Ecological Benefits and Structure of Mixed vs. Pure Forest Plantations in Subtropical China
Abstract
1. Introduction
2. Materials and Methods
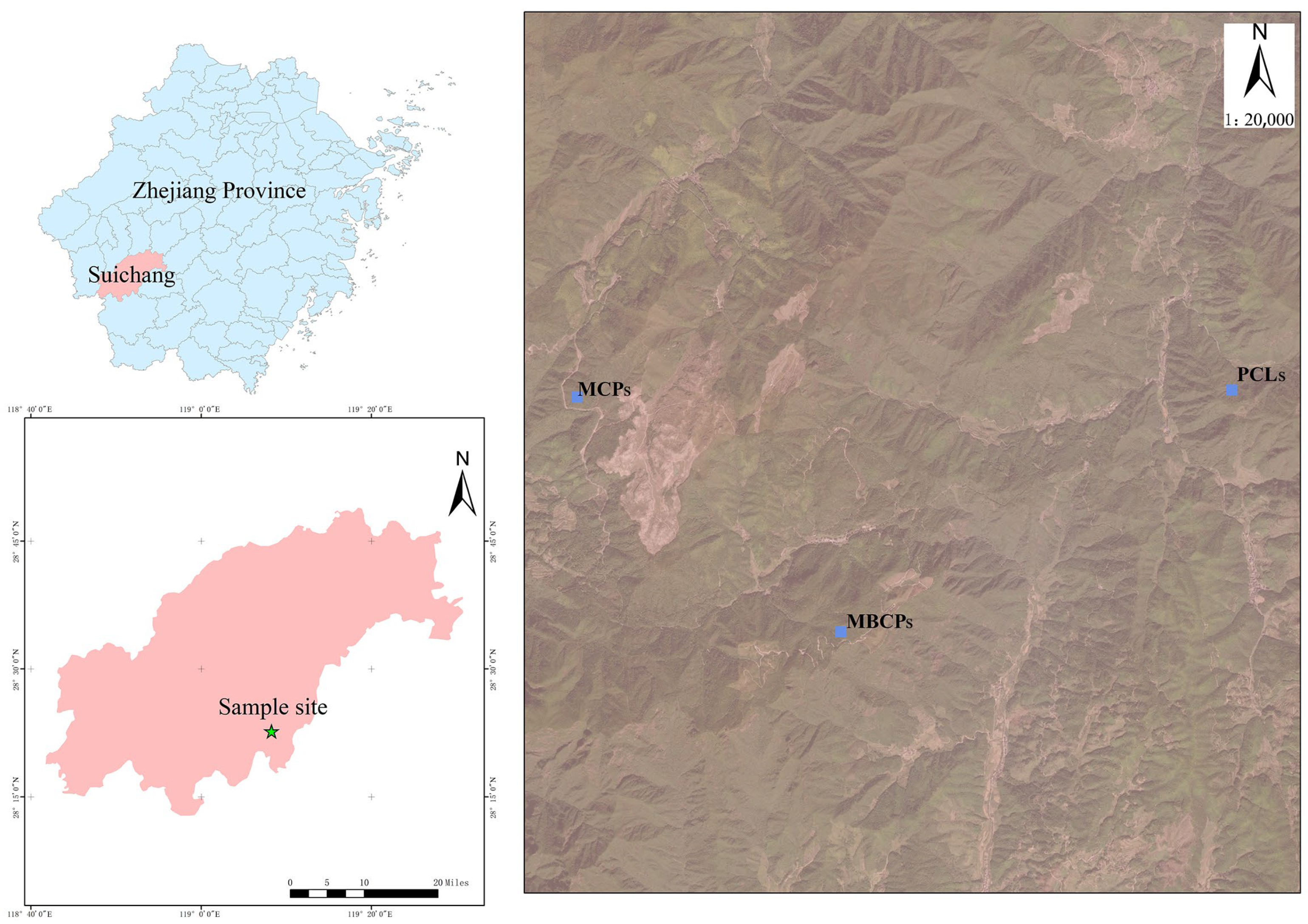
2.1. Study Area
2.2. Forest Management History
2.3. Field Survey and Sampling
2.4. Survey of Understory Shrubs and Herbaceous Plants
2.5. Investigation of Physical and Chemical Properties of Soil
2.6. Data Analysis
2.6.1. Analysis of Stand Biomass
2.6.2. Calculation of the Importance Value and Index for Forest Spatial Structure
- Uniform angle index ()
- Neighborhood Comparison ()
- Mingling degree ()
2.6.3. Species Diversity of Understory Shrubs and Herbs
2.6.4. Physico-Chemical Properties of Soil
2.7. Statistical Analysis
3. Results
3.1. Stand Growth
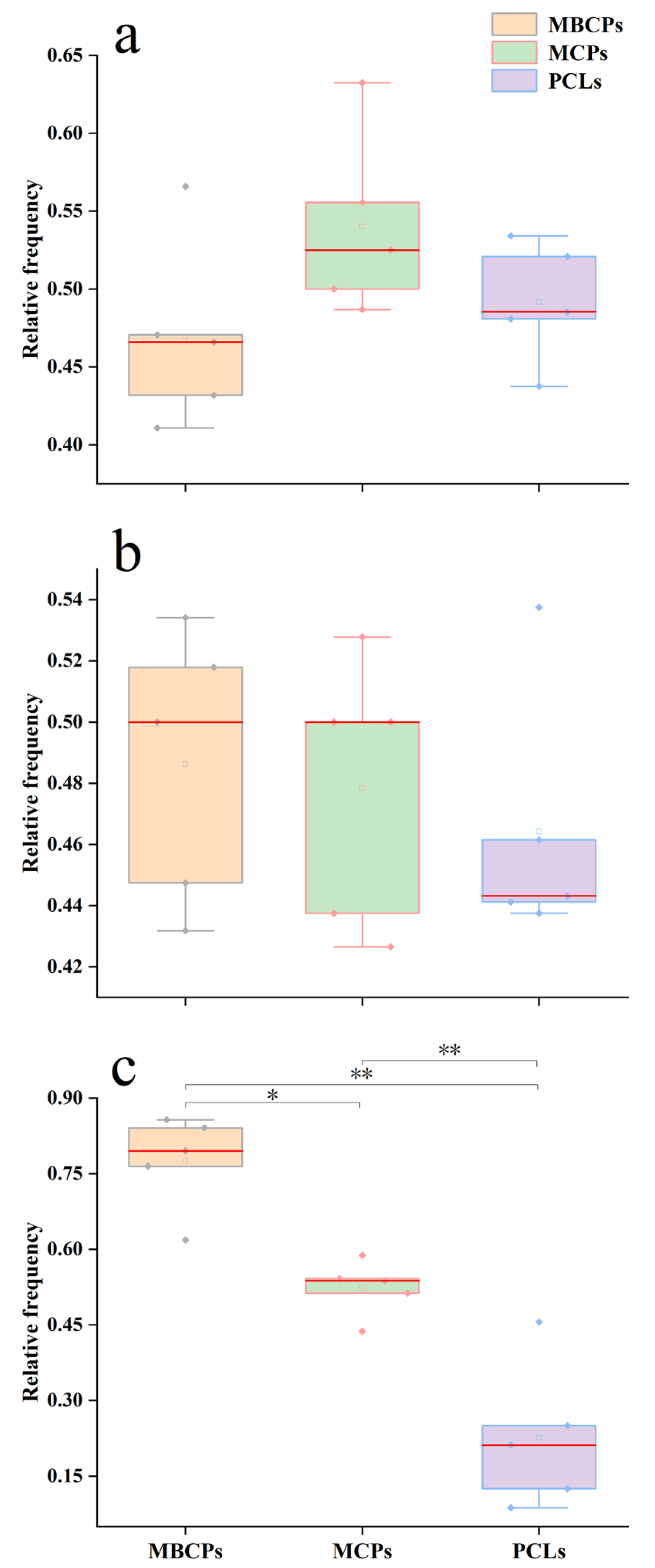
3.2. Stand Structure
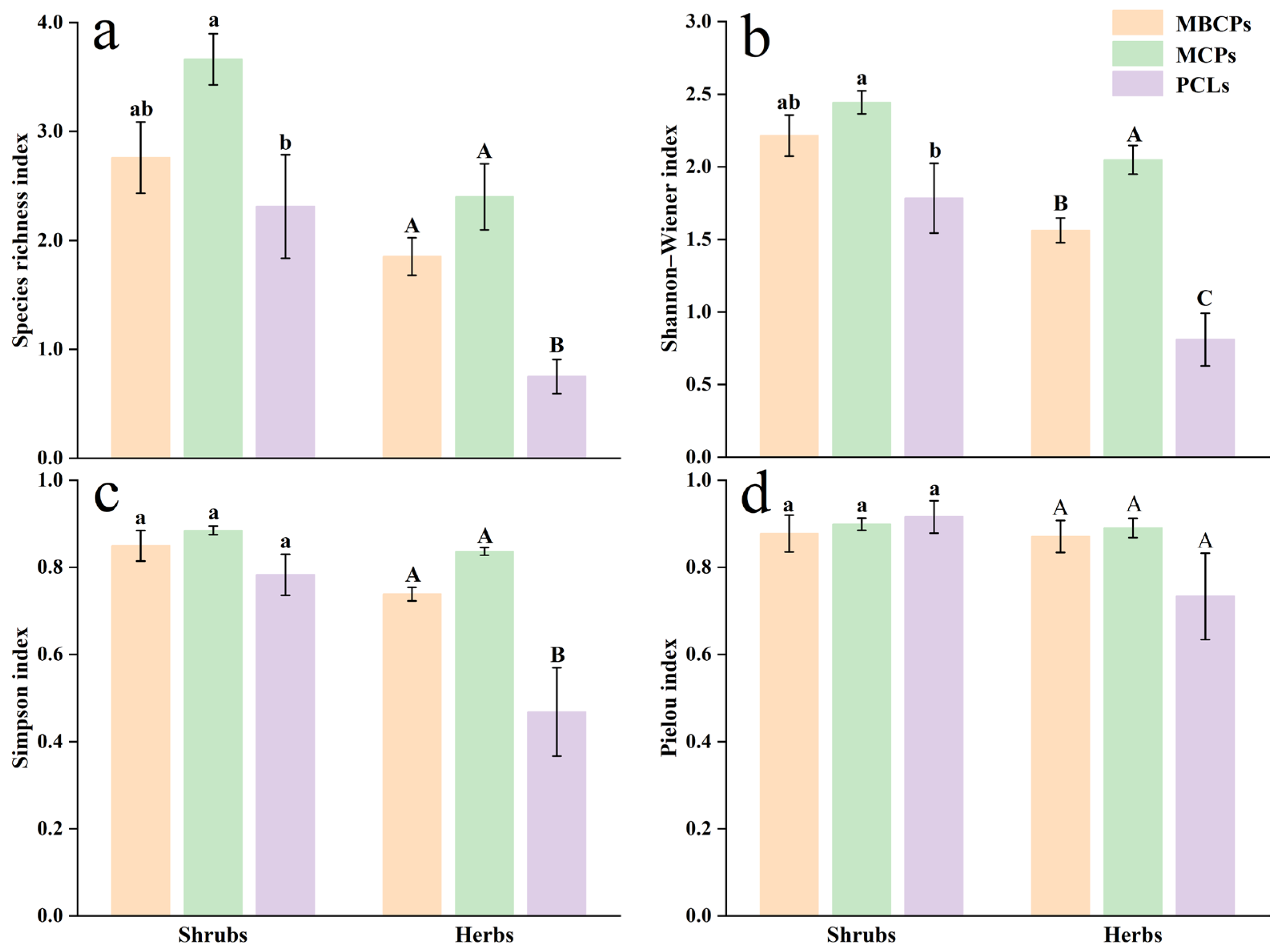
3.3. Species Composition and Diversity of Understory Shrubs and Herbaceous Plants
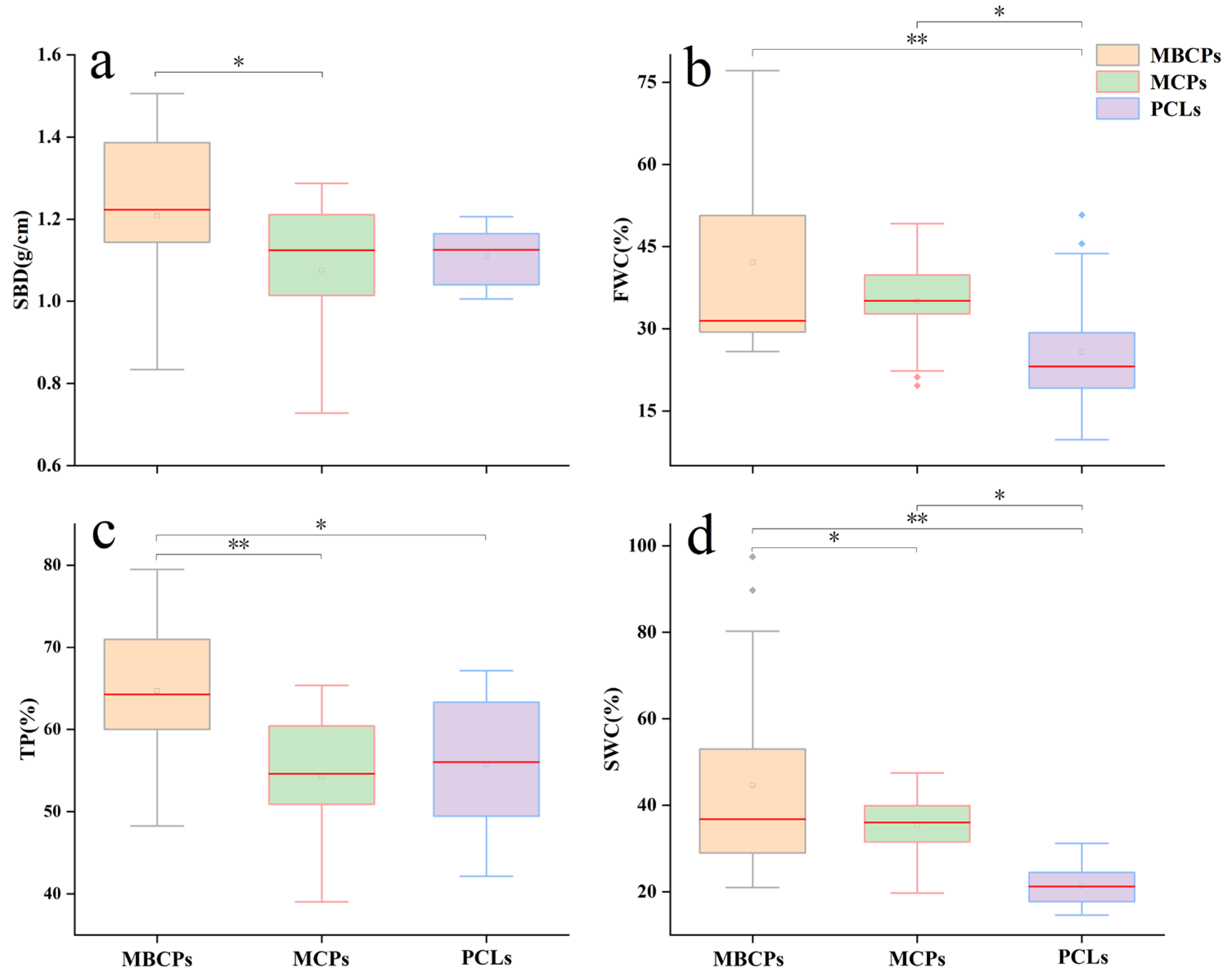
3.4. Soil Physical and Chemical Properties
4. Discussion
4.1. Analysis of Stand Growth Conditions and Spatial Structure
4.2. Understory Vegetation Diversity Analysis
4.3. Analysis of Soil Physico-Chemical Properties Analysis
4.4. Integrated Ecological Benefits of Mixed Plantation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- West, P.W. Growing Plantation Forests; Springer International Publishing: Cham, Switzerland, 2014; p. 276. [Google Scholar]
- Yin, Y.; Ma, D.; Wu, S. Climate change risk to forests in China associated with warming. Sci. Rep. 2018, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, N.; Li, M.; Xu, L.; Wang, L.; Zhu, J.; Sun, O.J. Carbon Sequestration of Chinese Forests from 2010 to 2060: Spatiotemporal Dynamics and Its Regulatory Strategies. Sci. Bull. 2022, 8, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.H.; Shakoor, A.; Wu, X.; Li, Y.; Rashid, M.H.U.; Zhang, X.; Gilani, M.M.; Kumar, U.; Chen, X.; Yan, W. Perspectives of Plantation Forests in the Sustainable Forest Development of China. iForest-Biogeosci. For. 2021, 14, 166–174. [Google Scholar] [CrossRef]
- Miao, L.; Zhu, F.; He, B.; Ferrat, M.; Liu, Q.; Cao, X.; Cui, X. Synthesis of China’s land use in the past 300 years. Glob. Planet. Change 2013, 100, 224–233. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- van der Plas, F.; Manning, P.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Hector, A.; Ampoorter, E.; Baeten, L.; et al. Jack-of-all-trades effects drive biodiversity-ecosystem multifunctionality relationships in European forests. Nat. Commun. 2016, 7, 11109. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1994, 379, 718–720. [Google Scholar] [CrossRef]
- Grossman, J.J.; Vanhellemont, M.; Barsoum, N.; Bauhus, J.; Bruelheide, H.; Castagneyrol, B.; Cavender-Bares, J.; Eisenhauer, N.; Ferlian, O.; Gravel, D.; et al. Synthesis and Future Research Directions Linking Tree Diversity to Growth, Survival, and Damage in a Global Network of Tree Diversity Experiments. Environ. Exp. Bot. 2018, 152, 68–89. [Google Scholar] [CrossRef]
- Raf, A.; Olivier, H. Forest restoration, biodiversity and ecosystem functioning. Ann. Occup. Environ. Med. 2011, 11, 29. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Jin, G. Mixed forests provide higher levels of growth and yield than monocultures across a broad range of conditions. Forest Ecol. Manag. 2024, 566, 122083. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Ou, Z.; Pang, S.; He, Q.; Peng, Y.; Huang, X.; Shen, W. Effects of vegetation restoration and environmental factors on understory vascular plants in a typical karst ecosystem in southern China. Sci. Rep. 2020, 10, 12011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guan, F.; Fan, S.; Zhang, X. Response of soil microbial community structure and diversity to mixed proportions and mixed tree species in bamboo–broad-leaved mixed forests. Forests 2024, 15, 921. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher Levels of Multiple Ecosystem Services Are Found in Forests with More Tree Species. Nat. Commun. 2011, 4, 1340. [Google Scholar] [CrossRef]
- Zhao, Z.; Hui, G.; Liu, W.; Hu, Y.; Zhang, G. A novel method for calculating stand structural diversity based on the relationship of adjacent trees. Forests 2022, 13, 343. [Google Scholar] [CrossRef]
- Chen, X.; Taylor, A.R.; Reich, P.B.; Hisano, M.; Chen, H.Y.H.; Chang, S.X. Publisher Correction: Tree Diversity Increases Decadal Forest Soil Carbon and Nitrogen Accrual. Nature 2023, 620, E16. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Huuskonen, S.; Domisch, T.; Finér, L.; Berninger, F.; Forsius, M.; Lindroos, A.-J.; Nieminen, T.; Piirainen, S.; Poikolainen, J.; Ukonmaanaho, L. What is the potential for replacing monocultures with mixed-species stands to enhance ecosystem services in boreal forests in Fennoscandia? For. Ecol. Manag. 2021, 479, 118548. [Google Scholar] [CrossRef]
- Bauhus, J.; Forrester, D.I.; Gardiner, B.; Jactel, H.; Vallejo, R.; Pretzsch, H. (Eds.) Mixed-Species Forests: Ecology and Management; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Cao, X.; Liu, Z.; Lv, Y. The Multivariate Distribution of Stand Spatial Structure and Tree Size Indices Using Neighborhood-Based Variables in Coniferous and Broad Mixed Forest. Forests 2023, 14, 2228. [Google Scholar] [CrossRef]
- Zhejiang Provincial Forestry Bureau. Technical Specifications for Long-Term Biodiversity Monitoring Plot Construction and Survey in Zhejiang Natural Protected Areas (Version 1.0); Zhejiang Standard Press: Hangzhou, China, 2022. [Google Scholar]
- Paquette, A.; Messier, C. The Effect of Biodiversity on Tree Productivity: From Temperate to Boreal Forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V. Comparison of Tree Species Effects on Microbial C and N Transformations and Dissolved Organic Matter Properties in Boreal Forest Floors. Appl. Soil Ecol. 2011, 49, 224–233. [Google Scholar] [CrossRef]
- Jian, Z.; Ni, Y.; Lei, L.; Chen, X.; Wang, H.; Wang, Y.; Zhang, Y.; Wu, J. Phosphorus is the key soil indicator controlling productivity in planted Masson pine forests across subtropical China. Sci. Total Environ. 2022, 822, 153525. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.A.; Vogt, D.J.; Brown, S.; Tilley, J.P.; Edmonds, R.L.; Silver, W.L.; Siccama, T.G. Dynamics of forest floor and soil organic matter accumulation in boreal, temperate, and tropical forests. In Soil Management and Greenhouse Effect; Kimble, J.M., Levine, E.R., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1995; p. 2. [Google Scholar] [CrossRef]
- Cook, A.M.; Daughton, C.G. Total Phosphorus Determination by Spectrophotometry. Methods Enzymol. 1981, 72, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Avramidis, P.; Nikolaou, K.; Bekiari, V. Total Organic Carbon and Total Nitrogen in Sediments and Soils: A Comparison of the Wet Oxidation–Titration Method with the Combustion-Infrared Method. Agric. Agric. Sci. Procedia 2015, 4, 425–430. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Chang, J.; Jiang, B.; Jiang, H.; Peng, C.; Zhu, J.; Yuan, W.; Qi, L.; Yu, S. Carbon storage by ecological service forests in Zhejiang Province, subtropical China. For. Ecol. Manag. 2007, 245, 64–75. [Google Scholar] [CrossRef]
- Zhang, G.; Hui, G.; Zhang, G.; Zhao, Z.; Hu, Y. Telescope method for characterizing the spatial structure of a pine-oak mixed forest in the Xiaolong Mountains, China. Scand. J. For. Res. 2019, 34, 751–762. [Google Scholar] [CrossRef]
- Hui, G.; Li, L.; Zhao, Z. The comparison of methods in analysis of the tree spatial distribution pattern. Acta Ecol. Sin. 2007, 27, 4717–4728. [Google Scholar] [CrossRef]
- Hui, G.; Gadow, K.; Albert, M. The neighbourhood pattern: A new structure parameter for describing distribution of forest tree position. Sci. Silv. Sin. 1999, 35, 37–42. [Google Scholar]
- Hui, G.; Gadow, K.; Albert, M. A new parameter for stand spatial structure neighbourhood comparison. For. Res. 1999, 12, 1–6. [Google Scholar]
- Gadow, K.; Hui, G. Characterizing Forest Spatial Structure and Diversity. In Proceedings of the International Workshop Organized at the University of Lund, Lund, Sweden, 7–9 April 1999; pp. 20–30. [Google Scholar]
- Curtis, J.T. Plant Ecology Workbook. Soil Sci. 1950, 69, 418. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Technol. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Magurran, A.E. (Ed.) Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.H. The Measurement of Linguistic Diversity. Language 1956, 32, 109–115. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Isbell, F.; Purves, D.W.; Loreau, M.; Hector, A. Understanding the value of plant diversity for ecosystem functioning through niche theory. Proc. Biol. Sci. 2016, 283, 20160536. [Google Scholar] [CrossRef]
- Houpert, L.; Rohner, B.; Forrester, D.I.; Mina, M.; Huber, M.O. Mixing Effects in Norway Spruce—European Beech Stands Are Modulated by Site Quality, Stand Age and Moisture Availability. Forests 2018, 9, 83. [Google Scholar] [CrossRef]
- Fien, E.K.P.; Fraver, S.; Teets, A.; Weiskittel, A.R.; Hollinger, D.Y. Drivers of Individual Tree Growth and Mortality in an Uneven-Aged, Mixed-Species Conifer Forest. For. Ecol. Manag. 2019, 449, 539. [Google Scholar] [CrossRef]
- Condés, S.; Pretzsch, H.; Río, M. Species Admixture Can Increase Potential Tree Growth and Reduce Competition. For. Ecol. Manag. 2023, 539, 120997. [Google Scholar] [CrossRef]
- Quiroga, P.; Souto, C. Ecological niche modeling, niche overlap, and good old Rabinowitz’s rarities applied to the conservation of gymnosperms in a global biodiversity hotspot. Landsc. Ecol. 2021, 37, 2571–2588. [Google Scholar] [CrossRef]
- Sterba, H.; Drinberger, G.; Riter, T. The contribution of forest structure to complementarity in mixed stands of Norway spruce (Picea abies L. Karst) and European larch (Larix decidua Mill.). Forests 2018, 9, 410. [Google Scholar] [CrossRef]
- Forrester, D.I. The Spatial and Temporal Dynamics of Species Interactions in Mixed-Species Forests: From Pattern to Process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Sardans, J.; Vallicrosa, H.; Zuccarini, P.; Farre-Armengol, G.; Fernandez-Martinez, M.; Peguero, G.; Gargallo-Garriga, A.; Ciais, P.; Janssens, I.A.; Obersteiner, M.; et al. Empirical support for the biogeochemical niche hypothesis in forest trees. Nat. Ecol. Evol. 2021, 5, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tan, W.; Gao, X.; Chai, Z. Close-to-Nature Management Positively Improves the Spatial Structure of Masson Pine Forest Stands. Web Ecol. 2021, 21, 45–54. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Cheng, R.; Ge, Z.; Zhang, Z. Effects of neighborhood competition and stand structure on the productivity of pure and mixed Larix principis-rupprechtii forests. Forests 2022, 13, 1318. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Ciais, P.; Jepsen, M.R.; Penuelas, J.; Wigneron, J.P.; Xiao, X.-P.; Song, X.-P.; Horion, S.; et al. Forest management in southern China generates short-term extensive carbon sequestration. Nat. Commun. 2020, 11, 129. [Google Scholar] [CrossRef]
- Yang, S.; Mao, K.; Yang, H.; Wang, Y.; Feng, Q.; Wang, S.; Miao, N. Stand characteristics and ecological benefits of Chinese fir, Chinese cedar, and mixed plantations in the mountainous areas of the Sichuan Basin. For. Ecol. Manag. 2023, 544, 121168. [Google Scholar] [CrossRef]
- Su, X.; Zheng, G.; Chen, H.Y.H. Understory diversity is driven by resource availability rather than resource heterogeneity in subtropical forests. For. Ecol. Manag. 2022, 503, 119781. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Cowley, E.S.; Kleiner, M.; Anantharaman, K. Diversity and Ecology of Microbial Sulfur Metabolism. Nat. Rev. Microbiol. 2025, 23, 122–140. [Google Scholar] [CrossRef]
- Williams, L.J.; Paquette, A.; Cavender-Bares, J.; Messier, C.; Reich, P.B. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nat. Ecol. Evol. 2017, 1, 63. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Yan, M.; Han, Y.; Wang, K.; Chen, Z.; Ruan, D.; Yu, Y.; Tu, Z. Differential Water Conservation Capacity in Broadleaved and Mixed Forest Restoration in Latosol Soil-Eroded Region, Hainan Province, China. Plants 2024, 13, 694. [Google Scholar] [CrossRef]
- Raguet, P.; Cade-Menun, B.; Mollier, A.; Abdi, D.; Ziadi, N.; Karam, A.; Morel, C. Mineralization and speciation of organic phosphorus in a sandy soil continuously cropped and phosphorus-fertilized for 28 years. Soil Biol. Biochem. 2023, 178, 108938. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Malhi, S.S.; Li, S.Q.; Gao, Y.J.; Tian, X.H. Nutrient and water management effects on crop production, and nutrient and water use efficiency in dryland areas of China. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2009; Volume 102, pp. 223–265. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Yuan, H.; Li, E.; Yang, X. The impact of artificial afforestation on the soil microbial community and function in desertified areas of NW China. Forests 2024, 15, 1140. [Google Scholar] [CrossRef]
- Piotto, D.; Víquez, E.; Montagnini, F.; Kanninen, M. Pure and mixed forest plantations with native species of the dry tropics of Costa Rica: A comparison of growth and productivity. For. Ecol. Manag. 2004, 190, 359–372. [Google Scholar] [CrossRef]
- Feng, L.; Li, Z.; Zhao, Z. Extreme Climate Shocks and Green Agricultural Development: Evidence from the 2008 Snow Disaster in China. Int. J. Environ. Res. Public Health 2021, 18, 12055. [Google Scholar] [CrossRef] [PubMed]
- Cantarello, E.; Jacobsen, J.B.; Lloret, F.; Lindner, M. Shaping and Enhancing Resilient Forests for a Resilient Society. Ambio 2024, 53, 1095–1108. [Google Scholar] [CrossRef]
- Bigelow, S.W.; North, M.P. Microclimate Effects of Fuels-Reduction and Group-Selection Silviculture: Implications for Fire Behavior in Sierran Mixed-Conifer Forests. For. Ecol. Manag. 2012, 264, 51–59. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Impacts of Mixed Forests on Controlling Soil Erosion in China. Catena 2022, 213, 106147. [Google Scholar] [CrossRef]


| Forest Types | Aspect | Slope (°) | Density of Trees (Trees/hm−2) | Canopy Density |
|---|---|---|---|---|
| MBCPs | Southwest | 20 | 2725 | 0.74 |
| East | 21 | 1550 | 0.77 | |
| West | 19 | 1450 | 0.77 | |
| Southeast | 25 | 1525 | 0.75 | |
| South | 16 | 2200 | 0.73 | |
| MCPs | South | 9 | 1850 | 0.67 |
| South | 11 | 1825 | 0.73 | |
| South | 14 | 1425 | 0.81 | |
| Southwest | 13 | 2075 | 0.68 | |
| Northeast | 14 | 1825 | 0.68 | |
| PCLs | West | 12 | 1550 | 0.31 |
| Northwest | 18 | 2025 | 0.67 | |
| North | 9 | 1925 | 0.64 | |
| West | 21 | 1250 | 0.68 | |
| North | 13 | 1000 | 0.71 |
| Forest Types | pH | AN (mg/kg) | NN (mg/kg) | TN (g/kg) | TP (g/kg) | AP (mg/kg) | TOC (g/kg) |
|---|---|---|---|---|---|---|---|
| MBCPs | 4.70 ± 0.22 b | 15.06 ± 7.43 b | 51.14 ± 31.48 a | 2.51 ± 0.64 b | 0.16 ± 0.12 b | 46.42 ± 13.45 b | 2.86 ± 1.10 b |
| MCPs | 4.68 ± 0.13 b | 26.38 ± 13.10 a | 23.25 ± 29.18 b | 3.12 ± 0.83 a | 0.18 ± 0.04 b | 55.23 ± 16.12 a | 3.63 ± 1.41 b |
| PCLs | 5.24 ± 0.26 a | 8.02 ± 5.04 c | 7.96 ± 16.32 b | 3.13 ± 0.57 a | 0.43 ± 0.14 a | 42.98 ± 9.07 b | 14.64 ± 12.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, P.; Han, Y.; Li, X.; Jin, S. Ecological Benefits and Structure of Mixed vs. Pure Forest Plantations in Subtropical China. Forests 2025, 16, 738. https://doi.org/10.3390/f16050738
Qian P, Han Y, Li X, Jin S. Ecological Benefits and Structure of Mixed vs. Pure Forest Plantations in Subtropical China. Forests. 2025; 16(5):738. https://doi.org/10.3390/f16050738
Chicago/Turabian StyleQian, Penghong, Yini Han, Xueqin Li, and Songheng Jin. 2025. "Ecological Benefits and Structure of Mixed vs. Pure Forest Plantations in Subtropical China" Forests 16, no. 5: 738. https://doi.org/10.3390/f16050738
APA StyleQian, P., Han, Y., Li, X., & Jin, S. (2025). Ecological Benefits and Structure of Mixed vs. Pure Forest Plantations in Subtropical China. Forests, 16(5), 738. https://doi.org/10.3390/f16050738





