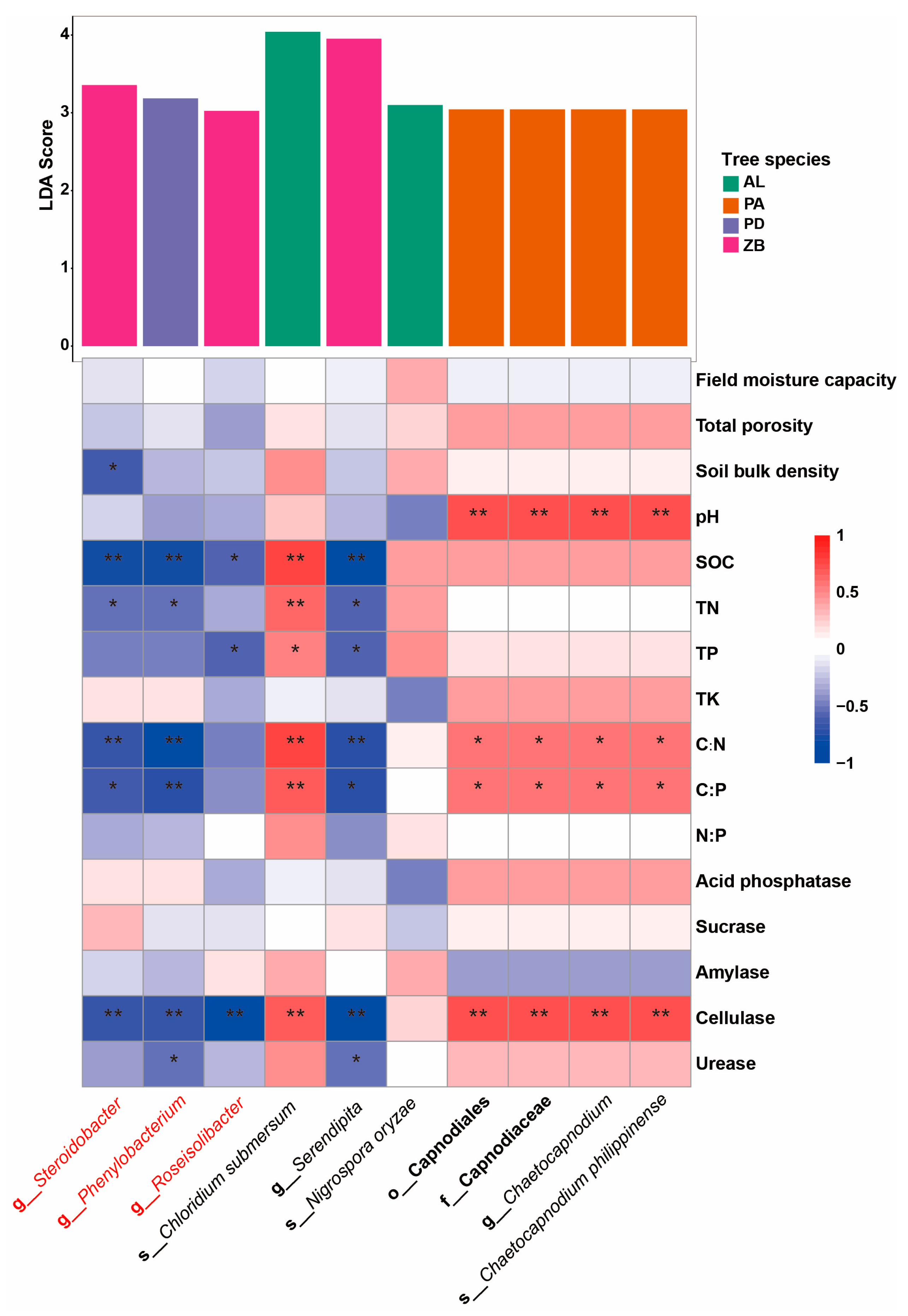
3.4. The Association Between Soil Properties and Microbial Composition
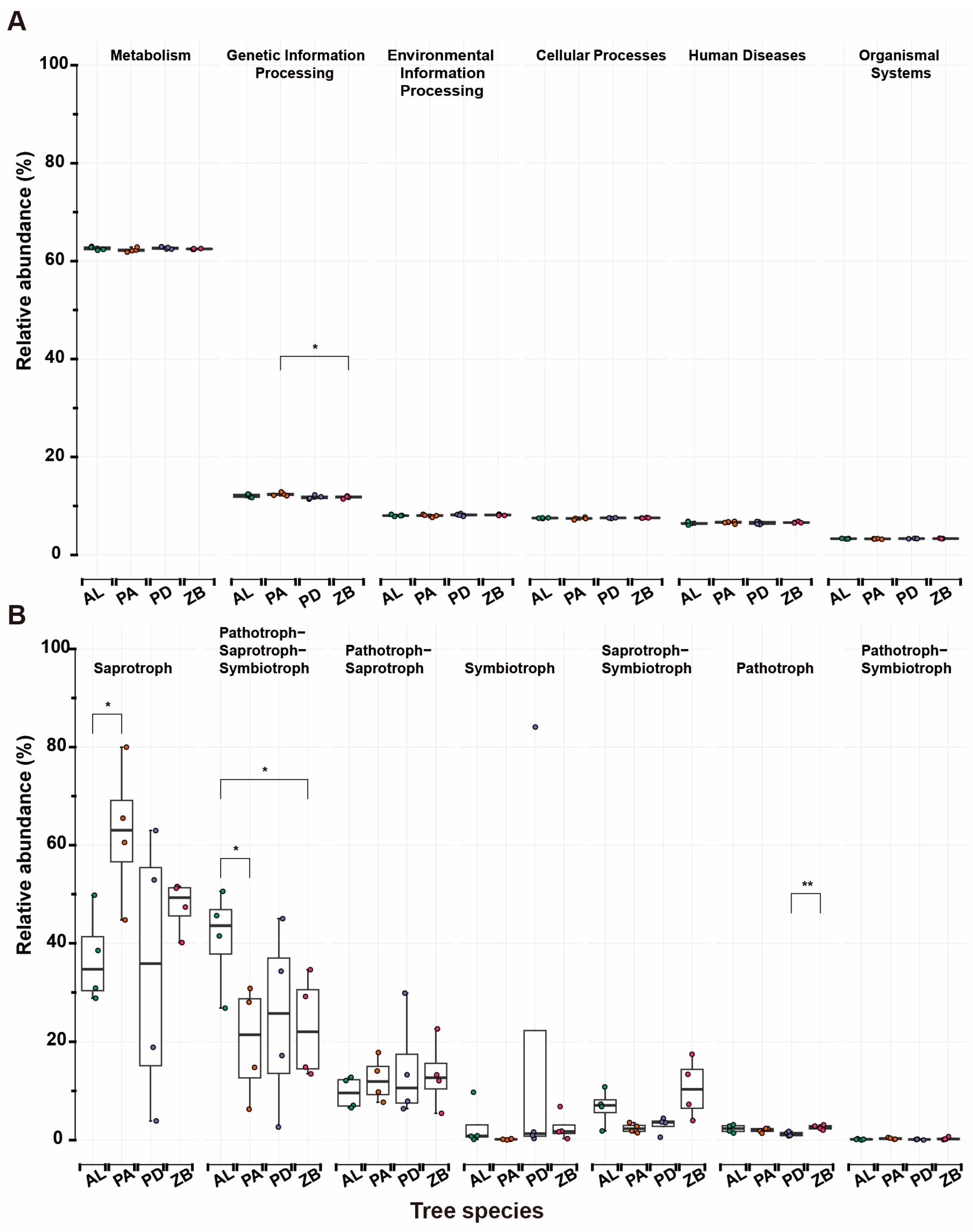
3.5. Functional Profiles of Microbes in Stands Afforested with Different Tree Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Z.; Yang, Y.; Zhang, Y.; Zhang, P.; Li, Y. Grain-for-green policy and its impacts on grain supply in West China. Land Use Policy 2005, 22, 301–312. [Google Scholar] [CrossRef]
- Li, S.; Liu, M. The development process, current situation and prospects of the conversion of farmland to forests and grasses project in China. J. Resour. Ecol. 2022, 13, 120–128. [Google Scholar]
- Li, Y.; Ma, W.; Jiang, G.; Li, G.; Zhou, D. The degree of cultivated land abandonment and its influence on grain yield in main grain producing areas of China. J. Nat. Resour. 2021, 36, 1439–1454. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Cheng, X.; Zhang, Q. Change in soil organic carbon following the ‘Grain-for-Green’ programme in China. Land Degrad. Dev. 2010, 21, 13–23. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Chen, J.; Zhang, Z. Assessing effects of the Returning Farmland to Forest Program on vegetation cover changes at multiple spatial scales: The case of northwest Yunnan, China. J. Environ. Manag. 2022, 304, 114303. [Google Scholar] [CrossRef] [PubMed]
- Delang, C.O.; Wang, W. Chinese forest policy reforms after 1998: The case of the Natural Forest Protection Program and the Slope Land Conversion Program. Int. For. Rev. 2013, 15, 290–304. [Google Scholar] [CrossRef]
- Gutiérrez Rodríguez, L.; Hogarth, N.; Zhou, W.; Putzel, L.; Xie, C.; Zhang, K. Socioeconomic and Environmental Effects of China’s Conversion of Cropland to Forest Program after 15 Years: A Systematic Review Protocol. Environ. Evid. 2015, 4, 6. [Google Scholar] [CrossRef]
- Hagen-Thorn, A.; Callesen, I.; Armolaitis, K.; Nihlgård, B. The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former Agricultural land. Forest Ecol. Manag. 2004, 195, 373–384. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; ten Brink, D.-J.; Brunet, J. Land use effects on soil N, P, C and pH persist over 40–80 Years of forest growth on agricultural soils. Forest Ecol. Manag. 2006, 225, 74–81. [Google Scholar] [CrossRef]
- Zhang, L.; Du, H.; Song, T.; Yang, Z.; Peng, W.; Gong, J.; Huang, G.; Li, Y. Conversion of farmland to forest or grassland improves soil carbon, nitrogen, and ecosystem multi-functionality in a subtropical karst region of southwest China. Sci. Rep. 2024, 14, 17745. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; He, X.; Zheng, Q.; Wang, H.; Wu, Y.; Zhong, Z. Changes in soil properties, X-ray-mineral diffractions and infrared-functional groups in bulk soil and fractions following afforestation of farmland, Northeast China. Sci. Rep. 2017, 7, 12829. [Google Scholar] [CrossRef]
- Qian, J.; Ji, C.; Yang, J.; Zhao, H.; Wang, Y.; Fu, L.; Liu, Q. The advantage of afforestation rsing native tree species to enhance soil quality in degraded forest ecosystems. Sci. Rep. 2024, 14, 20022. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, X.; Zhao, Y.; Cong, P.; Wang, H.; Wang, A.; Chang, S. Sloping farmlands conversion to mixed forest improves soil carbon pool on the Loess Plateau. Int. J. Environ. Res. Public Health 2022, 19, 5157. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Xu, M.; Gao, D.; Fu, S.; Lu, X.; Wu, S.; Han, X.; Yang, G.; Feng, Y. Long-term effects of vegetation and soil on the microbial communities following afforestation of farmland with Robinia pseudoacacia plantations. Geoderma 2020, 367, 114263. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; He, X.; Gao, N.; Li, Q.; Qiu, Z.; Hou, Y.; Shen, W. Soil pH amendment alters the abundance, diversity, and composition of microbial communities in two contrasting agricultural soils. Microbiol. Spectr. 2024, 12, e04165-23. [Google Scholar] [CrossRef] [PubMed]
- Sauze, J.; Ogée, J.; Maron, P.A.; Crouzet, O.; Nowak, V.; Wohl, S.; Kaisermann, A.; Jones, S.P.; Wingate, L. The interaction of soil phototrophs and fungi with pH and their impact on soil CO2, CO18O and OCS Exchange. Soil Biol. Biochem. 2017, 115, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fan, Y.; Jin, C.; Wang, Y.; Yan, F.; Wang, T.; Liu, Q.; Chen, Y.; Deng, F.; Lei, X.; et al. High-yield rice with rich nutrition and low toxicity can be obtained under potato–rice cropping system. J. Sci. Food Agric. 2024, 105, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- He, W.; Wang, Y.; Wang, X.; Wen, X.; Li, T.; Ye, M.; Chen, G.; Zhao, K.; Hou, G.; Li, X.; et al. Stand structure adjustment influences the biomass allocation in naturally generated Pinus massoniana seedlings through environmental factors. Front. Plant Sci. 2022, 13, 997795. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Tie, L.; Hu, J.; Peñuelas, J.; Sardans, J.; Wei, S.; Liu, X.; Zhou, S.; Huang, C. The amounts and ratio of nitrogen and phosphorus addition drive the rate of litter decomposition in a subtropical forest. Sci. Total Environ. 2022, 833, 155163. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Demkina, E.V.; Shanenko, E.F.; Nikolaev, Y.A.; El’-Registan, G.I. Model of the regulation of activity of immobilized enzymes (amylases) in soil. Microbiology 2017, 86, 231–240. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Y.; Zuo, Q.; Du, B.; Chen, W.; Wei, D.; Huang, Q. Contrasting responses of bacterial and fungal communities to aggregate-size fractions and long-term fertilizations in soils of Northeastern China. Sci. Total Environ. 2018, 635, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef]
- Girona-García, A.; Badía-Villas, D.; Martí-Dalmau, C.; Ortiz-Perpiñá, O.; Mora, J.L.; Armas-Herrera, C.M. Effects of Prescribed Fire for Pasture Management on Soil Organic Matter and Biological Properties: A 1-Year study case in the Central Pyrenees. Sci. Total Environ. 2018, 618, 1079–1087. [Google Scholar] [CrossRef]
- Sağlam, R.; Gökbulak, F. Effect of frequent clearcutting on some soil properties, temperatures, and herbaceous vegetation and the potential of their recovery in a short time. Environ. Monit. Assess. 2024, 196, 853. [Google Scholar] [CrossRef]
- Kooijman, A.M.; Weiler, H.A.; Cusell, C.; Anders, N.; Meng, X.; Seijmonsbergen, A.C.; Cammeraat, L.H. Litter quality and microtopography as key drivers to topsoil properties and understorey plant diversity in ancient broadleaved forests on decalcified marl. Sci. Total Environ. 2019, 684, 113–125. [Google Scholar] [CrossRef]
- Yang, H.; Yao, B.; Lian, J.; Su, Y.; Li, Y. Tree species-dependent effects of afforestation on soil fungal diversity, functional guilds and co-occurrence networks in northern China. Environ. Res. 2024, 263, 120258. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Srinivasa Rao, C.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 156, pp. 1–107. [Google Scholar]
- Abdalmoula, M.M.; Makineci, E.; Özturna, A.G.; Pehlivan, S.; Şahin, A.; Tolunay, D. Soil organic carbon accumulation and several physicochemical soil properties under stone pine and maritime pine plantations in coastal dune, Durusu-Istanbul. Environ. Monit. Assess. 2019, 191, 312. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Su, J.; Gao, Z.; Li, S.; Nan, Z. Temporal changes of metal bioavailability and extracellular enzyme activities in relation to afforestation of highly contaminated calcareous soil. Sci. Total Environ. 2018, 622–623, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Durka, W.; Fischer, M.; Dommert, S.; Schöps, R.; Buscot, F.; Wubet, T. Tree species, tree genotypes and tree genotypic diversity levels affect microbe-mediated soil ecosystem functions in a subtropical forest. Sci. Rep. 2016, 6, 36672. [Google Scholar] [CrossRef]
- Feng, J.; Xu, X.; Wu, J.; Zhang, Q.; Zhang, D.; Li, Q.; Long, C.; Chen, Q.; Chen, J.; Cheng, X. Inhibited enzyme activities in soil macroaggregates contribute to enhanced soil carbon sequestration under afforestation in central China. Sci. Total Environ. 2018, 640–641, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kwatcho Kengdo, S.; Peršoh, D.; Schindlbacher, A.; Heinzle, J.; Tian, Y.; Wanek, W.; Borken, W. Long-term soil warming alters fine root dynamics and morphology, and their ectomycorrhizal fungal community in a temperate forest soil. Glob. Change Biol. 2022, 28, 3441–3458. [Google Scholar] [CrossRef]
- Schwob, G.; Roy, M.; Manzi, S.; Pommier, T.; Fernandez, M.P. Green alder (Alnus viridis) encroachment shapes microbial communities in subalpine soils and impacts its bacterial or fungal symbionts differently. Environ. Microbiol. 2017, 19, 3235–3250. [Google Scholar] [CrossRef]
- Lee, J.E.; Eom, A.H. Diversity and community structure of ectomycorrhizal mycorrhizal fungi in roots and rhizosphere soil of Abies koreana and Taxus cuspidata in Mt. Halla. Mycobiology 2022, 50, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ferreras, J.; Hartmann, M. Thinning partially mitigates the impact of Atlantic forest replacement by pine monocultures on the soil microbiome. Front. Microbiol. 2020, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Goss-Souza, D.; Mendes, L.W.; Borges, C.D.; Rodrigues, J.L.M.; Tsai, S.M. Amazon forest-to-agriculture conversion alters rhizosphere microbiome composition while functions are kept. FEMS Microbiol. Ecol. 2019, 95, fiz009. [Google Scholar] [CrossRef]
- Sietiö, O.-M.; Santalahti, M.; Putkinen, A.; Adamczyk, S.; Sun, H.; Heinonsalo, J. Restriction of plant roots in boreal forest organic soils affects the microbial community but does not change the dominance from ectomycorrhizal to saprotrophic fungi. FEMS Microbiol. Ecol. 2019, 95, fiz133. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Pu, Z.; Krumins, V.; Dong, Y.; Li, B.; Hu, M. Paddy soil microbial communities driven by environment- and microbe-microbe interactions: A case study of elevation-resolved microbial communities in a rice terrace. Sci. Total Environ. 2018, 612, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microbiol. 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Zhao, K.H.; Zhao, Y.; Zhang, Y.L.; Fu, Q.; Huang, S.W. Nigrospora oryzae causing panicle branch rot disease on Oryza sativa (Rice). Plant Dis. 2021, 105, 2724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, L.; Wu, J. Genome sequencing and analysis of Nigrospora oryzae, a rice leaf disease fungus. J. Fungi 2024, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Hannula, S.E.; Hundscheid, M.P.J.; Klein Gunnewiek, P.J.A.; De Boer, W. Stimulated saprotrophic fungi in arable soil extend their activity to the rhizosphere and root microbiomes of crop seedlings. Environ. Microbiol. 2021, 23, 6056–6073. [Google Scholar] [CrossRef] [PubMed]
- Frossard, A.; Gerull, L.; Mutz, M.; Gessner, M.O. Disconnect of microbial structure and function: Enzyme activities and bacterial communities in nascent stream corridors. ISME J. 2012, 6, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wu, S.; Shi, W.; Hu, H.; Lin, T.; Zhao, K.; Hou, G.; Fan, C.; Li, X.; Chen, G. Combined effects of cadmium and simulated acid rain on soil microbial communities in the early cultivation of Populus beijingensis seedlings. Ecotox. Environ. Safe 2024, 280, 116583. [Google Scholar] [CrossRef] [PubMed]
- Langenheder, S.; Lindström, E.S.; Tranvik, L.J. Structure and function of bacterial communities emerging from different sources under identical conditions. Appl. Environ. Microb. 2006, 72, 212–220. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S. Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ling, N.; Zhang, R.; Huang, Q.; Xu, Y.; Shen, Q. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017, 37, 202–212. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph:saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Li, Y.; Bezemer, T.M.; Yang, J.; Lü, X.; Li, X.; Liang, W.; Han, X.; Li, Q. Changes in litter quality induced by N deposition alter soil microbial communities. Soil Biol. Biochem. 2019, 130, 33–42. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, Y.; Wang, B. Effects of simulated acid rain on soil respiration and its component in a mixed coniferous-broadleaved forest of the three gorges reservoir area in Southwest China. For. Ecosyst. 2019, 6, 32. [Google Scholar] [CrossRef]



| Tree Species | DBH (cm) | Height (m) | Density (Plant hm−2) | Canopy Density/Cover Density | Dominant Grass Species |
|---|---|---|---|---|---|
| AL | — | — | — | 0.98 | Setaria viridis, Imperata cylindrica, Alternanthera philoxeroides, Bidens pilosa, Pouzolzia zeylanica, Setaria palmifolia, Humulus scandens, Lysimachia christinae, Lactuca indica, Artemisia frigida |
| PA | 4.8 | 4.5 | 4230 | 0.80 | Alternanthera philoxeroides, Artemisia frigida, Setaria palmifolia, Imperata cylindrica, Setaria viridis, Chelidonium majus |
| PD | 10.6 | 11 | 2500 | 0.85 | Alternanthera philoxeroides, Equisetum hyemale, Artemisia frigida, Symphyotrichum subulatu, Phyllanthus urinaria, Trifolium repens |
| ZB | 4.4 | 3.7 | 1667 | 0.70 | Equisetum hyemale, Alternanthera philoxeroides, Trifolium repens, Artemisia argyi, Digitaria sanguinalis |
| Tree Species | Field Moisture Capacity (%) | Total Porosity (%) | Soil Bulk Density (g cm−3) | pH | SOC Content (g kg−1) | TN Content (g kg−1) | TP Content (g kg−1) | TK Content (g kg−1) | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AL | 278.9 ± 9.80 | 43.80 ± 0.55 ab | 1.50 ± 0.02 | 6.90 ± 0.17 b | 15.56 ± 0.34 ab | 1.86 ± 0.06 | 0.55 ± 0.02 | 12.22 ± 0.29 | 8.39 ± 0.10 b | 28.21 ± 0.79 b | 3.36 ± 0.06 a |
| PA | 263.88 ± 7.12 | 44.56 ± 1.00 a | 1.51 ± 0.02 | 8.07 ± 0.05 a | 19.01 ± 1.74 a | 1.77 ± 0.22 | 0.52 ± 0.04 | 13.10 ± 0.21 | 11.03 ± 1.21 a | 36.81 ± 2.44 a | 3.39 ± 0.20 a |
| PD | 274.93 ± 9.56 | 44.98 ± 0.31 a | 1.47 ± 0.04 | 7.15 ± 0.10 b | 8.99 ± 1.98 c | 1.21 ± 0.21 | 0.49 ± 0.05 | 13.31 ± 0.67 | 7.26 ± 0.40 b | 17.77 ± 2.82 c | 2.41 ± 0.27 b |
| ZB | 258.18 ± 6.72 | 40.89 ± 1.54 b | 1.46 ± 0.02 | 7.16 ± 0.14 b | 11.75 ± 1.38 bc | 1.42 ± 0.16 | 0.41 ± 0.02 | 12.43 ± 0.20 | 8.25 ± 0.05 b | 28.78 ± 2.49 b | 3.49 ± 0.29 a |
| Tree Species | Acid Phosphatase (μg g−1 2 h−1) | Sucrase (mg g−1 24 h−1) | Amylase (mg g−1 24 h−1) | Cellulase (mg g−1 72 h−1) | Urease (mg g−1 24 h−1) |
|---|---|---|---|---|---|
| AL | 12.22 ± 0.29 | 2.65 ± 0.63 | 0.97 ± 0.11 a | 0.29 ± 0.03 a | 0.26 ± 0.01 b |
| PA | 13.1 ± 0.21 | 3.64 ± 0.92 | 0.65 ± 0.1 b | 0.35 ± 0.03 a | 0.45 ± 0.10 a |
| PD | 13.31 ± 0.67 | 3.51 ± 1.14 | 0.54 ± 0.09 b | 0.20 ± 0.03 b | 0.22 ± 0.03 b |
| ZB | 12.43 ± 0.20 | 4.32 ± 1.53 | 0.82 ± 0.06 ab | 0.17 ± 0.03 b | 0.26 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, Y.; Lin, J.; Mu, C.; Wang, Y.; He, Z.; Chen, G.; Ding, W. Short-Term Effects of Three Tree Species on Soil Physicochemical Properties and Microbial Communities During Land-Use Change from Farmland to Forests. Forests 2025, 16, 362. https://doi.org/10.3390/f16020362
Jian Y, Lin J, Mu C, Wang Y, He Z, Chen G, Ding W. Short-Term Effects of Three Tree Species on Soil Physicochemical Properties and Microbial Communities During Land-Use Change from Farmland to Forests. Forests. 2025; 16(2):362. https://doi.org/10.3390/f16020362
Chicago/Turabian StyleJian, Yi, Jing Lin, Changlong Mu, Yuqi Wang, Zhenyang He, Gang Chen, and Wei Ding. 2025. "Short-Term Effects of Three Tree Species on Soil Physicochemical Properties and Microbial Communities During Land-Use Change from Farmland to Forests" Forests 16, no. 2: 362. https://doi.org/10.3390/f16020362
APA StyleJian, Y., Lin, J., Mu, C., Wang, Y., He, Z., Chen, G., & Ding, W. (2025). Short-Term Effects of Three Tree Species on Soil Physicochemical Properties and Microbial Communities During Land-Use Change from Farmland to Forests. Forests, 16(2), 362. https://doi.org/10.3390/f16020362







