Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest
Abstract
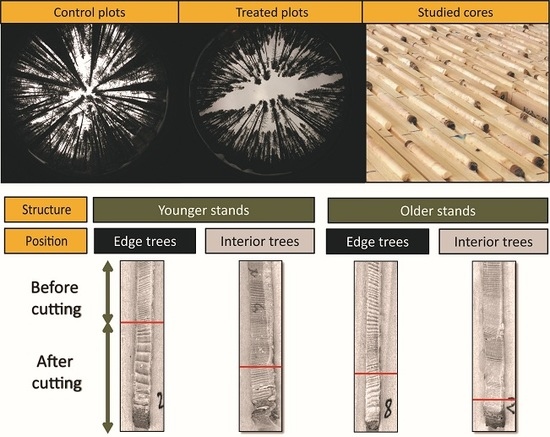
:1. Introduction
- (i)
- Shelterwood and seed-tree treatments will show a significant increase in radial growth compared to untreated control plots.
- (ii)
- No significant differences will be found in radial growth among shelterwood treatments; however, seed-trees will produce a greater growth response than shelterwood because of a higher harvest intensity.
- (iii)
- Younger and denser stands will have a faster and greater growth response, due to the growth decrease with age.
- (iv)
- Edge trees will manifest greater differences in terms of radial growth, because they have less competition and more accessibility to nutrients and light compared to interior residual trees.
- (v)
- Suppressed trees before cutting will display stronger growth responses after treatment than dominant trees due to tree selection in the residual strip.
2. Material & Methods
2.1. Study Area
2.2. Experimental Design
2.3. Silvicultural Treatments
2.4. Plot Measurements and Compilation
2.5. Dendroecological Data
2.6. Data Analysis
2.6.1. Radial Growth Model
2.6.2. Factors Influencing Growth Response
3. Results
3.1. Stand Attributes
3.2. Radial Growth Response
4. Discussion
4.1. Radial Growth Response
4.2. Factors Influencing Growth Response
4.2.1. Initial Stand Age and Density
4.2.2. Silvicultural Treatment
4.2.3. Edge Effect
4.2.4. Time Response
4.2.5. Growth before Cutting
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.; Schepaschenko, D. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Smeets, E.M.; Faaij, A.P. Bioenergy potentials from forestry in 2050. Clim. Chang. 2007, 81, 353–390. [Google Scholar] [CrossRef]
- Groot, A.; Lussier, J.M.; Mitchell, A.; MacIsaac, D. A silvicultural systems perspective on changing Canadian forestry practices. For. Chron. 2005, 81, 50–55. [Google Scholar] [CrossRef]
- Rosenvald, R.; Lohmus, A. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For. Ecol. Manag. 2008, 255, 1–15. [Google Scholar] [CrossRef]
- Bouchard, M.; Pothier, D. Long-term influence of fire and harvesting on boreal forest age structure and forest composition in eastern Québec. For. Ecol. Manag. 2011, 261, 811–820. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F. Conserving Forest Biodiversity: A Comprehensive Multiscaled Approach; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Fuller, A.K.; Harrison, D.J.; Lachowski, H.J. Stand scale effects of partial harvesting and clearcutting on small mammals and forest structure. For. Ecol. Manag. 2004, 191, 373–386. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. State of the World’s Forest: Enhancing the Socioeconomic Benefits from Forests; FAO: Rome, Italy, 2014; p. 133. [Google Scholar]
- Puettmann, K.J.; Coates, K.D.; Messier, C.C. A Critique of Silviculture. Managing for Complexity, 1st ed.; Island Press: Washington, DC, USA, 2009; p. 208. [Google Scholar]
- Gauthier, S.V.; Vaillancourt, M.-A.; Leduc, A.; De Granpré, L.; Kneeshaw, D.; Morin, H.; Drapeau, P.; Bergeron, Y. Ecosystem Management in the Boreal Forest, 1st ed.; Presses de l’Université de Québec: Quebec City, QC, Canada, 2009. [Google Scholar]
- Fenton, N.; Bescond, H.; Imbeau, L.; Boudreault, C.; Drapeau, P.; Bergeron, Y. Silvicultural and ecological evaluation of partial harvest in the boreal forest on the claybelt, Québec. In Ecosystem Management in the Boreal Forest; Gauthier, S., Leduc, A., De Granpré, L., Kneeshaw, D., Morin, H., Drapeau, P., Bergeron, Y., Eds.; Presses de l’Université du Québec: Quebec City, QC, Canada, 2009; pp. 373–393. [Google Scholar]
- Thorpe, H.; Thomas, S. Partial harvesting in the Canadian boreal: Success will depend on stand dynamic responses. For. Chron. 2007, 83, 319–325. [Google Scholar] [CrossRef]
- Latham, P.; Tappeiner, J. Response of old-growth conifers to reduction in stand density in western Oregon forests. Tree Physiol. 2002, 22, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, L.; Raymond, P.; Camiré, C.; Munson, A.D. Impact of precommercial thinning in balsam fir stands on soil nitrogen dynamics, microbial biomass, decomposition, and foliar nutrition. Can. J. For. Res. 2000, 30, 229–238. [Google Scholar] [CrossRef]
- Pape, R. Influence of thinning and tree diameter class on the development of basic density and annual ring width in Picea abies. Scand. J. For. Res. 1999, 14, 27–37. [Google Scholar] [CrossRef]
- Mehtätalo, L.; Peltola, H.; Kilpeläinen, A.; Ikonen, V.P. The response of basal area growth of Scots pine to thinning: A longitudinal analysis of tree-specific series using a nonlinear mixed-effects model. For. Sci. 2014, 60, 636–644. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Isomäki, A. Radial, height and volume increment variation in Picea abies (L.) Karst. stands with varying thinning intensities. Scand. J. For. Res. 2002, 17, 304–316. [Google Scholar] [CrossRef]
- Bella, I.E.; DeFranceschi, J. Commercial Thinning Improves Growth of Jack Pine; Information Report NOR-X-112; Canadian Forest Service, Northern Forest Research Center: Edmonton, AB, Canada, 1974; p. 23.
- Pamerleau-Couture, É.; Krause, C.; Pothier, D.; Weiskittel, A. Effect of three partial cutting practices on stand structure and growth of residual black spruce trees in north-eastern Quebec. Forestry 2015, 88, 471–483. [Google Scholar] [CrossRef]
- Vincent, M.; Krause, C.; Zhang, S. Radial growth response of black spruce roots and stems to commercial thinning in the boreal forest. Forestry 2009, 82, 557–571. [Google Scholar] [CrossRef]
- Krause, C.; Laplante, S.; Plourde, P.-Y. Transversal tracheid dimension in thinned black spruce and jack pine stands in the boreal forest. Scand. J. For. Res. 2011, 26, 477–487. [Google Scholar] [CrossRef]
- Thorpe, H.; Thomas, S.; Caspersen, J. Residual-tree growth responses to partial stand harvest in the black spruce (Picea mariana) boreal forest. Can. J. For. Res. 2007, 37, 1563–1571. [Google Scholar] [CrossRef]
- Alteyrac, J.; Zhang, S.; Cloutier, A.; Ruel, J.C. Influence of stand density on ring width and wood density at different sampling heights in black spruce (Picea mariana (Mill.) BSP). Wood Fiber Sci. 2005, 37, 83–94. [Google Scholar]
- Mailly, D.; Turbis, S.; Pothier, D. Predicting basal area increment in a spatially explicit, individual tree model: A test of competition measures with black spruce. Can. J. For. Res. 2003, 33, 435–443. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Esseen, P.A. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Sandoval, S.; Cancino, J. Modeling the edge effect in even-aged Monterrey pine (Pinus radiata D. Don) stands incorporating a competition index. For. Ecol. Manag. 2008, 256, 78–87. [Google Scholar] [CrossRef]
- Baker, S.C.; Spies, T.A.; Wardlaw, T.J.; Balmer, J.; Franklin, J.F.; Jordan, G.J. The harvested side of edges: Effect of retained forests on the re-establishment of biodiversity in adjacent harvested areas. For. Ecol. Manag. 2013, 302, 107–121. [Google Scholar] [CrossRef]
- Chen, J.; Franklin, J.F.; Spies, T.A. Contrasting microclimates among clearcut, edge, and interior of old-growth douglas-fir forest. Agric. For. Meteorol. 1993, 63, 219–237. [Google Scholar] [CrossRef]
- Genet, A.; Pothier, D. Modeling tree spatial distributions after partial harvesting in uneven-aged boreal forests using inhomogeneous point processes. For. Ecol. Manag. 2013, 305, 158–166. [Google Scholar] [CrossRef]
- Thorpe, H.; Thomas, S.; Caspersen, J. Tree mortality following partial harvests is determined by skidding proximity. Ecol. Appl. 2008, 18, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Labelle, E.R.; Jaeger, D. Soil compaction caused by cut-to-length forest operations and possible short-term natural rehabilitation of soil density. Soil Sci. Soc. Am. J. 2011, 75, 2314–2329. [Google Scholar] [CrossRef]
- Thorpe, H.; Vanderwel, M.; Fuller, M.; Thomas, S.; Caspersen, J. Modelling stand development after partial harvests: An empirically based, spatially explicit analysis for lowland black spruce. Ecol. Model. 2010, 221, 256–267. [Google Scholar] [CrossRef]
- Fraver, S.; D’Amato, A.W.; Bradford, J.B.; Jonsson, B.G.; Jönsson, M.; Esseen, P.A. Tree growth and competition in an old-growth Picea abies forest of boreal Sweden: Influence of tree spatial patterning. J. Veg. Sci. 2014, 25, 374–385. [Google Scholar] [CrossRef]
- Mayaka, T.B. A family of segmented polynomial functions for modelling the border effect on the diameter growth of Ayous (Triplochiton scleroxylon K. Schum). For. Ecol. Manag. 1994, 70, 275–283. [Google Scholar] [CrossRef]
- Cancino, J. Modelling the edge effect in even-aged Monterey pine (Pinus radiata D. Don) stands. For. Ecol. Manag. 2005, 210, 159–172. [Google Scholar] [CrossRef]
- Bowering, M.; LeMay, V.; Marshall, P. Effects of forest roads on the growth of adjacent lodgepole pine trees. Can. J. For. Res. 2006, 36, 919–929. [Google Scholar] [CrossRef]
- Saucier, J.P.; Bergeron, J.F.; Grondin, P.; Robitaille, A. Les régions écologiques du Québec méridional (3th version): Un des éléments du système hiérarchique de classification écologique du territoire mis au point par le Ministère des Ressources Naturelles du Québec. L’aubelle 1998, 124, 1–12. [Google Scholar]
- Rossi, S.; Morin, H.; Deslauriers, A.; Plourde, P.Y. Predicting xylem phenology in black spruce under climate warming. Glob. Chang. Biol. 2011, 17, 614–625. [Google Scholar] [CrossRef]
- Robitaille, A.; Saucier, J.P. Paysages Régionaux du Québec Méridional; Les Publications du Québec: Québec City, QC, Canada, 1998. [Google Scholar]
- Waldron, K.; Ruel, J.-C.; Gauthier, S. The effects of site characteristics on the landscape-level windthrow regime in the North Shore region of Quebec, Canada. Forestry 2013, 86, 159–171. [Google Scholar] [CrossRef]
- Matthews, J.D. Silvicultural Systems; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology, 9th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Raymond, P.L.I.; Guay, L.; Godbout, C. Chapter 19. La coupe progressive régulière. In Le Guide Sylvicole du Québec. Les Concepts et L’application de la Sylviculture, 1st ed.; Larouche, C., Raymond, F.G.P., Saucier, J.P., Eds.; Gouvernament du Québec: Québec City, QC, Canada, 2013; Volume 2, pp. 456–515. [Google Scholar]
- Krause, C.; Morin, H. Changes in radial increment in stems and roots of balsam fir [Abies balsamea (L.) Mill.] after defoliation spruce budworm. For. Chron. 1995, 71, 747–754. [Google Scholar] [CrossRef]
- Guay, R.; Gagnon, R.; Morin, H. A new automatic and interactive tree ring measurement system based on a line scan camera. For. Chron. 1992, 68, 138–141. [Google Scholar] [CrossRef]
- Piepho, H.; Williams, E.; Fleck, M. A note on the analysis of designed experiments with complex treatment structure. HortScience 2006, 41, 446–452. [Google Scholar]
- Selya, A.S.; Rose, J.S.; Dierker, L.C.; Hedeker, D.; Mermelstein, R.J. A practical guide to calculating Cohen’s f2, a measure of local effect size, from proc mixed. Front. Psychol. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, G.J.; Abrams, M.D. Radial-growth averaging criteria for reconstructing disturbance histories from presettlement-origin oaks. Ecol. Monogr. 1997, 67, 225–249. [Google Scholar] [CrossRef]
- Copenheaver, C.A.; Abrams, M.D. Dendroecology in young stands: Case studies from jack pine in northern lower Michigan. For. Ecol. Manag. 2003, 182, 247–257. [Google Scholar] [CrossRef]
- Payette, S.; Filion, L.; Delwaide, A. Disturbance regime of a cold temperate forest as deduced from tree-ring patterns: The Tantaré ecological reserve, Quebec. Can. J. For. Res. 1990, 20, 1228–1241. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Baldwin, V., Jr. Regeneration Following Shelterwood Cutting in a New Brunswick Softwood Stand; Information Report M-X-76; Maritime Forest Research Center, Canadian Forest Service: Fredericton, NB, Canada, 1977.
- Burgess, D.; Pinto, F.; Wetzel, S. Some Management Implications from an Eastern White Pine Regeneration Experiment; Transfer, T., Ed.; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2002; Volume 28, p. 6.
- Youngblood, A.P. Radial growth after a shelterwood seed cut in a mature stand of white spruce in interior alaska. Can. J. For. Res. 1991, 21, 410–413. [Google Scholar] [CrossRef]
- Goudiaby, V.; Brais, S.; Berninger, F.; Schneider, R. Vertical patterns in specific volume increment along stems of dominant jack pine (Pinus banksiana) and black spruce (Picea mariana) after thinning. Can. J. For. Res. 2012, 42, 733–748. [Google Scholar] [CrossRef]
- Soucy, M.; Lussier, J.M.; Lavoie, L. Long-term effects of thinning on growth and yield of an upland black spruce stand. Can. J. For. Res. 2012, 42, 1669–1677. [Google Scholar] [CrossRef]
- Seymour, R.S.; Kenefic, L.S. Influence of age on growth efficiency of Tsuga canadensis and Picea rubens trees in mixed-species, multiaged northern conifer stands. Can. J. For. Res. 2002, 32, 2032–2042. [Google Scholar] [CrossRef]
- Yoder, B.; Ryan, M.; Waring, R.; Schoettle, A.; Kaufmann, M. Evidence of reduced photosynthetic rates in old trees. For. Sci. 1994, 40, 513–527. [Google Scholar]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carrer, M. Age-dependent xylogenesis in timberline conifers. New Phytol. 2008, 177, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.A.; Macdonald, S.E.; Mayerhofer, M.S.; Biswas, S.R.; Esseen, P.A.; Hylander, K.; Stewart, K.J.; Mallik, A.U.; Drapeau, P.; Jonsson, B.G. Edge influence on vegetation at natural and anthropogenic edges of boreal forests in Canada and Fennoscandia. J. Ecol. 2015, 103, 550–562. [Google Scholar] [CrossRef]
- MCDonald, R.I.; Urban, D.L. Forest edges and tree growth rates in the North Carolina Piedmont. Ecology 2004, 85, 2258–2266. [Google Scholar] [CrossRef]
- Picchio, R.; Neri, F.; Maesano, M.; Savelli, S.; Sirna, A.; Blasi, S.; Baldini, S.; Marchi, E. Growth effects of thinning damage in a corsican pine (Pinus laricio Poiret) stand in central italy. For. Ecol. Manag. 2011, 262, 237–243. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Silvics of North America; U.S. Dept. of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2, p. 654.
- Groot, A.; Horton, B.J. Age and size structure of natural and second-growth peatland Picea mariana stands. Can. J. For. Res. 1994, 24, 225–233. [Google Scholar] [CrossRef]
- Belien, E.; Rossi, S.; Morin, H.; Deslauriers, A. High-resolution analysis of stem radius variations in black spruce [Picea mariana (Mill.) BSP] subjected to rain exclusion for three summers. Trees 2014, 28, 1257–1265. [Google Scholar] [CrossRef]
- Krause, C.; Morin, H. Adventive-root development in mature black spruce and balsam fir in the boreal forests of Quebec, Canada. Can. J. For. Res. 2005, 35, 2642–2654. [Google Scholar] [CrossRef]
- Roberts, S.D.; Harrington, C.A. Individual tree growth response to variable-density thinning in coastal Pacific northwest forests. For. Ecol. Manag. 2008, 255, 2771–2781. [Google Scholar] [CrossRef]
- Urban, S.; Lieffers, V.; MacDonald, S. Release in radial growth in the trunk and structural roots of white spruce as measured by dendrochronology. Can. J. For. Res. 1994, 24, 1550–1556. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Dunn, A.J. The effects of wind speed and direction on radial growth of structural roots. In The Supporting Roots of Trees and Woody Plants: Form, Function and Physiology; Springer: Dordrecht, The Netherlands, 2000; pp. 219–225. [Google Scholar]
- Ruel, J.C.; Larouche, C.; Achim, A. Changes in root morphology after precommercial thinning in balsam fir stands. Can. J. For. Res. 2003, 33, 2452–2459. [Google Scholar] [CrossRef]
- Bebber, D.P.; Thomas, S.C.; Cole, W.G.; Balsillie, D. Diameter increment in mature eastern white pine Pinus strobus L. following partial harvest of old-growth stands in Ontario, Canada. Trees 2004, 18, 29–34. [Google Scholar]
- Chen, J.; Franklin, J.F.; Spies, T.A. Growing-season microclimatic gradients from clearcut edges into old-growth douglas-fir forests. Ecol. Appl. 1995, 5, 74–86. [Google Scholar] [CrossRef]
- Weiner, J.; Thomas, S.C. Size variability and competition in plant monocultures. Oikos 1986, 47, 211–222. [Google Scholar] [CrossRef]







| Treatment | Partial Cutting | Basal Area Harvested (%) | Residual Strip | Skidding Trail | Secondary Trail | Edge Surface (b) (%) | ||
|---|---|---|---|---|---|---|---|---|
| Width (m) | Intact Surface (%) | Width (m) | Surface (%) | |||||
| Mini-strip (MS) | Yes | 50 | 5 | 100 | 5 | 50 | No | 50 |
| Distant selection (DS) | Yes | 50 | 25 | 20 | 5 or 10 (a) | 17 | Yes | 24.5 |
| Close selection (CS) | Yes | 50 | 15 | 33 | 5 | 25 | No | 16.3 |
| Seed-trees (ST) | No | 75 | 5 | 100 | 15 | 75 | No | 50 |
| Treatment | Density (Tree/ha) | Basal Area (m2/ha) | Volume (m3/ha) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Residual | Harvested (%) | Initial | Residual | Harvested (%) | Initial | Residual | Harvested (%) | |||||||
| Control | |||||||||||||||
| -Younger | 2316.7 | ±464.6 | 2316.7 | ±464.6 | 0 | 38.6 | ±2.5 | 38.6 | ±2.5 | 0 | 192.9 | ±15.8 | 192.9 | ±15.8 | 0 |
| -Older | 1272.2 | ±398.6 | 1272.2 | ±398.6 | 0 | 25.2 | ±6.9 | 25.2 | ±6.9 | 0 | 138.9 | ±42.2 | 138.8 | ±42.2 | 0 |
| Mini-strip | |||||||||||||||
| -Younger | 2355.6 | ±209.1 | 1427.8 | ±138.9 | 39.4 | 35.8 | ±4.2 | 21.4 | ±3.2 | 40.2 | 169.4 | ±36.6 | 100.2 | ±22.4 | 40.9 |
| -Older | 1888.9 | ±502.4 | 888.9 | ±317.6 | 53.0 | 33.8 | ±8.2 | 15.5 | ±5.8 | 54.1 | 174.2 | ±39.4 | 78.1 | ±29.3 | 55.2 |
| Distant selection | |||||||||||||||
| -Younger | 2894.4 | ±373.3 | 1722.2 | ±352.5 | 40.5 | 41.5 | ±3.4 | 23.2 | ±5.2 | 44.1 | 188.2 | ±10.5 | 99.9 | ±23.9 | 47.0 |
| -Older | 1461.1 | ±231.8 | 838.9 | ±198.2 | 42.6 | 32.6 | ±5.8 | 18.3 | ±6.1 | 43.9 | 187.8 | ±37.2 | 104.7 | ±40.1 | 44.2 |
| Close selection | |||||||||||||||
| -Younger | 2794.4 | ±382.0 | 1483.3 | ±285.9 | 47.0 | 49.5 | ±5.4 | 26.3 | ±2.7 | 46.9 | 255.9 | ±28.8 | 136.0 | ±10.6 | 46.9 |
| -Older | 1566.7 | ±337.2 | 900.0 | ±279.0 | 42.6 | 30.1 | ±5.8 | 15.5 | ±4.9 | 48.5 | 162.0 | ±27.9 | 78.3 | ±24.6 | 51.7 |
| Seed-trees | |||||||||||||||
| -Younger | 2683.3 | ±211.7 | 850.0 | ±78.8 | 68.3 | 40.5 | ±3.0 | 11.7 | ±1.4 | 71.1 | 190.1 | ±32.6 | 51.6 | ±10.0 | 72.9 |
| -Older | 1538.9 | ±174.9 | 400.0 | ±50.9 | 74.0 | 32.9 | ±3.4 | 8.3 | ±0.9 | 74.8 | 185.3 | ±17.4 | 46.2 | ±4.1 | 75.0 |
| Effect | df | ddf | F | Pr > F |
|---|---|---|---|---|
| Structure | 1 | 4 | 10.74 | 0.0306 |
| Treatment/Position—factor | 8 | 32 | 2.82 | 0.0172 |
| Treatment | 4 | 32 | 2.91 | 0.0368 |
| -Control vs treated plots | 1 | 32 | 5.72 | 0.0228 |
| -Partial cuttings vs seed-tree | 1 | 32 | 2.01 | 0.1662 |
| -Close selection vs seed-tree | 1 | 32 | 4.54 | 0.0408 |
| -Mini-strip vs distant selection | 1 | 32 | 1.24 | 0.2733 |
| Position | 1 | 32 | 8.52 | 0.0064 |
| Year | 9 | 36 | 15.54 | <0.0001 |
| Structure × Year | 9 | 36 | 4.74 | 0.0003 |
| Treatment/Position × Year | 72 | 288 | 1.96 | <0.0001 |
| Structure × Treatment/Position × Year | 72 | 288 | 1.40 | 0.03 |
| GBC | 1 | 9368 | 906.07 | <0.0001 |
| GBC × Structure | 1 | 9368 | 5.14 | 0.0234 |
| GBC × Treatment/Position | 8 | 9368 | 2.62 | 0.0073 |
| GBC × Year | 9 | 9368 | 13.16 | <0.0001 |
| GBC × Structure × Year | 9 | 9368 | 3.39 | 0.0004 |
| GBC × Treatment/Position × Year | 80 | 9368 | 2.05 | <0.0001 |
| GBC × Structure × Treatment/Position × Year | 80 | 9368 | 1.34 | 0.0226 |
| R2 | N | Parameter | Estimate | SE | t Ratio | VIF | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Control | 0.73 | 218 | stand age | 0.20 | 0.04 | 4.88 | 1.07 | <0.0001 |
| GBC | 0.93 | 0.06 | 15.66 | 1.61 | <0.0001 | |||
| Edge | 0.56 | 418 | treatment | −0.09 | 0.03 | −2.74 | 1 | 0.0064 |
| structure | 0.08 | 0.04 | 2.34 | 2.32 | 0.0190 | |||
| stand age | −0.13 | 0.04 | −3.37 | 2.45 | 0.0008 | |||
| mortality | 0.12 | 0.02 | 4.11 | 1.72 | <0.0001 | |||
| GBC | 0.73 | 0.04 | 16.42 | 1.2 | <0.0001 | |||
| Interior | 0.61 | 403 | stand age | −0.19 | 0.05 | −3.45 | 1.48 | 0.0006 |
| harvest density | 0.73 | 0.30 | 2.45 | 3.15 | 0.0149 | |||
| DBH b.c. | 0.02 | 0.005 | 3.42 | 4.54 | 0.0007 | |||
| mortality | 0.18 | 0.06 | 3.12 | 5.17 | 0.0019 | |||
| GBC | 0.84 | 0.04 | 19.90 | 1.18 | <0.0001 |
| Structure | Years | GBC (mm/Year) | df | ddf | F | Pr > F |
|---|---|---|---|---|---|---|
| Younger | 0–5 | 0.2 | 8 | 36 | 1.24 | 0.3027 |
| Younger | 6–10 | 0.2 | 8 | 36 | 5.41 | 0.0002 |
| Older | 0–5 | 0.2 | 8 | 36 | 0.39 | 0.9170 |
| Older | 6–10 | 0.2 | 8 | 36 | 1.43 | 0.2189 |
| Younger | 0–5 | 0.6 | 8 | 36 | 0.44 | 0.8857 |
| Younger | 6–10 | 0.6 | 8 | 36 | 2.73 | 0.0183 |
| Older | 0–5 | 0.6 | 8 | 36 | 0.98 | 0.4663 |
| Older | 6–10 | 0.6 | 8 | 36 | 0.49 | 0.8555 |
| Younger | 0–5 | 1.0 | 8 | 36 | 1.40 | 0.2282 |
| Younger | 6–10 | 1.0 | 8 | 36 | 2.69 | 0.0197 |
| Older | 0–5 | 1.0 | 8 | 36 | 1.78 | 0.1144 |
| Older | 6–10 | 1.0 | 8 | 36 | 0.64 | 0.7395 |
| Position Class | Treatment | GBC (mm/Year) | ||
|---|---|---|---|---|
| 0.2 | 0.6 | 1.0 | ||
| Interior | Control | n.s. | ||
| Mini-strip | n.s. | |||
| Distant selection | n.s. | |||
| Close selection | n.s. | |||
| Seed-trees | n.s. | |||
| Edge | Control | c | c | c |
| Mini-strip | a | ab | bc | |
| Distant selection | a | a | a | |
| Close selection | ab | bc | c | |
| Seed-trees | ab | a | ab | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoro Girona, M.; Morin, H.; Lussier, J.-M.; Walsh, D. Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest. Forests 2016, 7, 240. https://doi.org/10.3390/f7100240
Montoro Girona M, Morin H, Lussier J-M, Walsh D. Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest. Forests. 2016; 7(10):240. https://doi.org/10.3390/f7100240
Chicago/Turabian StyleMontoro Girona, Miguel, Hubert Morin, Jean-Martin Lussier, and Denis Walsh. 2016. "Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest" Forests 7, no. 10: 240. https://doi.org/10.3390/f7100240
APA StyleMontoro Girona, M., Morin, H., Lussier, J.-M., & Walsh, D. (2016). Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest. Forests, 7(10), 240. https://doi.org/10.3390/f7100240