Landscape Biology of Western White Pine: Implications for Conservation of a Widely-Distributed Five-Needle Pine at Its Southern Range Limit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Current Population Structure and Trends
2.3. Genetic Structure and Diversity
2.4. Evaluating Disease Resistance and Cr2 Allele Frequency
3. Results
3.1. Western White Pine and Forest Conditions
3.2. Population Structure and Trends
3.3. Genetic Structure and Diversity
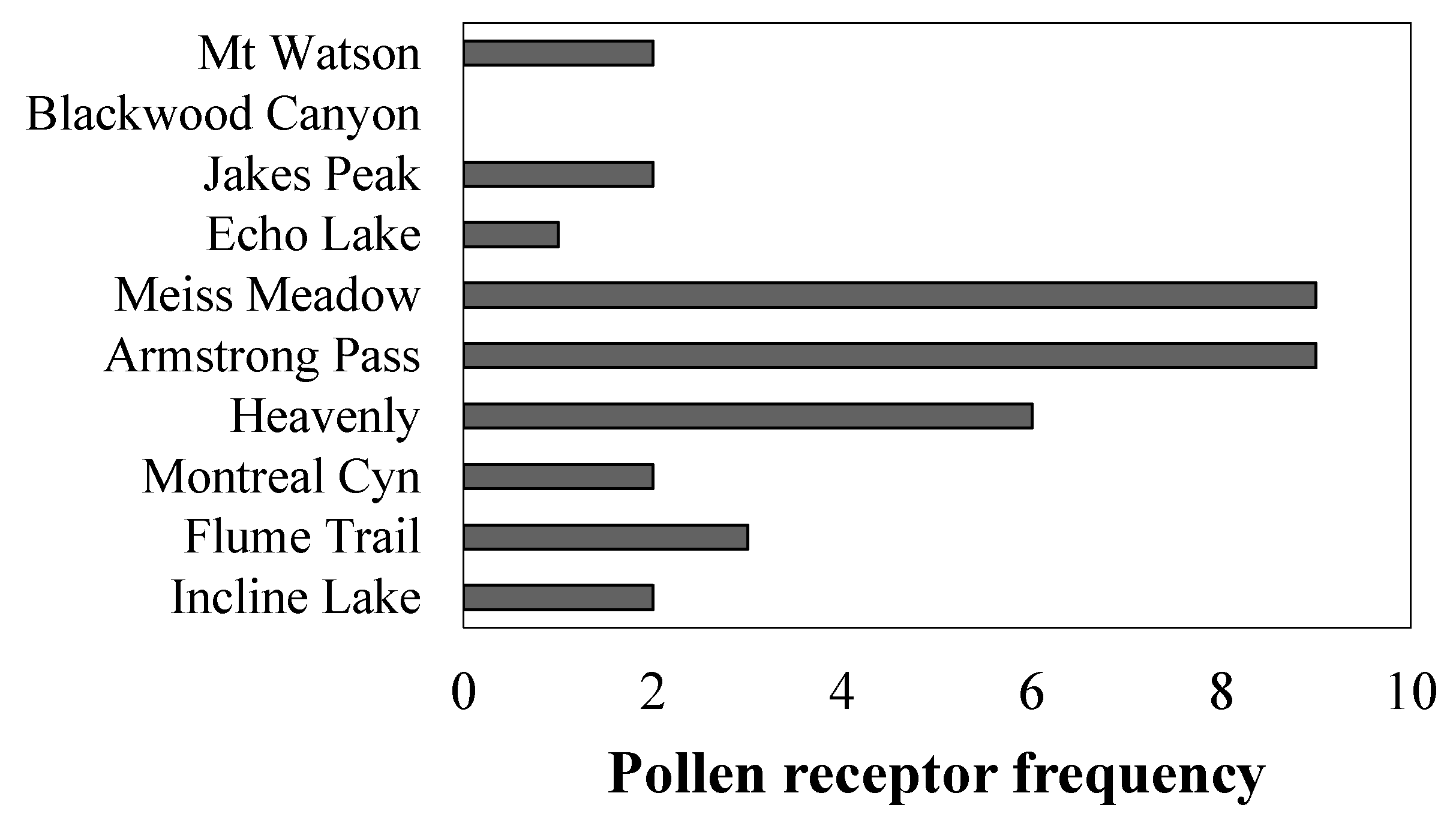
3.4. Frequency of Disease Resistance
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laudenslayer, W.F.J.; Darr, H.H. Historical effects of logging on the forests of the Cascade and Sierra Nevada ranges in California. Trans. West. Sect. Wild. Soc. 1990, 26, 12–23. [Google Scholar]
- Fins, L.; Byler, J.; Ferguson, D.; Harvey, A.; Mahalovich, M.F.; McDonald, G.; Miller, D.; Schwandt, J.; Zack, A. Return of the giants: Restoring western white pine to the inland Northwest. J. For. 2002, 100, 20–26. [Google Scholar]
- Taylor, A.H. Identifying forest reference conditions on early cut-over lands of the Lake Tahoe Basin, USA. Ecol. Appl. 2004, 14, 1903–1920. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Keifer, M.; Keeley, J. Effects of an introduced pathogen and fire exclusion on the demography of sugar pine. Ecol. Appl. 2004, 14, 1590–1602. [Google Scholar] [CrossRef]
- Shaw, C.G.; Geils, B.W. Special Issue: White Pines, Ribes, and Blister Rust. For. Pathol. 2010, 40, 145–418. [Google Scholar] [CrossRef]
- Maloney, P.E.; Vogler, D.R.; Eckert, A.J.; Jensen, C.E.; Neale, D.B. Population biology of sugar pine (Pinus lambertiana Dougl.) with reference to historical disturbances in the Lake Tahoe Basin: Implications for restoration. For. Ecol. Manag. 2011, 262, 770–779. [Google Scholar] [CrossRef]
- Goheen, E.M.; Goheen, D.J. Status of Sugar and Western White Pines on Federal Forest Lands in Southwest Oregon: Inventory Query and Natural Stand Survey Results; Report No. SWOFIDSC-14-01; USDA Forest Service, Pacific Northwest Region: Portland, OR, USA, 2014; p. 71. [Google Scholar]
- Gernandt, D.S.; Geada López, G.; Ortiz García, S.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef]
- Sudworth, J.B. Unpublished Field Note Books of Sierra Nevada Forest Reserve Inventory; University of California, Berkeley, Bioscience and Natural Resources Library: Berkeley, CA, USA, 1899. [Google Scholar]
- McKelvey, K.S.; Johnston, J.D. Historical perspectives on forests of the Sierra Nevada and the Transverse Ranges of southern California: Forest conditions at the turn of the century. General Technical Report; GTR-PSW-133; Verner, J., McKelvey, K.S., Noon, B.R., Gutierrez, R.J., Gould, G.I.J., Beck, T.W., Eds.; Tech. Coords., U.S. Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 1992; pp. 225–246. [Google Scholar]
- Ansley, J.; Battles, J.J. Forest composition, structure, and change in an old-growth mixed conifer forest in the northern Sierra Nevada. J. Torr. Bot. Soc. 1998, 125, 297–308. [Google Scholar] [CrossRef]
- Stephens, S.L. Mixed conifer and red fir forest structure and uses in 1899 from the central and northern Sierra Nevada, California. Madroño 2000, 47, 43–52. [Google Scholar]
- Maloney, P.E. Incidence and distribution of white pine blister rust in the high-elevation forests of California. For. Pathol. 2011, 41, 308–316. [Google Scholar] [CrossRef]
- Homans, G.M. Seventh Biennial Report of the State Board of Forestry of the State of California; California State Printing Office: Sacramento, CA, USA, 1919. [Google Scholar]
- Neuenschwander, L.F.; Byler, J.W.; Harvey, A.E.; McDonald, G.I.; Ortiz, D.S.; Osborne, H.L.; Snyder, G.C.; Zack, A. White Pine in the American West: A Vanishing Species-Can We Save It? General Technical Report; RMRS-GTR-35; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 1999; p. 20. [Google Scholar]
- Smith, R.S. Spread and intensification of blister rust in the range of sugar pine. In Sugar Pine: Status, Values, and Roles in Ecosystems: Proceedings of a Symposium presented by the California Sugar Pine Management; Publication 3362; Kinloch, B.B.J., Marosy, M., Huddleston, M.E., Eds.; University of California, Division of Agriculture and Natural Resources: Davis, CA, USA, 1996; pp. 112–118. [Google Scholar]
- Kinloch, B.B.J.; Sniezko, R.A.; Dupper, G.E. Origin and distribution of Cr2, a gene for resistance to white pine blister rust in natural populations of western white pine. Phytopatologyh 2003, 93, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, B.B.J.; Littlefield, J.L. White pine blister rust: Hypersensitive resistance in sugar pine. Can. J. Bot. 1976, 55, 1148–1155. [Google Scholar] [CrossRef]
- Taylor, A.H.; Halpern, C.B. The structure and dynamics of Abies magnifica forests in the southern Cascade Range, USA. J. Veg. Sci. 1991, 2, 189–200. [Google Scholar] [CrossRef]
- Taylor, A.H. Fire history and structure of red fir (Abies magnifica) forests, Swain Mountain Experimental Forest, Cascade Range, northeastern California. Can. J. For. Res. 1993, 23, 1672–1678. [Google Scholar] [CrossRef]
- Stephens, S.L. Fire history differences in adjacent Jeffrey pine and upper montane forests in the eastern Sierra Nevada. Int. J. Wildland Fire 2001, 10, 161–167. [Google Scholar] [CrossRef]
- North, M.P.; van de Water, K.M.; Stephens, S.L.; Collins, B.M. Climate, rain shadow, and human-use influences on fire regimes in the eastern Sierra Nevada, California, USA. Fire Ecol. 2009, 5, 20–34. [Google Scholar] [CrossRef]
- Perry, D.A.; Hessburg, P.; Skinner, C.N.; Spies, T.A.; Stephens, S.L.; Taylor, A.H.; Franklin, J.; McComb, B.; Riegel, G. The ecology of mixed severity fire regimes in Washington, Oregon, and Northern California. For. Ecol. Manag. 2011, 262, 703–717. [Google Scholar] [CrossRef]
- Meyer, M.D. Natural Range of Variation of Subalpine Forests in the Bioregional Assessment Area; Unpublished Report; USDA Forest Service, Pacific Southwest Region: Vallejo, CA, USA, 2013. [Google Scholar]
- Unites States Department of Agriculture, Natural Resources Conservation Service. Soil Survey of the Tahoe Basin Area, California and Nevada; USDA NRCS: Washington, DC, USA, 2007. [Google Scholar]
- Kinloch, B.B.J.; Scheuner, W.H. Pinus lambertiana Dougl., sugar pine. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 370–379. [Google Scholar]
- Manley, P.N.; Fites-Kaufman, J.A.; Barbour, M.G.; Schlesinger, M.D.; Rizzo, D.M. Biological Integrity. In Lake Tahoe Basin Watershed Assessment, Volume 1; Gen. Tech. Rep. PSW-GTR-175; Murphy, D.D., Knopp, C.M., Eds.; Pacific Southwest Research Station, USDA Forest Service: Albany, CA, USA, 2000; pp. 403–600. [Google Scholar]
- Neale, D.B. Population Genetic Structure of the Douglas-Fir Shelterwood Regeneration System in Southwest Oregon. Ph.D. Dissertation, Oregon State University, Corvallis, OR, USA, 1983. [Google Scholar]
- Adams, W.T.; Birkes, D.S. Estimating mating patterns in forest tree populations. In Proceedings of the International Workshop on Plant Biology, Biochemical Markers in Population Genetics of Forest Trees, Porano-Orvieto, Italy, 11–13 October 1988; Hattemer, H.H., Fineschi, S., Eds.; Institute of Agroforestry and Natural Resources Council Italy (CNR), SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 152–172. [Google Scholar]
- Daly, C.; Neilson, R.P.; Phillips, D.L. A statistical model for mapping climatological precipitation over mountainous terrain. J. Appl. Meteorol. 1994, 33, 140–158. [Google Scholar] [CrossRef]
- Lefkovitch, L.P. The study of population growth in organisms grouped by stage. Biometrics 1965, 21, 1–18. [Google Scholar] [CrossRef]
- Ettl, G.J.; Cottone, N. Whitebark pine (Pinus albicaulis) in Mt. Rainier National Park, Washington, USA: Response to blister rust infection. In RAMAS GIS; Akçakaya, H.R., Ed.; Applied Mathematics: Setauket, NY, USA; New York, NY, USA, 2002; pp. 36–48. [Google Scholar]
- Caswell, H. Matrix Population Models, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- MATLAB; The Mathworks, Inc.: 3 Apple Hill Dr., Natick, MA, USA, 2009.
- SAS Institute. JMP Start Statistics: JMP Statistics and Graphics Guide; release 8.0.1; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Jermstad, K.D.; Eckert, A.J.; Wegrzyn, J.L.; Mix, A.D.; Davis, D.A.; Burton, D.C.; Neale, D.B. Comparative mapping in Pinus: Sugar pine (Pinus lambertiana Dougl.) and loblolly pine (Pinus taeda). Tree Gen. Genom. 2011, 7, 457–468. [Google Scholar] [CrossRef]
- Eckert, A.J.; Ersoz, E.S.; Pande, B.; Wright, M.H.; Rashbrook, V.K.; Nicolet, C.M.; Neale, D.B. High-throughput genotyping and mapping of single nucleotide polymorphisms in loblolly pine (Pinus taeda L.). Tree Gen. Genom. 2009, 5, 225–234. [Google Scholar] [CrossRef]
- Goudet, J. Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Available online: http://www.R-project.org (accessed on 15 January 2007).
- Nicholson, G.; Smith, A.V.; Jonsson, F.; Gustafsson, O.; Stefansson, K.; Donnelly, P. Assessing population differentiation and isolation from single-nucleotide polymorphism data. J. R. Stat. Soc. B 2002, 64, 695–715. [Google Scholar] [CrossRef]
- Balding, D.; Nichols, R. A method for quantifying differentiation between populations at multi-allelic loci and its implications for investigating identity and paternity. Genetica 1995, 96, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ledig, F.T. Genetic variation in Pinus. In Ecology and Biogeography of Pinus; Richardson, D.M., Ed.; Cambridge University Press: Cambridge, UK, 1990; pp. 251–280. [Google Scholar]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene flow and local adaptation in forest trees. Annu. Rev. Ecol. Evol. Systemat. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Hirt, R.R. The Relation of Certain Meterological Factors to the Infection of Eastern White Pine by the Blister-Rust Fungus; Tech. Pub. No. 59; The New York State College of Forestry, Syracuse University: Syracuse, NY, USA, 1942. [Google Scholar]
- Van Arsdel, E.P.; Riker, A.J.; Patton, R.F. The effects of temperature and moisture on the spread of white pine blister rust. Phytopathology 1956, 6, 307–318. [Google Scholar]
- McDonald, G.I. Ecotypes of blister rust and management of sugar pine in California. In Sugar Pine: Status, Values, and Roles in Ecosystems; Publication 3362; Proceedings of a Symposium presented by the California Sugar Pine Management, Davis, CA, USA, 30 March–1 April 1992; Kinloch, B.B.J., Marosy, M., Huddleston, M.E., Eds.; University of California, Division of Agriculture and Natural Resources: Davis, CA, USA, 1996; pp. 137–147. [Google Scholar]
- Kim, M.-S.; Richardson, B.A.; McDonald, G.I.; Klopfenstein, N.B. Genetic diversity and structure of western white pine (Pinus monticola) in North America: A baseline study for conservation, restoration, and addressing impacts of climate change. Tree Gen. Genom. 2011, 7, 11–21. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W. Conservation genetics of endemic plant species. In Conservation Genetics: Case Histories from Nature; Avise, J.C., Hamrick, J.L., Eds.; Chapman & Hall: New York, NY, USA, 1996; pp. 281–304. [Google Scholar]
- Hamrick, J.L. Response of forest trees to global environmental changes. For. Ecol. Manag. 2004, 197, 323–335. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Hoff, R.J.; Steinhoff, R.J. Geographic patterns of genetic variation in Pinus monitcola. Bot. Gaz. 1984, 145, 229–239. [Google Scholar] [CrossRef]
- Richardson, B.A.; Rehfeldt, G.E.; Kim, M.-S. Congruent climate-related genecological responses from molecular markers and quantitative traits for western white pine (Pinus monticola). Int. J. Plant Sci. 2009, 170, 1120–1131. [Google Scholar] [CrossRef]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hicke, J.; Hayes, J.L.; Kelsey, R.; Negrón, J.; Seybold, S.J. Climate change and bark beetles of the western US and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Boone, C.K.; Aukema, B.H.; Bohlman, J.; Carroll, A.L.; Raffa, K.F. Efficacy of tree defense physiology varies with bark beetle population density: A basis for positive feedback in eruptive species. Can. J. For. Res. 2011, 41, 1174–1188. [Google Scholar] [CrossRef]
- Barbour, M.G.; Minnich, R.A. California upland forests and woodlands. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 2000; pp. 161–202. [Google Scholar]
- SNEP Science Team. Status of the Sierra Nevada: Final Report to Congress of the Sierra Nevada Ecosystem Project; 3 Volume, Rep 36; Wildland Resources Center, University of California: Davis, CA, USA, 1996. [Google Scholar]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Rüger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.L.; Ruth, L.W. Federal forest fire policy in the United States. Ecol. Appl. 2005, 15, 532–542. [Google Scholar] [CrossRef]
- Stephens, S.L.; Agee, J.; Fulé, P.; North, M.; Romme, W.; Swetnam, T.; Turner, M. Managing forests and fire in changing climates. Science 2013, 342, 41–42. [Google Scholar] [CrossRef] [PubMed]






| Location | Density (inds. ha−1) | Basal Area (m2 ha−1) | d.b.h. (cm) | WPBR (%) | MPB (%) | Mortality (%) | Elev | Ann ppt | Tmin (°C) | Tmax (°C) | WC 15 bar | Sand (%) | CEC | Soil Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incline Lake | 74 | 21.9 | 38.8 | 10 | 17 | 9 | 2578 | 1394 | −7.6 | 21.9 | 3.4 | 79.0 | 2.9 | G |
| Flume Trail | 70 | 9.27 | 32.0 | 13 | 23 | 5 | 2414 | 797 | −7.8 | 23.4 | 3.8 | 84.9 | 1.9 | G |
| Montreal Cyn | 94 | 11.6 | 35.6 | 9 | 15 | 12 | 2439 | 710 | −6.8 | 24.3 | 10.2 | 34.7 | 22.5 | M |
| Heavenly | 97 | 18.3 | 41.3 | 0 | 40 | 17 | 2503 | 815 | −7.4 | 22.5 | 3.1 | 81.0 | 1.9 | G |
| Armstrong Pass | 76 | 19.1 | 38.2 | 4 | 17 | 6 | 2675 | 1100 | −9.0 | 21.6 | 5.5 | 90.6 | 2.9 | G |
| Meiss Meadow | 111 | 14.6 | 24.8 | 4 | 6 | 2 | 2687 | 1310 | −8.3 | 21.3 | 3.6 | 66.2 | 12.5 | A, TB |
| Echo Lake | 56 | 5.27 | 24.6 | 6 | 6 | 4 | 2292 | 1292 | −6.6 | 23.0 | 1.6 | 84.0 | 1.67 | G |
| Jakes Peak | 76 | 14.8 | 40.0 | 1 | 11 | 2 | 2370 | 1218 | −6.8 | 22.7 | 5.57 | 86.9 | 2.6 | G |
| Blackwood Cyn | 74 | 14.9 | 39.4 | 45 | 17 | 17 | 2155 | 1472 | −6.7 | 23.1 | 7.8 | 66.1 | 25.9 | TL, V |
| Mt Watson | 97 | 7.1 | 20.4 | 23 | 9 | 7 | 2413 | 1035 | −7.2 | 23.7 | 7.4 | 66.0 | 16.3 | A |
| Population | Fecundity | Survival | λ | HO | HE | FIS | ci | Cr2 | δ13C |
|---|---|---|---|---|---|---|---|---|---|
| Incline Lake | 0.207 | 0.912 | 0.991 | 0.244 | 0.247 | 0.025 | 0.0244 | 0.00 | −31.04 |
| (0.09) | (0.502, 1.625) | (0.038) | (0.031) | (0.048) | (0.0149–0.0362) | ||||
| Flume Trail | 0.150 | 0.949 | 1.024 | 0.253 | 0.262 | 0.037 | 0.0185 | 0.00 | −29.92 |
| (0.05) | (0.667, 1.550) | (0.039) | (0.032) | (0.055) | (0.0096–0.0296) | ||||
| Montreal Cyn | 1.036 | 0.880 | 1.001 | 0.238 | 0.257 | 0.075 | 0.0066 | 0.07 | −30.92 |
| (0.12) | (0.494, 1.639) | (0.034) | (0.031) | (0.066) | (0.0008–0.0144) | ||||
| Heavenly | 0.211 | 0.828 | 0.997 | 0.235 | 0.267 | 0.109 | 0.0062 | 0.00 | −30.38 |
| (0.17) | (0.315, 1.719) | (0.030) | (0.030) | (0.070) | (0.0009–0.0138) | ||||
| Armstrong Pass | 0.202 | 0.936 | 1.011 | 0.243 | 0.259 | 0.067 | 0.0120 | 0.02 | −31.09 |
| (0.06) | (0.753, 1.517) | (0.036) | (0.031) | (0.058) | (0.0049–0.0210) | ||||
| Meiss Meadow | 0.357 | 0.979 | 1.073 | 0.231 | 0.245 | 0.066 | 0.0216 | 0.00 | −30.59 |
| (0.02) | (0.693, 1.545) | (0.034) | (0.031) | (0.061) | (0.0124–0.0332) | ||||
| Echo Lake | 0.112 | 0.958 | 1.056 | 0.259 | 0.266 | 0.049 | 0.0166 | 0.00 | −30.79 |
| (0.04) | (0.760, 1.461) | (0.041) | (0.030) | (0.067) | (0.0088–0.0260) | ||||
| Jakes Peak | 0.076 | 0.983 | 1.061 | 0.252 | 0.272 | 0.112 | 0.0115 | 0.00 | −30.89 |
| (0.02) | (0.784, 1.470) | (0.035) | (0.026) | (0.094) | (0.0053–0.0189) | ||||
| Blackwood Cyn | 0.263 | 0.833 | 0.946 | 0.244 | 0.272 | 0.146 | 0.0178 | 0.00 | −31.02 |
| (0.17) | (0.679, 1.539) | (0.038) | (0.031) | (0.091) | (0.009–0.272) | ||||
| Mt Watson | 1.437 | 0.929 | 1.005 | 0.253 | 0.262 | 0.050 | 0.0090 | 0.00 | −31.33 |
| (0.07) | (0.653, 1.779) | (0.036) | (0.029) | (0.068) | (0.0024–0.0176) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloney, P.E.; Eckert, A.J.; Vogler, D.R.; Jensen, C.E.; Delfino Mix, A.; Neale, D.B. Landscape Biology of Western White Pine: Implications for Conservation of a Widely-Distributed Five-Needle Pine at Its Southern Range Limit. Forests 2016, 7, 93. https://doi.org/10.3390/f7050093
Maloney PE, Eckert AJ, Vogler DR, Jensen CE, Delfino Mix A, Neale DB. Landscape Biology of Western White Pine: Implications for Conservation of a Widely-Distributed Five-Needle Pine at Its Southern Range Limit. Forests. 2016; 7(5):93. https://doi.org/10.3390/f7050093
Chicago/Turabian StyleMaloney, Patricia E., Andrew J. Eckert, Detlev R. Vogler, Camille E. Jensen, Annette Delfino Mix, and David B. Neale. 2016. "Landscape Biology of Western White Pine: Implications for Conservation of a Widely-Distributed Five-Needle Pine at Its Southern Range Limit" Forests 7, no. 5: 93. https://doi.org/10.3390/f7050093
APA StyleMaloney, P. E., Eckert, A. J., Vogler, D. R., Jensen, C. E., Delfino Mix, A., & Neale, D. B. (2016). Landscape Biology of Western White Pine: Implications for Conservation of a Widely-Distributed Five-Needle Pine at Its Southern Range Limit. Forests, 7(5), 93. https://doi.org/10.3390/f7050093





