Ecological Variability and Carbon Stock Estimates of Mangrove Ecosystems in Northwestern Madagascar
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
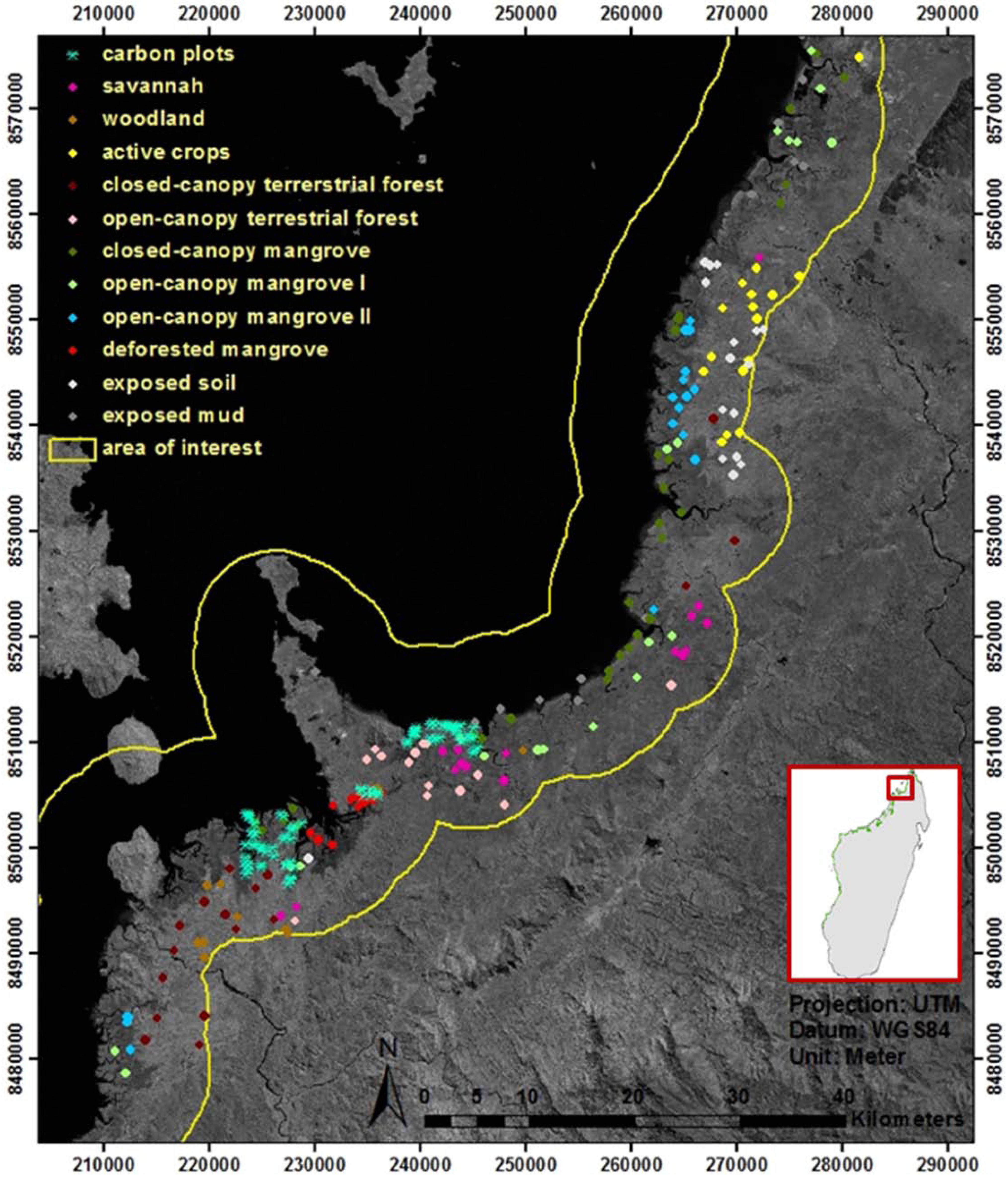
2.2. Inventory of Existing Mangrove Maps and Assessment of Mangrove Dynamics
2.3. Acquisition and Pre-Processing of Remotely Sensed Data
2.4. Definition and Refinement of Mangrove and Surrounding Land-Cover Categories
| Class | Description of typical constituents | Class can also include |
|---|---|---|
| Savannah | mosaic of dry grass, exposed soil and extremely sparse trees/shrubs | senesced rice; reeds |
| Woodland | mosaic of dry grass and scattered trees/shrubs; canopy <30% closed | orchard agriculture |
| Active cultivation | sugar cane, rice, reeds | |
| Closed-canopy terrestrial forest | closed-canopy terrestrial forest; canopy >60% closed | |
| Open-canopy terrestrial forest | open-canopy terrestrial forest; canopy 30%–60% closed | |
| Closed-canopy mangrove | tall, mature stands; canopy >60% closed | extremely dense younger stands |
| Open-canopy mangrove I | young, short-medium trees; canopy 30%–60% closed; influenced by background soil/mud | naturally open; very degraded tall |
| Open-canopy mangrove II | stunted short trees, very sparse; canopy ≥10% closed; dominated by background soil/mud | |
| Deforested mangrove | mosaic of stumps, scattered trees; canopy <30% closed; greatly influenced by exposed soil/mud | |
| Exposed soil | inactive agri/aquacultural fields; extremely patchy savannah; extremely dry tanne (mud-flats) | |
| Exposed mud | mangrove/ocean interface; river sediment; wet tannes (mud-flats); inactive aquaculture ponds |
2.5. Image Classification
2.6. Mangrove Carbon Stocks
| Species | Allometric equation | Wood density [119] | References |
|---|---|---|---|
| Avicennia marina | B = 0.1848 × dbh2.3524 | 0.661 | [120] |
| Bruguiera gymnorrhiza (leaves) | B = 0.0679 × dbh1.4914 | 0.741 | [121] |
| Bruguiera gymnorrhiza (stem) | B = 0.464 × (dbh2 × H)0.94275 × p | 0.741 | [22] |
| Ceriops tagal (dbh 2–18 cm) | B = 10−0.7247 × dbh2.3379 | 0.803 | [121] |
| Ceriops tagal (dbh 18–25 cm) | B = 10−0.494 × dbh2.056 | 0.803 | [122] |
| Heritiera littoralis (leaves) | B = 0.0679 × dbh1.4914 | 1.074 | [121] |
| Heritiera littoralis (stem) | B = 0.464 × (dbh2 × H)0.94275 × p | 1.074 | [22] |
| Lumnitzera racemosa | B = 0.0214 × (dbh2 × H)1.05655 × p | 0.565 | [22] |
| Rhizophora mucronata (leaves) | B = 0.0139 × D2.1072 | 0.867 | [121] |
| Rhizophora mucronata (root) | B = 0.0068 × dbh3.1353 | 0.867 | [121] |
| Rhizophora mucronata (stem) | B = 0.0311 × (dbh2 × H)1.00741 × p | 0.867 | [22] |
| Sonneratia alba | B = 0.0825 × (dbh2 × H)0.89966 × p | 0.78 | [22] |
| Xylocarpus granatum | B = 0.0830 × (dbh2 × H)0.89806 × p | 0.7 | [22] |
3. Results and Discussion
3.1. Overview of Existing Mangrove Maps

3.2. Spectral Separability and Classification Results


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | User’s (%) | Commission (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Savannah (1) | 54 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 58 | 93 | 7 |
| Woodland (2) | 0 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 100 | 0 |
| Active crops (3) | 0 | 0 | 51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 51 | 100 | 0 |
| Closed-canopy terrestrial forest (4) | 0 | 0 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 100 | 0 |
| Open-canopy terrestrial forest (5) | 0 | 4 | 0 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 58 | 93 | 7 |
| Closed-canopy mangrove (6) | 0 | 0 | 0 | 0 | 0 | 79 | 9 | 0 | 0 | 0 | 0 | 88 | 90 | 10 |
| Open-canopy mangrove I (7) | 0 | 0 | 0 | 0 | 0 | 11 | 72 | 0 | 2 | 0 | 0 | 85 | 85 | 15 |
| Open-canopy mangrove II (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 52 | 100 | 0 |
| Deforested mangrove (9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 60 | 100 | 0 |
| Exposed soil (10) | 0 | 8 | 3 | 0 | 0 | 0 | 0 | 2 | 1 | 53 | 0 | 67 | 79 | 21 |
| Exposed mud (11) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 54 | 100 | 0 |
| Total | 54 | 54 | 54 | 54 | 54 | 90 | 81 | 54 | 63 | 54 | 54 | 666 | ||
| Producer’s (%) | 100 | 72 | 94 | 100 | 100 | 88 | 89 | 96 | 95 | 98 | 100 | Overall | 93.4 | |
| Omission (%) | 0 | 28 | 6 | 0 | 0 | 12 | 11 | 4 | 5 | 2 | 0 | Kappa | 0.9 |
3.3. Carbon Plot Locations and Ecological Characteristics of Mapped Mangrove Classes


3.4. Carbon Stocks of Mapped Mangrove Classes
| Class | Sub-type | Spp dominance | Height (m) (±1 SD) | Trees/ha (±1 SD) | d (cm) (±1 SD) |
|---|---|---|---|---|---|
| Closed-canopy (n = 24) | intact, tall, mature stands | Avicennia marina | 8.6 (n = 1) | 1250 (n = 1) | 14.9 (n = 1) |
| intact, tall, mature stands | Ceriops tagal | 7.3 (±1.2) (n = 3) | 2625 (±318) (n = 2) | 10.1 (±0.5) (n = 3) | |
| intact, tall, mature stands | Rhizophora mucronata | 7 (±1.3) (n = 14) | 4719 (±1133) (n = 12) | 10.1 (±3) (n = 14) | |
| intact, tall, mature stands | Sonneratia alba | 5.6 (n = 1) | 5300 (n = 1) | 10.6 (n = 1) | |
| very dense medium-tall stands | Rhizophora mucronata | 4.8 (±0.1) (n = 2) | 5600 (±1838) (n = 2) | 7.8 (±1.1) (n = 2) | |
| intact, tall, mature stands | mixed | 6.7 (±1.6) (n = 2) | 1825 (±248) (n = 2) | 11.3 (±2.5) (n = 2) | |
| Open-canopy I (n = 28) | medium stands | Ceriops tagal | 4.6 (±0.8) (n = 7) | 3300 (±849) (n = 2) | 7.5 (±1.6) (n = 7) |
| medium stands | Rhizophora mucronata | 4.2 (±0.6) (n = 6) | 2160 (±498) (n = 5) | 7.3 (±1.3) (n = 6) | |
| naturally open/very degraded tall | variable | 5.7 (±0.3) (n = 4) | 1525 (±35) (n = 2) | 10.1 (±1.2) (n = 4) | |
| very dense short stands | Ceriops tagal | 2.5 (±0.3) (n = 9) | 2780 (±750) (n = 5) | 5.1 (±0.9) (n = 9) | |
| medium stands | mixed | 4.8 (±0.1) (n = 2) | 1800 (±141) (n = 2) | 9.5 (±2) (n = 2) | |
| Open-canopy II (n = 4) | stunted, scrub ecosystems | Avicennia marina | 1.7 (±0.5) (n = 4) | 1306 (±554) (n = 4) | 4.6 (±0.2) (n = 4) |

| Class | Vegetation carbon | Soil organic carbon | Total carbon |
|---|---|---|---|
| Closed-canopy (n = 23; 22) | 146.8(10.2) | 446.2 (36.9) | 593 (39) |
| Open-canopy I (n = 28; 24) | 42.9 (5.8) | 324.3 (36.5) | 367.2 (37.3) |
| Open-canopy II (n = 4; 4) | 20.8 (4.6) | 517.1 (76) | 537.9 (75.2) |
| Class | Sample depth (cm) | Carbon (%) | Bulk density (g/cm3) | Carbon mass (Mg/ha) | |||
|---|---|---|---|---|---|---|---|
| Closed-canopy (n = 22) | 0–15 | 4.8 | (0.9) | 0.77 | (0.08) | 41.1 | (3.5) |
| 15–30 | 4.1 | (0.4) | 0.73 | (0.08) | 41.3 | (3.7) | |
| 30–50 | 4.1 | (0.3) | 0.73 | (0.06) | 58.4 | (3.9) | |
| 50–100 | 4.2 | (0.4) | 0.72 | (0.06) | 155.6 | (14.9) | |
| 100–150 | 3.9 | (0.5) | 0.70 | (0.09) | 149.8 | (17.4) | |
| Total | 446.2 | (36.9) | |||||
| Open-canopy I (n = 24) | 0–15 | 3.4 | (2.9) | 0.78 | (0.08) | 32.5 | (3.8) |
| 15–30 | 4.4 | (3.6) | 0.72 | (0.07) | 43.1 | (5.3) | |
| 30–50 | 3.2 | (2.6) | 0.75 | (0.07) | 45.2 | (5.6) | |
| 50–100 | 3.2 | (2.6) | 0.60 | (0.08) | 120.7 | (16.9) | |
| 100–150 | 1.9 | (2.2) | 0.52 | (0.10) | 82.9 | (18.3) | |
| Total | 324.3 | (36.5) | |||||
| Open-canopy II (n = 4) | 0–15 | 0.6 | (0.1) | 1.39 | (0.04) | 12.1 | (0.7) |
| 15–30 | 0.6 | (0.3) | 1.28 | (0.05) | 10.6 | (2.6) | |
| 30–50 | 0.8 | (0.6) | 1.35 | (0.06) | 22.5 | (7.4) | |
| 50–100 | 2.2 | (1.2) | 1.10 | (0.03) | 120.0 | (28.9) | |
| 100–150 | 6.1 | (2.4) | 1.18 | (0.08) | 351.9 | (58.1) | |
| Total | 517.1 | (76.0) | |||||

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar]
- Blasco, F.; Bellan, M.F.; Chaudhury, M.U. Estimating the extent of floods in bangladesh—Using SPOT data. Remote Sens. Environ. 1992, 39, 167–178. [Google Scholar] [CrossRef]
- Marshall, N. Mangrove conservation in relation to overall environmental considerations. Hydrobiologia 1994, 285, 303–309. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of world’s mangrove forest. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Jayatissa, L.P.; di Nitto, D.; Bosire, J.O.; Lo Seen, D.; Koedam, N. How effective were mangroves as a defence against the recent tsunami? Curr. Biol. 2005, 15, R443–R447. [Google Scholar] [CrossRef]
- Barbier, E.B. Natural barriers to natural disasters: Replanting mangroves after tsunami. Front. Ecol. Environ. 2006, 4, 124–131. [Google Scholar] [CrossRef]
- Food and Agricultural Organization (FAO). The World’s Mangroves 1980–2005; FAO Forestry Paper 153; FAO: Rome, Italy, 2007. [Google Scholar]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate chang. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.J.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habit function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon payments for mangrove conservation: Ecosystem constraints and uncertainties of sequestration potential. Environ. Sci. Policy 2011, 14, 462–470. [Google Scholar] [CrossRef]
- Kuezner, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef]
- Primavera, J.H. Socio-economic impacts of shrimp culture. Aquac. Res. 1997, 28, 815–827. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Mumby, P.J.; Edwards, A.J.; Arias-Gonzáles, E.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. Mangrove enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kumianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marba, N.; et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 2012, 7, e43542. [Google Scholar] [CrossRef] [Green Version]
- Twilley, R.R.; Chen, R.H.; Hargis, T. Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut. 1992, 64, 265–288. [Google Scholar] [CrossRef]
- Kirui, K.B.; Karachi, M.; Kairo, J.G. Allometric equations for estimating aboveground biomass of Rhizophora mucronata at Gazi bay Kenya. West. Indian. Ocean J. Mar. Sci. 2006, 5, 27–34. [Google Scholar]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer Science and Business Media BV: New York, NY, USA, 2009. [Google Scholar]
- Kairo, J.G.; Bosire, J.; Langat, J.; Kirui, B.; Koedam, N. Allometry and biomass distribution in replanted mangrove plantations at Gazi Bay, Kenya. Aquat. Conserv. 2009, 19, S63–S69. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Cole, T.G. Micronesian mangrove forest structure and tree responses to a severe typhoon. Wetlands 2010, 30, 1077–1084. [Google Scholar] [CrossRef]
- Camacho, L.D.; Gevana, D.T.; Carandang, A.P.; Sofronio, C.C.; Combalicer, E.A.; Rebugio, L.L.; Youn, Y.-C. Tree biomass and carbon stock of a community-managed mangrove forest in Bohol, Phillipines. For. Sci. Technol. 2011, 7, 161–167. [Google Scholar]
- Mitra, A.; Sengupta, K.; Banerjee, K. Standing biomass and carbon storage of above-ground structures in dominant mangrove trees in the Sundarbans. For. Ecol. Manag. 2011, 261, 1325–1335. [Google Scholar] [CrossRef]
- Abino, A.C.; Castillo, J.A.A.; Lee, Y.J. Assessment of species diversity, biomass and carbon sequestration potential of a natural mangrove stand in Samar, the Philippines. For. Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Hutchison, J.; Manica, A.; Swetnam, R.; Balmford, A.; Spalding, M. Predicting global patterns in mangrove forest biomass. Conserv. Lett. 2013. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Heider, C.; Cole, T.G.; Dwire, K.A.; Donato, D.C. Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 2011, 31, 343–352. [Google Scholar] [CrossRef]
- Ray, R.; Ganguly, D.; Chowdhury, C.; Dey, M.; Das, S.; Dutta, M.K.; Mandal, S.K.; Majumder, N.; De, T.K.; Mukhopadhyay, S.K.; et al. Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmos. Environ. 2011, 45, 5016–5024. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, X.; Tam, N.F.Y.; Lu, W.; Luo, Z.; Du, X.; Wang, J. Comparing carbon sequestration and stand structure of monoculture and mixed mangrove plantations of Sonneratia caseolaris and S. apetala in Southern China. For. Ecol. Manag. 2012, 284, 222–229. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Mackenzie, R.A.; Ainsworth, A.; Pfleeger, A.Z. Whole-island carbon stocks in the tropical Pacific: Implications for mangrove conservation and upland restoration. J. Environ. Manag. 2012, 97, 89–96. [Google Scholar] [CrossRef]
- Matsui, N.; Morimune, K.; Meepol, W.; Chukwamdee, J. Ten year evaluation of carbon stock in mangrove plantation reforested from an abandoned shrimp pond. Forests 2012, 3, 431–444. [Google Scholar] [CrossRef]
- Adame, M.F.; Kauffman, J.B.; Medina, I.; Gamboa, J.N.; Torres, O.; Caamal, J.P.; Reza, M.; Herrera-Silveira, J.A. Carbon stocks of tropical coastal wetlands within the Karstic landscape of the Mexican Caribbean. PLoS One 2013, 8, e56569. [Google Scholar] [CrossRef]
- Wang, G.; Dongsheng, G.; Peart, M.R.; Chen, Y.; Peng, Y. Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdon Province of South China. For. Ecol. Manag. 2013, 310, 539–546. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Heider, C.; Norfolk, J.; Payton, F. Carbon stocks of intact mangroves and carbon emissions arising from their conversion in the Dominican Republic. Ecol. Appl. in press. [CrossRef]
- Golley, F.; Odum, H.T.; Wilson, R.F. The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 1962, 43, 9–19. [Google Scholar] [CrossRef]
- Eong, O.J. Mangroves—A carbon source and sink. Chemosphere 1993, 27, 1097–1107. [Google Scholar] [CrossRef]
- Matsui, N. Estimated stocks of organic carbon in mangrove roots and sediments in Hinchinbrook Channel, Australia. Mangroves Salt Marshes 1998, 2, 199–204. [Google Scholar] [CrossRef]
- Fujimoto, K.; Imaya, A.; Tabuchi, R.; Kuramoto, S.; Utsugi, H.; Murofushi, T. Belowground C storage of Micronesian mangrove forests. Ecol. Res. 1999, 14, 409–413. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Donato, D.C. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests. Working Paper 86; CIFOR: Bogor, Indonesia, 2012. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. Mangrove extinction risk and geographic areas of global concern. PLoS One 2010, 5, e10095. [Google Scholar] [CrossRef]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove forests: One of the world’s threatened major tropical environments. Bioscience. 2001, 51, 807–815. [Google Scholar] [CrossRef]
- Wilkie, M.L.; Foruna, S. Status and Trends in Mangrove Area Extent Worldwide; Forest Resources Assessment Working Paper 63; Food and Agriculture Organization of the United Nations: Roma, Italy, 2003. [Google Scholar]
- Giesen, W.; Wulffraat, S.; Zieren, M.; Scholten, L. Mangrove Guidebook for Southeast Asia; Food and Agricultural Organisation and Wetlands International: Bangkok, Thailand, 2007. [Google Scholar]
- Duke, N.C.; Meynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; Earthscan: London, UK, 2010. [Google Scholar]
- Farnsworth, E.J.; Ellison, A.M. The global conservation status of mangroves. AMBIO 1997, 26, 328–334. [Google Scholar]
- Primavera, J.H. Development and conservation of Philippine mangroves: Institutional issues. Ecol. Econ. 2000, 35, 91–106. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F. The use of remote sensing and GIS in the sustainable management of tropical coastal ecosystems. Environ. Dev. Sustain. 2002, 4, 93–112. [Google Scholar] [CrossRef]
- Primavera, J.H. Mangroves, fishponds, and the quest for sustainability. Science 2005, 310, 57–59. [Google Scholar] [CrossRef]
- Gopal, B.; Chauhan, M. Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquat. Sci. 2006, 68, 338–354. [Google Scholar] [CrossRef]
- Primavera, J.H. Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast. Manag. 2006, 49, 531–545. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Walters, B.B.; Rönnbäck, P.; Kovacs, J.M.; Crona, B.; Hussain, S.A.; Badola, R.; Primavera, J.H.; Barbier, E.; Dahdouh-Guebas, F. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236. [Google Scholar] [CrossRef]
- Field, C.D. Impact of expected climate change on mangroves. Hydrobiologia 1995, 295, 75–81. [Google Scholar] [CrossRef]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; Lopez-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Chan, H.T.; Baba, S. Manual on Guidelines for Rehabilitation of Coastal Forests Damaged by Natural Hazards in the Asia-Pacific Region; International Society for Mangrove Ecosystems (ISME) and International Tropical Timber Organization (ITTO): Okinawa, Japan, 2009; p. 66. [Google Scholar]
- Suzuki, T.; Zijlema, M.; Burger, B.; Meijer, M.C.; Narayan, S. Wave dissipation by vegetation with layer schematization in SWAN. Coast. Eng. 2012, 59, 64–71. [Google Scholar] [CrossRef]
- De la Cruz, A.A. Tropical wetlands as a carbon source. Aquat. Bot. 1986, 25, 109–115. [Google Scholar] [CrossRef]
- Hooijer, A.; Silvius, M.; Wösten, H.; Page, S. PEAT-CO2: Assessment of CO2 Emissions from Drained Peatlands in SE Asia, Delft Hydraulics, Report Q3943, 1st ed.; Wetlands International, Delft Hydraulics: Delft, The Netherlands, 2006. [Google Scholar]
- Grimsditch, G.; Alder, J.; Nakamura, T.; Kenchington, R.; Tamelander, J. The blue carbon special edition—Introduction and overview. Ocean Coast. Manag. 2013, 83, 1–4. [Google Scholar] [CrossRef]
- United Nations Environmental Program. Global Environment Outlook Yearbook 2004; United Nations Environment Programme: Nairobi, Kenya, 2004. [Google Scholar]
- Laffoley, D.; Grimsditch, G. The Management of Natural COASTAL Carbon Sinks; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2009; p. 53. [Google Scholar]
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdés, L.; de Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon—A Rapid Response Assessment. United Nations Environment Programme, GRID-Arendal. 2009. Available online: http://www.grida.no (accessed on 1 December 2012).
- Gordon, D.; Murray, B.C.; Pendleton, L.; Victor, B. Financing Options for Blue Carbon: Opportunities and Lessons from the REDD+ Experience; Nicholas Institute Report; Nicholas Institute: Durham, NC, USA, 2011. [Google Scholar]
- Ullman, R.; Bilbao-Bastida, V.; Grimsditch, G. Including blue carbon in climate market mechanisms. Ocean Coast. Manag. 2012, 83, 15–18. [Google Scholar]
- Jones, T.G. Shining a light on Madagascar’s mangroves. Madag. Conserv. Dev. 2013, 8, 4–6. [Google Scholar]
- Heumann, B.W. Satellite remote sensing of mangrove forests: Recent advances and future opportunities. Prog. Phys. Geogr. 2011, 35, 87–108. [Google Scholar] [CrossRef]
- Manson, F.J.; Loneragan, N.R.; McLeod, I.M.; Kenyon, R.A. Assessing techniques for estimating the extent of mangroves: Topographic maps, aerial photographs, and Landsat TM images. Mar. Freshw. Res. 2001, 52, 787–792. [Google Scholar] [CrossRef]
- Ruiz-Luna, A.; Berlanga-Robles, C.A. Land use, land cover changes and coastal lagoon surface reduction associated with urban growth in northwest Mexico. Landsc. Ecol. 2003, 18, 159–171. [Google Scholar] [CrossRef]
- Cornejo, R.H.; Koedam, N.; Luna, A.R.; Troell, M.; Dahdouh-Guebas, F. Remote sensing and ethno- botanical assessment of the mangrove forest changes in the Navachiste-San Ignacio-Macapule lagoon complex, Sinaloa, Mexico. Ecol. Soc. 2005, 10, 16. [Google Scholar]
- Beland, M.; Goita, K.; Bonn, F.; Pham, T.T.H. Assessment of land-cover changes related to shrimp aquaculture using remote sensing data: A case study in the Giao Thuy District, Vietnam. Int. J. Remote Sens. 2006, 27, 1491–1510. [Google Scholar] [CrossRef]
- Giri, C.; Pengra, B.; Zhu, Z.L.; Singh, A.; Tieszen, L.L. Monitoring mangrove forest dynamics of the Sundarbans in Bangladesh and India using multi-temporal satellite data from 1973 to 2000. Estuar. Coast. Shelf Sci. 2007, 73, 91–100. [Google Scholar] [CrossRef]
- Giri, C.; Zhu, Z.; Tieszen, L.L.; Singh, A.; Gillette, S.; Kelmelis, J.A. Mangrove forest distributions and dynamics (1975–2005) of the tsunami-affected region of Asia. J. Biogeogr. 2008, 35, 519–528. [Google Scholar] [CrossRef]
- Liu, K.; Li, X.; Shi, X.; Wang, S.G. Monitoring mangrove forest changes using remote sensing and GIS data with decision-tree learning. Wetlands 2008, 28, 336–346. [Google Scholar] [CrossRef]
- Paling, E.I.; Kobryn, H.T.; Humphreys, G. Assessing the extent of mangrove change caused by Cyclone Vance in the eastern Exmouth Gulf, northwestern Australia. Estuar. Coast. Shelf Sci. 2008, 77, 603–613. [Google Scholar] [CrossRef]
- Alsaaideh, B.; Al-Hanbali, A.; Tateishi, R.; Kobayashi, T.; Hoan, N.T. Mangrove forests mapping in the southern part of Japan using Landsat ETM+ with DEM. J. Geogr. Inf. Syst. 2013, 5, 369–377. [Google Scholar]
- Li, M.S.; Mao, L.J.; Shen, W.J.; Liu, S.Q.; Wei, A.I. Change and fragmentation trends of Zhanjiang mangrove forests in southern China using multi-temporal Landsat imagery (1977–2010). Estuar. Coast. Shelf Sci. 2013, 130, 111–120. [Google Scholar] [CrossRef]
- Nguyen, H.; McAlpine, C.; Pullar, D.; Johansen, K.; Duke, N. The relationship of spatial-temporal changes in fringe mangrove extent and adjacent land-use: Case study of Ken Giang coast, Vietnam. Ocean Coast. Manag. 2013, 76, 12–22. [Google Scholar] [CrossRef]
- Giri, C.; Muhlhausen, J. Mangrove forest distributions and dynamics in Madagascar (1975–2005). Sensors 2008, 8, 2104–2117. [Google Scholar] [CrossRef]
- Rasolofoharinoro, M.; Blasco, F.; Bellan, M.F.; Aizpuru, M.; Gauquelin, T.; Denis, J. A remote sensing based methodology for mangrove studies in Madagascar. Int. J. Remote Sens. 1998, 19, 1873–1886. [Google Scholar] [CrossRef]
- Pasqualini, V.; Iltis, J.; Dessay, N.; Lointier, M.; Guelorget, O.; Polidori, L. Mangrove mapping in North-Western Madagascar using SPOT-XS and SIR-C radar data. Hydrobiologia 1999, 413, 127–133. [Google Scholar] [CrossRef]
- Guillet, M.; Renou, E.; Robin, M.; Debaine, F.; Ratsivalaka, S. Suivi et analyze de l’evolution de la mangrove de Mahajamba (Nord-ouest de Madagascar). In Proceedings of the International Pluridisciplinary Conference, Lille, France, 16–18 January 2008.
- Raharimahefa, T.; Kusky, T.M. Environmental monitoring of Bombetoka Bay and the Betsiboka Estuary, Madagascar, using multi-temporal satellite data. J. Earth Sci. 2010, 21, 210–226. [Google Scholar] [CrossRef]
- Rakotomavo, A.; Fromard, F. Dynamics of mangrove forests in the Mangoky River delta, Madagascar, under the influence of natural and human factors. For. Ecol. Manag. 2010, 259, 1161–1169. [Google Scholar] [CrossRef]
- Giri, C. National-Level Mangrove Cover Data-Sets for 1990, 2000 and 2010; United States Geological Survey: Sioux Falls, SD, USA, 2011. [Google Scholar]
- Rasolofo, M.V.; Ramilijaona, O. Variability in the abundance and recruitment of Fenneropenaeus indicus and Metapenaeus monoceros postlarvae and juveniles in Ambaro Bay mangroves of Madagascar. Nat. Faune 2009, 24, 103–109. [Google Scholar]
- Rasofolo, M.V. Use of mangroves by traditional fishermen in Madagascar. Mangroves Salt Marshes 1997, 1, 243–253. [Google Scholar] [CrossRef]
- Mayaux, P.; Gond, V.; Bartholome, E. A near-real time forest-cover map of Madagascar derived from SPOT-4 VEGETATION data. Int. J. Remote Sens. 2000, 21, 3139–3144. [Google Scholar] [CrossRef]
- Critical Ecosystem Partnership Fund (CEPF). Madagascar Vegetation Mapping Project; CEPF: Arlington, VA, USA, 2007. [Google Scholar]
- Harper, G.J.; Steininger, M.K.; Tucker, C.J.; Juhn, D.; Hawkins, F. Fifty years of deforestation and forest fragmentation in Madagascar. Environ. Conserv. 2007, 34, 325–333. [Google Scholar]
- USGS Earth Explorer. Available online: http://earthexplorer.usgs.gov/ (accessed on 1 December 2011).
- Gutman, G.; Byrnes, R.; Masek, J.; Covington, S.; Justice, C.; Franks, S.; Kurtz, R. Towards monitoring land-cover and land-use changes at a global scale: The Global Land Survey 2005. Photogramm. Eng. Remote Sens. 2008, 74, 6–10. [Google Scholar]
- Chavez, P.S. Image-based atmospheric corrections: Revisited and improved. Photogramm. Eng. Remote Sens. 1996, 62, 1025–1036. [Google Scholar]
- Kirui, K.B.; Kairo, J.G.; Bosire, J.; Viergever, K.M.; Rudra, S.; Huxham, M.; Briers, R.A. Mapping of mangrove forest land cover change along the Kenya coastline using Landsat imagery. Ocean Coast. Manag. 2013, 83, 19–24. [Google Scholar] [CrossRef]
- Simard, M.; Zhang, K.Q.; Rivera-Monroy, V.H.; Ross, M.S.; Ruiz, P.L.; Castaneda-Moya, E.; Twilley, R.R.; Rodriguez, E. Mapping height and biomass of mangrove forests in Everglades National Park with SRTM elevation data. Photogramm. Eng. Remote Sens. 2006, 72, 299–311. [Google Scholar] [CrossRef]
- Fatoyinbo, T.E.; Simard, M.; Washington-Allen, R.A.; Shugart, H.H. Landscape-scale extent, height, biomass, and carbon estimation of Mozambique’s mangrove forests with Landsat ETM+ and Shuttle Radar Topography Mission elevation data. J. Geophys. Res. Biogeosci. 2008, 113. [Google Scholar] [CrossRef]
- Simard, M.; Rivera-Monroy, V.H.; Mancera-Pineda, J.E.; Castaneda-Moya, E.; Twilley, R.R. A systematic method for 3D mapping of mangrove forests based on Shuttle Radar Topography Mission elevation data, ICEsat/GLAS waveforms and field data: Application to Cienaga Grande de Santa Marta, Colombia. Remote Sens. Environ. 2008, 112, 2131–2144. [Google Scholar] [CrossRef]
- Bhattarai, B.; Giri, C. Assessment of mangrove forests in the Pacific region using Landsat imagery. J. Appl. Remote Sens. 2011. [Google Scholar] [CrossRef]
- Long, J.B.; Giri, C. Mapping the Philippines’ mangrove forests using Landsat imagery. Sensors 2011, 11, 2972–2981. [Google Scholar] [CrossRef]
- Fatoyinbo, T.E.; Simard, M. Height and biomass of mangroves in Africa from ICESat/GLAS and SRTM. Int. J. Remote Sens. 2013, 34, 668–681. [Google Scholar] [CrossRef]
- Blasco, F.; Gauquelin, T.; Rasolofoharinoro, M.; Denis, J.; Aizpuru, M.; Caldairou, V. Recent advances in mangrove studies using remote sensing data. Mar. Freshw. Res. 1998, 49, 287–296. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Melbourne, Australia, 1986. [Google Scholar]
- Aschbacher, J.; Ofren, R.; Delsol, J.P.; Suselo, T.B.; Vibulsresth, S.; Charrupat, T. An integrated comparative approach to mangrove vegetation mapping using advanced remote sensing and GIS technologies: Preliminary results. Hydrologica 1995, 295, 285–295. [Google Scholar]
- Gao, J.A. Hybrid method toward accurate mapping of mangroves in a marginal habitat from SPOT Multispectral data. Int. J. Remote Sens. 1998, 19, 1887–1899. [Google Scholar] [CrossRef]
- Green, E.P.; Clark, C.D.; Mumby, P.J.; Edwards, A.J.; Ellis, A.C. Remote sensing techniques for mangrove mapping. Int. J. Remote Sens. 1998, 19, 935–956. [Google Scholar] [CrossRef]
- Gao, J.A. Comparative study on spatial and spectral resolutions of satellite data in mapping mangrove forests. Int. J. Remote Sens. 1999, 20, 2823–2833. [Google Scholar] [CrossRef]
- Saito, H.; Bellan, M.F.; Al-Habshi, A.; Aizpuru, M.; Blasco, F. Mangrove research and coastal ecosystem studies with SPOT-4 HRVIR and TERRA ASTER in Arabian Gulf. Int. J. Remote Sens. 2003, 24, 4073–4092. [Google Scholar] [CrossRef]
- Tong, P.H.; Auda, Y.; Populus, J.; Aizpura, M.; Habshi, A.A.; Blasco, F. Assessment from space of mangroves evolution in the Mekong Delta, in relation to extensive shrimp farming. Int. J. Remote Sens. 2004, 25, 4795–4812. [Google Scholar] [CrossRef]
- Jensen, L.S.; Mueller, T.; Tate, K.R.; Ross, D.J.; Magid, J.; Nielsen, N.E. Soil surface CO2 flux as an index of soil respiration in situ: A comparison of two chamber methods. Soil Biol. Biochem. 1996, 28, 1297–1306. [Google Scholar] [CrossRef]
- Fromard, F.; Puig, H.; Mougin, E.; Marty, G.; Betoulle, J.L.; Cadamuro, L. Structure, above-ground biomass and dynamics of mangrove ecosystems: New data from French Guiana. Oecologia 1998, 115, 39–53. [Google Scholar] [CrossRef]
- Smith, T.J., III; Whelan, K.R.T. Development of allometric relations for three mangrove species in South Florida for use in the Greater Everglades Ecosystem restoration. Wetl. Ecol. Manag. 2006, 14, 409–419. [Google Scholar] [CrossRef]
- Komiyama, A.; Poungparn, S.; Kato, S. Common allometric equations for estimate the tree weight of mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Schumacher, B. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; Ecological Risk Assessment Support Center, Office of Research and Development. US Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Mikhailova, E.A.; Noble, R.R.P.; Post, C.J. Comparison of soil organic carbon recovery by Walkley-Black and dry combustion methods in the Russian Chernozem. Commun. Soil Sci. Plant Anal. 2003, 34, 1853–1860. [Google Scholar] [CrossRef]
- De Vos, B.; Letterns, S.; Muys, B.; Deckers, J.A. Walkley-Black analysis of forest soil organic carbon: Recovery, limitations and uncertainty. Soil Use Manag. 2007, 23, 221–229. [Google Scholar] [CrossRef]
- Meersmans, J.; van Wesemael, B.; van Molle, M. Determining soil organic carbon for agricultural soils: A comparison between the Walkley & Black and the dry combustion methods (north Belgium). Soil Use Manag. 2009, 25, 346–353. [Google Scholar] [CrossRef]
- Simpson, W.T. Method to Estimate Dry-Kiln Schedules and Species Groupings: Tropical and Temperate Hardwoods; US Department of Agriculture, Forest Service, Forest Products Laboratory: Washington, DC, USA, 1996. [Google Scholar]
- Dharmawan, I.W.S.; Siregar, C.A. Soil carbon and carbon estimation of Avicennia marina (Forsk.). Vierh. Stand at Ciasem, Purwakarta. J. Penelit. Hutan dan Konservasi Alam. 2008, 5, 317–328. [Google Scholar]
- Clough, B.F.; Scott, K. Allometric relationships for estimating above-ground biomass in six mangrove species. For. Ecol. Manag. 1989, 27, 117–127. [Google Scholar] [CrossRef]
- Comley, B.W.T.; McGuinness, K.A. Above- and below-ground biomass, and allometry, of four common northern Australian mangroves. Aust. J. Bot. 2005, 53, 431–436. [Google Scholar] [CrossRef]
- Sinclair, T.T.; Hoffer, R.M.; Schreiber, M.M. Reflectance and internal structure of leaves from several crops during a growing season. Agron. J. 1971, 63, 864–868. [Google Scholar] [CrossRef]
- Elvidge, C.D. Visible and near-infrared reflectance characteristics of dry plant materials. Int. J. Remote Sens. 1990, 11, 1775–1795. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Tamura, M.; Kikushima, K. Extraction of mangrove forests using a satellite image and a digital elevation model. In Proceedings of the SPIE 7104, Remote Sensing for Agriculture, Ecosystems, and Hydrology X, Cardiff, UK, 15 September 2008; Volume 7104.
- Lippitt, C.D.; Rogan, J.; Zhe, L.; Eastman, R.J.; Jones, T.G. Mapping selective logging in mixed deciduous forest: A comparison of machine learning algorithms. Photogramm. Eng. Remote Sens. 2008, 74, 1201–1211. [Google Scholar] [CrossRef]
- Plugge, D.; Baldauf, T.; Ratsimba, H.R.; Rajoelison, G.; Köhl, M. Combined biomass inventory in the scope of REDD (Reducing Emissions from Deforestation and Forest Degradation. Madag. Conserv. Dev. 2010, 5, 23–34. [Google Scholar]
- Bredbenner, A. Profiles in Blue Carbon Field Work; Conservation International: Washington, DC, USA, 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jones, T.G.; Ratsimba, H.R.; Ravaoarinorotsihoarana, L.; Cripps, G.; Bey, A. Ecological Variability and Carbon Stock Estimates of Mangrove Ecosystems in Northwestern Madagascar. Forests 2014, 5, 177-205. https://doi.org/10.3390/f5010177
Jones TG, Ratsimba HR, Ravaoarinorotsihoarana L, Cripps G, Bey A. Ecological Variability and Carbon Stock Estimates of Mangrove Ecosystems in Northwestern Madagascar. Forests. 2014; 5(1):177-205. https://doi.org/10.3390/f5010177
Chicago/Turabian StyleJones, Trevor G., Harifidy Rakoto Ratsimba, Lalao Ravaoarinorotsihoarana, Garth Cripps, and Adia Bey. 2014. "Ecological Variability and Carbon Stock Estimates of Mangrove Ecosystems in Northwestern Madagascar" Forests 5, no. 1: 177-205. https://doi.org/10.3390/f5010177
APA StyleJones, T. G., Ratsimba, H. R., Ravaoarinorotsihoarana, L., Cripps, G., & Bey, A. (2014). Ecological Variability and Carbon Stock Estimates of Mangrove Ecosystems in Northwestern Madagascar. Forests, 5(1), 177-205. https://doi.org/10.3390/f5010177





