Variations in Leaf Photosynthesis and Its Limitations at Different Canopy Positions in Mature Camphor Trees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Environmental Measurements
2.4. Gas Exchange and Chlorophyll Fluorescence Measurements
2.5. Estimation of Mesophyll Conductance and Biochemical Parameters
2.6. Quantitative Limitation to the Light-Saturated Photosynthesis(Amax)
2.7. Concentration of Foliar Carbon, Nitrogen, and Photosynthetic Pigments
2.8. Statistical Analysis
3. Results
3.1. Growth Environment
3.2. Effect of Canopy Position and Season on Leaf Photosynthetic Traits
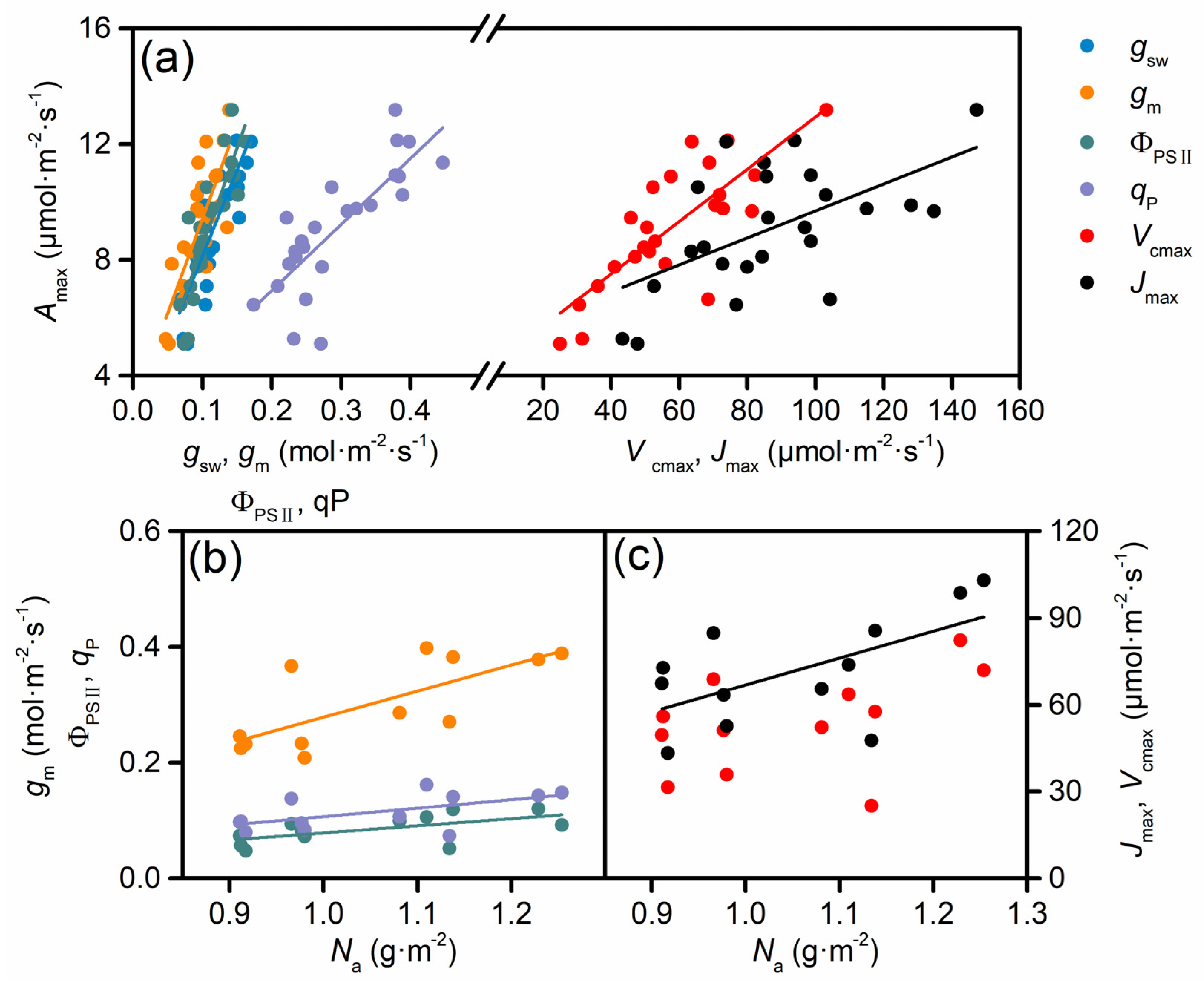
3.3. Correlations Among Amax and Other Photosynthetic Traits
3.4. Quantitative Limitation to Photosynthesis Throughout the Canopy
4. Discussion
4.1. Foliar Physiological and Biochemical Traits Regulated by Light Availability
4.2. Strategies of Canopy Resource Allocation to Photosynthetic Traits
4.3. Effect of Season on Canopy Photosynthesis and Its Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozanne, C.M.P.; Anhuf, D.; Boulter, S.L.; Keller, M.; Kitching, R.L.; Ko, C.; Meinzer, F.C.; Mitchell, A.W.; Nakashizuka, T.; Dias, P.L.S.; et al. Biodiversity meets the atmosphere: A global view of forest canopies. Science 2003, 301, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F. Light heterogeneity and plants: From ecophysiology to species coexistence and biodiversity. In Progress in Botany; Esser, K., Lüttge, U., Beyschlag, W., Hellwig, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 439–471. [Google Scholar]
- Niinemets, Ü.; Anten, N.P.R. Packing the photosynthesis machinery: From leaf to canopy. In Photosynthesis in Silico; Laisk, A., Nedbal, L., Govindjee, Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 363–399. [Google Scholar]
- Bachofen, C.; D’Odorico, P.; Buchmann, N. Light and VPD gradients drive foliar nitrogen partitioning and photosynthesis in the canopy of European beech and silver fir. Oecologia 2020, 192, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Iio, A.; Fukasawa, H.; Nose, Y.; Kato, S.; Kakubari, Y. Vertical, horizontal and azimuthal variations in leaf photosynthetic characteristics within a Fagus crenata crown in relation to light acclimation. Tree Physiol. 2005, 25, 533–544. [Google Scholar] [CrossRef]
- Hanba, Y.T.; Kogami, H.; Terashima, I. The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ. 2002, 25, 1021–1030. [Google Scholar] [CrossRef]
- Kenichi, Y. Irradiance heterogeneity within crown affects photosynthetic capacity and nitrogen distribution of leaves in Cedrela sinensis. Plant Cell Environ. 2010, 33, 750–758. [Google Scholar] [CrossRef]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between saplings and adult trees: An integration of field results by meta-analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef]
- Valladares, F.; Pearcy, R.W. The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia 1998, 114, 1–10. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Field, C. Allocating leaf nitrogen for the maximization of carbon gain: Leaf age as a control on the allocation program. Oecologia 1983, 56, 341–347. [Google Scholar] [CrossRef]
- Lloyd, J.; Wong, S.; Styles, J.M.; Batten, D.; Priddle, R.; Turnbull, C.; McConchie, C. Measuring and modelling whole-tree gas exchange. Funct. Plant Biol. 1995, 22, 987–1000. [Google Scholar] [CrossRef]
- Hirtreiter, J.N.; Potts, D.L. Canopy structure, photosynthetic capacity and nitrogen distribution in adjacent mixed and monospecific stands of Phragmites australis and Typha latifolia. Plant Ecol. 2012, 213, 821–829. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef]
- Niinemets, Ü. Variation in leaf photosynthetic capacity within plant canopies: Optimization, structural, and physiological constraints and inefficiencies. Photosyn Res. 2023, 158, 131–149. [Google Scholar] [CrossRef]
- Peltoniemi, M.S.; Duursma, R.A.; Medlyn, B.E. Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiol. 2012, 32, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Aasamaa, K.; Sõber, A.; Hartung, W.; Niinemets, Ü. Drought acclimation of two deciduous tree species of different layers in a temperate forest canopy. Trees 2004, 18, 93–101. [Google Scholar] [CrossRef]
- Sellin, A.; Õunapuu, E.; Kupper, P. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula). Physiol. Plant. 2008, 134, 412–420. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Sellin, A.; Kupper, K. Effects of light availability versus hydraulic constraints on stomatal responses within a crown of silver birch. Oecoligia 2005, 142, 388–397. [Google Scholar] [CrossRef]
- Sellin, A.; Kupper, K. Effects of enhanced hydraulic supply for foliage on stomatal responses in little-leaf linden (Tilia cordata Mill). Eur. J. For. Res. 2007, 126, 241–251. [Google Scholar] [CrossRef]
- Niinemets, Ü. Optimization of foliage photosynthetic capacity in tree canopies: Towards identifying missing constraints. Tree Physiol. 2012, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.L.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance and leaf hydraulic conductance in response to changing light across species: Coordination of CO2 diffusion and H2O transport inside leaves. Plant Cell Environ. 2017, 41, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Ubierna, N.; Gandin, A.; Cousins, A. The response of mesophyll conductance to short-term variation in CO2 in the C4 plants Setaria viridis and Zea mays. J. Exp. Bot. 2018, 69, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Li, S.L.; Tan, T.T.; Fan, Y.F.; Muhammad Ali, R.; Wang, Z.L.; Wang, B.B.; Zhang, J.W.; Tan, X.M.; Chen, P.; Iram, S.; et al. Responses of leaf stomatal and mesophyll conductance to abiotic stress factors. J. Integr. Agric. 2022, 21, 2787–2804. [Google Scholar] [CrossRef]
- Grassi, G.; Meir, P.; Cromer, R.; Tompkins, D.; Jarvis, P.G. Photosynthetic parameters in seedlings of Eucalyptus grandis as affected by rate of nitrogen supply. Plant Cell Environ. 2002, 25, 1677–1688. [Google Scholar] [CrossRef]
- Bachofen, C.; Hülsmann, L.; Revil, A.; Buchmann, N.; D’Odorico, P. Accounting for foliar gradients in Vcmax and Jmax improves estimates of net CO2 exchange of forests. Agric. For. Meteorol. 2022, 314, 108771. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Walker, A.P.; Quaife, T.; van Bodegom, P.M.; de Kauwe, M.G.; Keenan, T.F.; Joiner, J.; Lomas, M.R.; MacBean, N.; Xu, C.; Yang, X.; et al. The impact of alternative trait-scaling hypotheses for the maximum photosynthetic carboxylation rate (Vcmax) on global gross primary production. New Phytol. 2017, 215, 1370–1386. [Google Scholar] [CrossRef]
- Guo, J.M.; Beverly, D.P.; Ewers, B.E.; Williams, D.G. Stomatal, mesophyll and biochemical limitations to photosynthesis and their relationship with leaf structure over an elevation gradient in two conifers. Photosyn Res. 2023, 157, 85–101. [Google Scholar] [CrossRef]
- Crous, K.Y.; Campany, C.; López, R.; Cano, F.J.; Ellsworth, D.S. Canopy position affects photosynthesis and anatomy in mature Eucalyptus trees in elevated CO2. Tree Physiol. 2020, 41, 206–222. [Google Scholar] [CrossRef]
- Campany, C.E.; Tjoelker, M.G.; von Caemmerer, S.; Duursma, R.A. Coupled response of stomatal and mesophyll conductance to light enhances photosynthesis of shade leaves under sunflecks. Plant Cell Environ. 2016, 39, 2762–2773. [Google Scholar] [CrossRef] [PubMed]
- Cano, F.J.; Sánchez-Gómez, D.; Rodríguez-Calcerrada, J.; Warren, C.R.; Gil, L.; Aranda, I. Effects of drought on mesophyll conductance and photosynthetic limitations at different tree canopy layers. Plant Cell Environ. 2013, 36, 1961–1980. [Google Scholar] [CrossRef]
- Zhong, J.J.; Li, X.M.; Wang, B.Y.; Xu, X.B.; Yan, W. Investigation and Analysis of the Woody Plants in Shanghai Parks. Chin. Landsc. Archit. 2019, 35, 123–127. (In Chinese) [Google Scholar] [CrossRef]
- Evans, J.R.; Loreto, F. Acquisition and diffusion of CO2 in higher plant leaves. In Photosynthesis: Physiology and Metabolism; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Kluwer Academic: Dordrecht, The Netherland, 2000; pp. 321–351. [Google Scholar]
- Harley, P.C.; Loreto, F.; Dimarco, G.; Sharkey, T.D. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Pimentel, C.; Longi, S.P. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 2003, 26, 1419–1430. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Schmiege, S.C.; Griffin, K.L.; Boelman, N.T.; Vierling, L.A.; Bruner, S.G.; Min, E.; Maguire, A.J.; Jensen, J.; Eitel, J.U.H. Vertical gradients in photosynthetic physiology diverge at the latitudinal range extremes of white spruce. Plant Cell Environ. 2022, 46, 45–63. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Evergreen trees do not maximize instantaneous photosynthesis. Trends Plant Sci. 2004, 9, 270–274. [Google Scholar] [CrossRef]
- González-Zurdo, P.; Escudero, A.; Babiano, J.; García-Ciudad, A.; Mediavilla, S. Costs of leaf reinforcement in response to winter cold in evergreen species. Tree Physiol. 2016, 36, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Cao, X.; Ma, K. Leaf functional traits vary with the adult height of plant species in forest communities. J. Plant Ecol. 2014, 7, 68–76. [Google Scholar] [CrossRef]
- Gardner, A.; Ellsworth, D.S.; Pritchard, J.; MacKenzie1, A.R. Are chlorophyll concentrations and nitrogen across the vertical canopy profile affected by elevated CO2 in mature Quercus trees? Trees 2022, 36, 1797–1809. [Google Scholar] [CrossRef]
- Sharwood, R.E.; Crous, K.Y.; Whitney, S.M.; Ellsworth, D.S.; Ghannoum, O. Linking photosynthesis and leaf N allocation under future elevated CO2 and climate warming in Eucalyptus globulus. J. Exp. Bot. 2017, 68, 1157–1167. [Google Scholar] [CrossRef]
- Lennon, A.M.; Lewis, V.R.; Farrell, A.D.; Umaharan, P. Photochemical responses to light in sun and shade leaves of Theobroma cacao L. (West African Amelonado). Sci. Hortic. 2021, 276, 109747. [Google Scholar] [CrossRef]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef]
- Magney, T.S.; Eitel, J.U.H.; Griffin, K.L.; Boelman, N.T.; Greaves, H.E.; Prager, C.M.; Logan, B.A.; Zheng, G.; Ma, L.; Fortin, E.A.; et al. LiDAR canopy radiation model reveals patterns of photosynthetic partitioning in an Arctic shrub. Agric. For. Meteorol. 2016, 221, 78–93. [Google Scholar] [CrossRef]
- Crous, K.Y.; Ellsworth, D.S. Canopy position affects photosynthetic adjustments to long-term elevated CO2 concentration (FACE) in aging needles in a mature Pinus taeda forest. Tree Physiol. 2004, 24, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Medlyn, B.E.; Dukes, J.S.; Bonan, G.; von Caemmerer, S.; Dietze, M.C.; Zaehle, S. A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol. 2017, 213, 22–42. [Google Scholar] [CrossRef]
- Bauerle, W.L.; McCullough, C.; Iversen, M.; Hazlett, M. Leaf age and position effects on quantum yield and photosynthetic capacity in Hemp crowns. Plants 2020, 9, 271. [Google Scholar] [CrossRef]
- Warren, J.M.; Jensen, A.M.; Medlyn, B.E.; Norby, R.J.; Tissue, D.T. Carbon dioxide stimulation of photosynthesis in Liquidambar styracifua is not sustained during a 12-year field experiment. AoB Plants 2015, 7, plu074. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.L.; Liu, X.; Liu, L.; Douthe, C.; Li, Y.; Peng, S.B.; Liang, H.J. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ. 2015, 38, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Quebbeman, J.A.; Ramirez, J.A. Optimal allocation of leaf-level nitrogen: Implications for covariation of Vcmax and Jmax and photosynthetic downregulation. J. Geophys. Res. Biogeosci. 2016, 121, 2464–2475. [Google Scholar] [CrossRef]
- Rajsnerová, P.; Klem, K.; Holub, P.; Novotná, K.; Večeřová, K.; Kozáčiková, M.; Rivas-Ubach, A.; Sardans, J.; Marek, M.V.; Peñuelas, J.; et al. Morphological, biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude. Tree Physiol. 2015, 35, 47–60. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt Georg Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits -Vcmax and Jmax- to leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef]
- Buckley, T.N. Optimal carbon partitioning helps reconcile the apparent divergence between optimal and observed canopy profiles of photosynthetic capacity. New Phytol. 2021, 230, 2246–2260. [Google Scholar] [CrossRef]
- Yu, L.; Luo, X.; Croft, H.; Rogers, C.A.; Chen, J.M. Seasonal variation in the relationship between leaf chlorophyll content and photosynthetic capacity. Plant Cell Environ. 2024, 47, 3953–3965. [Google Scholar] [CrossRef]
- Bown, H.E.; Watt, M.S.; Mason, E.G.; Cliton, P.W.; Whitehead, D. The influence of nitrogen and phosphorus supply and genotype on mesophyll conductance limitations to photosynthesis in Pinus radiata. Tree Physiol. 2009, 29, 1143–1151. [Google Scholar] [CrossRef]
- Hacke, U.G.; Plavcová, L.; Almeida-Rodriguez, A.; King-Jones, S.; Zhou, W.; Cooke, J.E.K. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol. 2010, 30, 1016–1025. [Google Scholar] [CrossRef]
- Ligaba, A.; Katsuhara, M.; Shibasaka, M.; Djira, G. Abiotic stresses modulate expression of major intrinsic proteins in barley (Hordeum vulgare). C R. Biol. 2011, 334, 127–139. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef]
- Ryan, M.J.; Yoder, B.J. Hydraulic limits to tree height and tree growth. BioSci. 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Domec, J.C.; Lachenbruch, B.; Meinzer, F.C.; Woodruff, D.R.; Warren, J.M.; McCulloh, K.A. Maximum height in a conifer is associated with conflicting requirements for xylem design. Proc. Natl. Acad. Sci. USA 2008, 105, 12069–12074. [Google Scholar] [CrossRef]
- Givnish, T.J.; Wong, S.C.; Stuart-Williams, H.; Holloway-Phillips, M.; Farquhar, G.D. Determinants of maximum tree height in Eucalyptus species along a rainfall gradient in Victoria, Australia. Ecology 2014, 95, 2991–3007. [Google Scholar] [CrossRef]
- Wright, I.J.; Leishman, M.R.; Read, C.; Westoby, M. Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct. Plant Biol. 2006, 33, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Koike, N.; Ishida, A. Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Environ. 2002, 25, 1341–1356. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides×nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef]
- Buckley, T.N.; Martorell, S.; Diaz-Espejo, A.; Tomàs, M.; Medrano, H. Is stomatal conductance optimized over both time and space in plant crowns? A field test in grapevine (Vitis vinifera). Plant Cell Environ. 2014, 37, 2707–2712. [Google Scholar] [CrossRef]
- Uehlein, N.; Sperling, H.; Heckwolf, M.; Kaldenhoff, R. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ. 2012, 35, 1077–1083. [Google Scholar] [CrossRef]
- Momayyezi, M.; Guy, R.D. Substantial role for carbonic anhydrase in latitudinal variation in mesophyll conductance of Populus trichocarpa Torr. & Gray. Plant Cell Environ. 2017, 40, 138–149. [Google Scholar] [CrossRef]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Boex-Fontvieille, E.; Jossier, M.; Davanture, M.; Zivy, M.; Hodges, M.; Tcherkez, G. Differential protein phosphorylation regulates chloroplast movement in response to strong light and darkness in Arabidopsis thaliana. Plant Mol. Biol. Rep. 2014, 32, 987–1001. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef]
- Pons, T.L.; Welschen, R.A.M. Midday depression of net photosynthesis in the tropical rainforest tree Eperua grandiflora: Contributions of stomatal and internal conductances, respiration and Rubisco functioning. Tree Physiol. 2003, 23, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R.; Dreyer, E. Temperature response of photosynthesis and internal conductance to CO2: Results from two independent approaches. J. Exp. Bot. 2006, 57, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
- Montpied, P.; Granier, A.; Dreyer, E. Seasonal time-course of gradients of photosynthetic capacity and mesophyll conductance to CO2 across a beech (Fagus sylvatica L.) canopy. J. Exp. Bot. 2009, 60, 2407–2418. [Google Scholar] [CrossRef]
- Tosens, T.; Niinemets, Ü.; Vislap, V.; Eichelmann, H.; Castro Díez, P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: How structure constrains function. Plant Cell Environ. 2012, 35, 839–856. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Tjoelker, M.G.; Aspinwall, M.J.; Battaglia, M.; Cano, F.J.; Carter, K.R.; Cavaleri, M.A.; Cernusak, L.A.; et al. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 2019, 222, 768–784. [Google Scholar] [CrossRef]

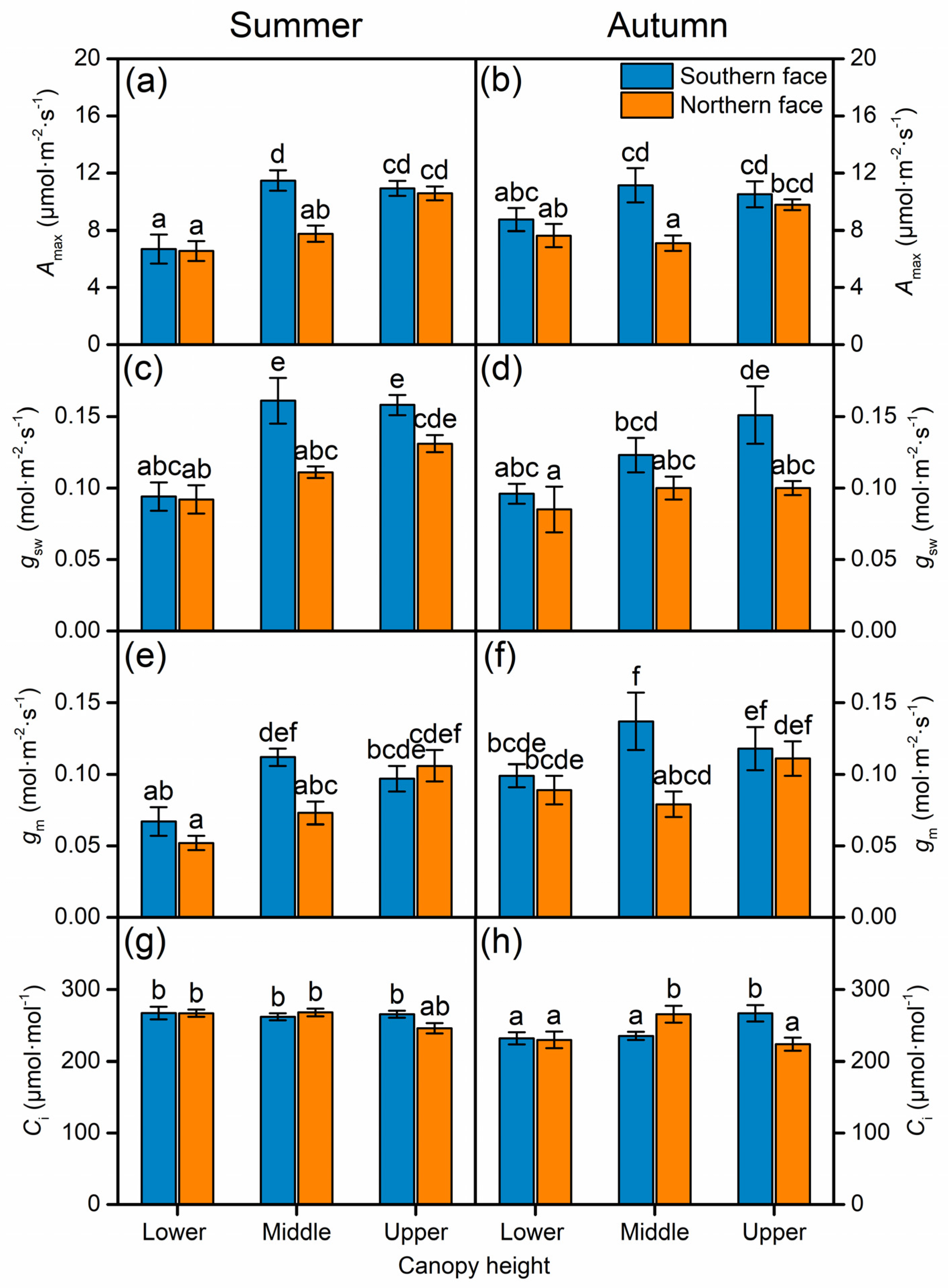
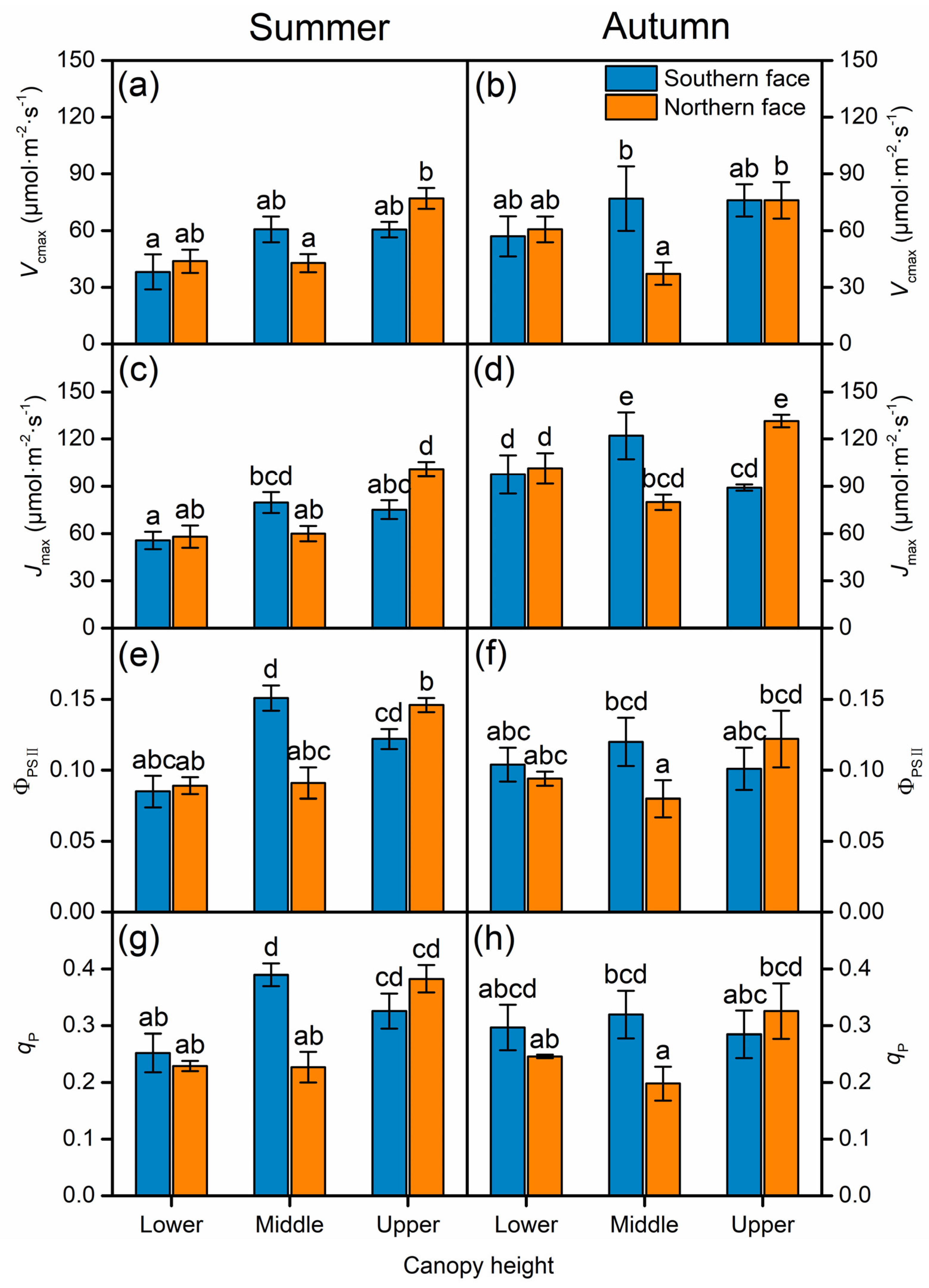
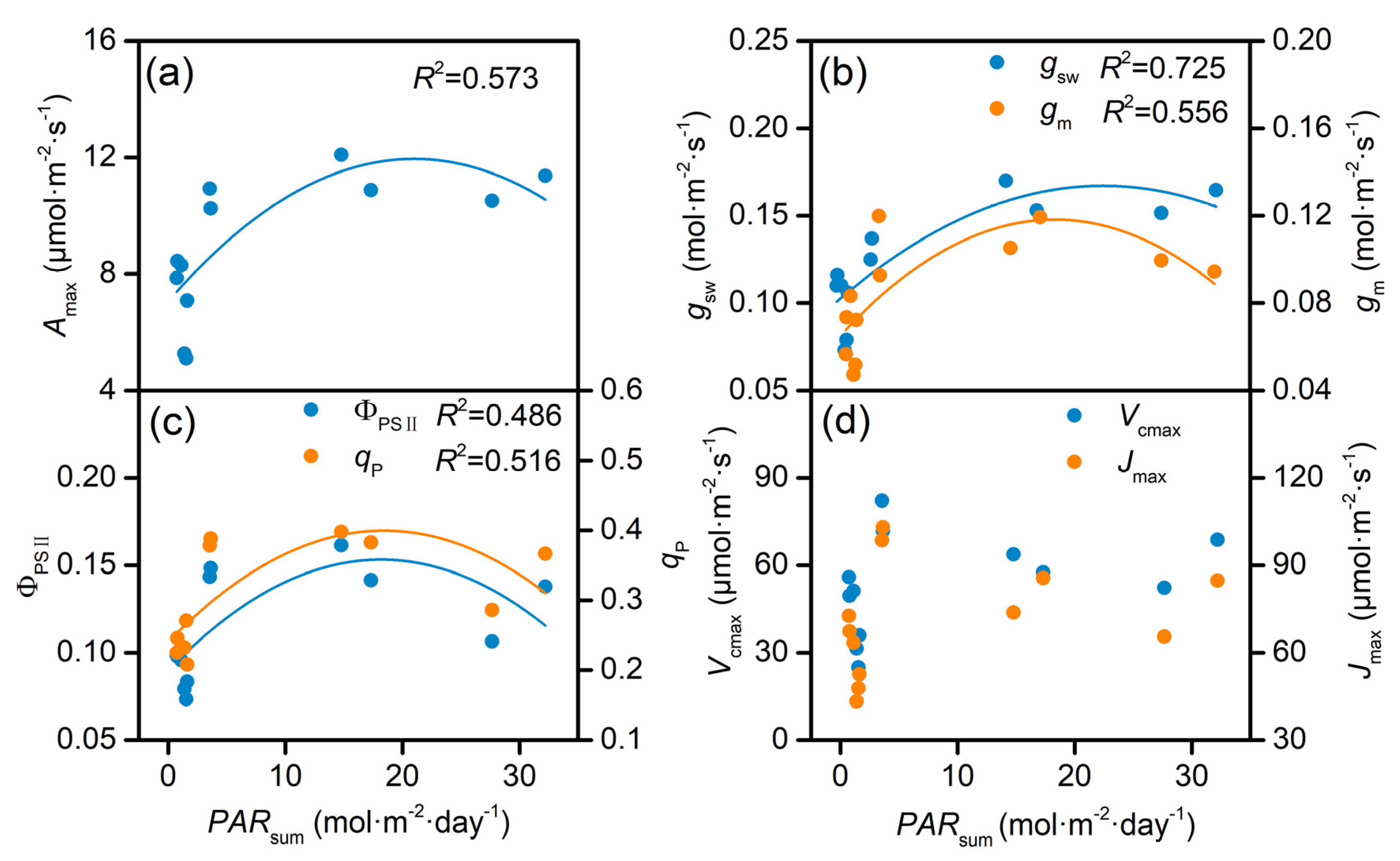
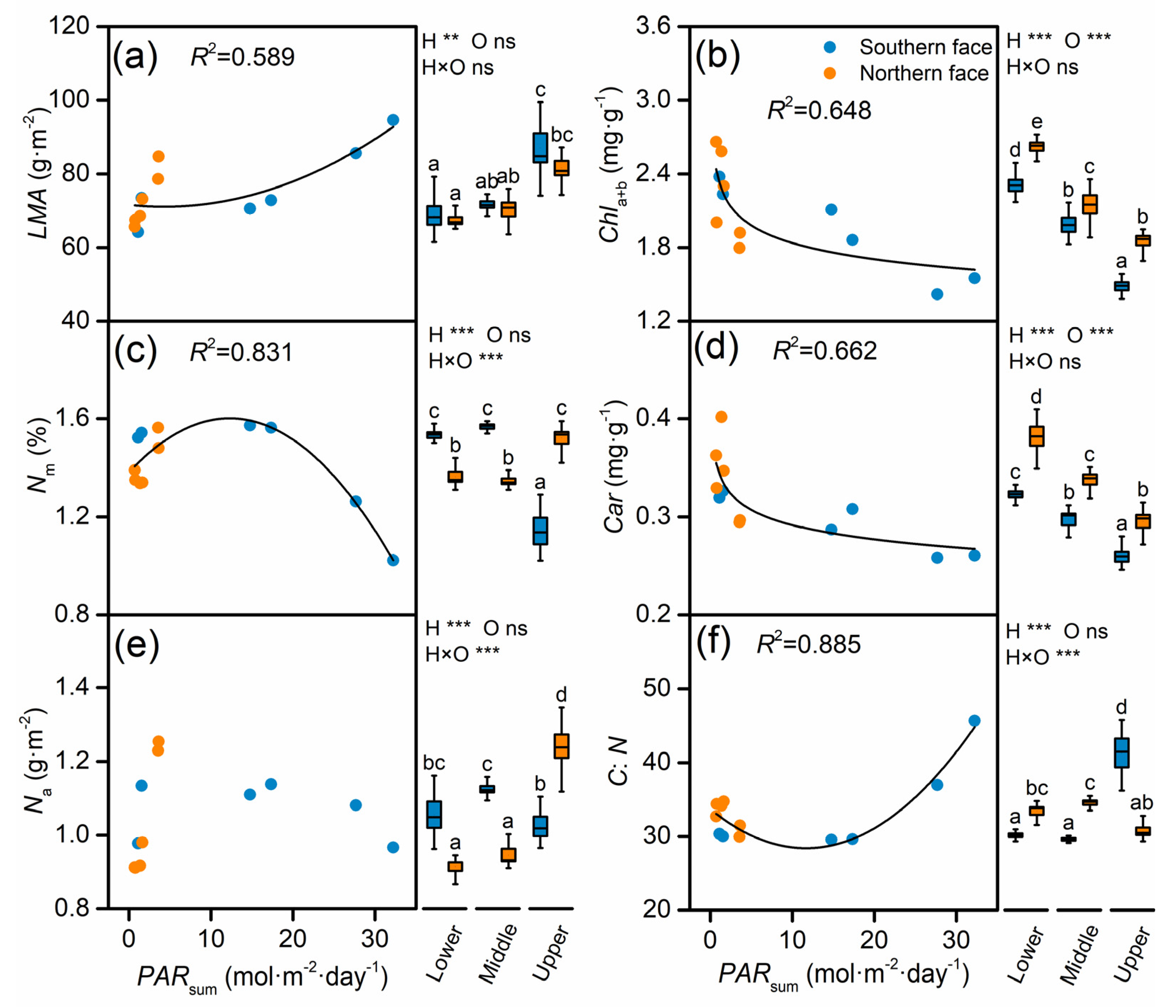

| Date | Canopy Height | Canopy Orientation | H × O | D × H × O | |
|---|---|---|---|---|---|
| Amax | 0.07 | 11.73 *** | 13.53 ** | 6.06 ** | 0.32 |
| gsw | 6.41 * | 10.34 ** | 12.94 ** | 1.74 | 1.98 |
| gm | 13.23 ** | 5.78 * | 8.29 ** | 3.91 * | 1.13 |
| Ci | 10.55 ** | 2.75 | 0.27 | 14.60 *** | 1.22 |
| Cc | 0.50 | 0.65 | 0.49 | 3.25 | 0.83 |
| Ci-Cc | 15.08 ** | 0.28 | 0.01 | 0.04 | 1.63 |
| Vcmax | 0.60 | 4.81 * | 0.54 | 11.39 *** | 0.14 |
| Jmax | 45.81 *** | 9.19 ** | 0.06 | 25.65 *** | 0.49 |
| ΦPSII | 4.11 | 4.93 * | 2.37 | 10.15 ** | 1.79 |
| qP | 2.95 | 5.23 * | 4.48 * | 8.03 ** | 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, H.; Zhou, L.; Yan, W. Variations in Leaf Photosynthesis and Its Limitations at Different Canopy Positions in Mature Camphor Trees. Forests 2025, 16, 581. https://doi.org/10.3390/f16040581
Leng H, Zhou L, Yan W. Variations in Leaf Photosynthesis and Its Limitations at Different Canopy Positions in Mature Camphor Trees. Forests. 2025; 16(4):581. https://doi.org/10.3390/f16040581
Chicago/Turabian StyleLeng, Hanbing, Lingyan Zhou, and Wei Yan. 2025. "Variations in Leaf Photosynthesis and Its Limitations at Different Canopy Positions in Mature Camphor Trees" Forests 16, no. 4: 581. https://doi.org/10.3390/f16040581
APA StyleLeng, H., Zhou, L., & Yan, W. (2025). Variations in Leaf Photosynthesis and Its Limitations at Different Canopy Positions in Mature Camphor Trees. Forests, 16(4), 581. https://doi.org/10.3390/f16040581








