Living and Dead Aboveground Biomass in Mediterranean Forests: Evidence of Old-Growth Traits in a Quercus pubescens Willd. s.l. Stand
Abstract
:1. Introduction
2. Materials and Methods
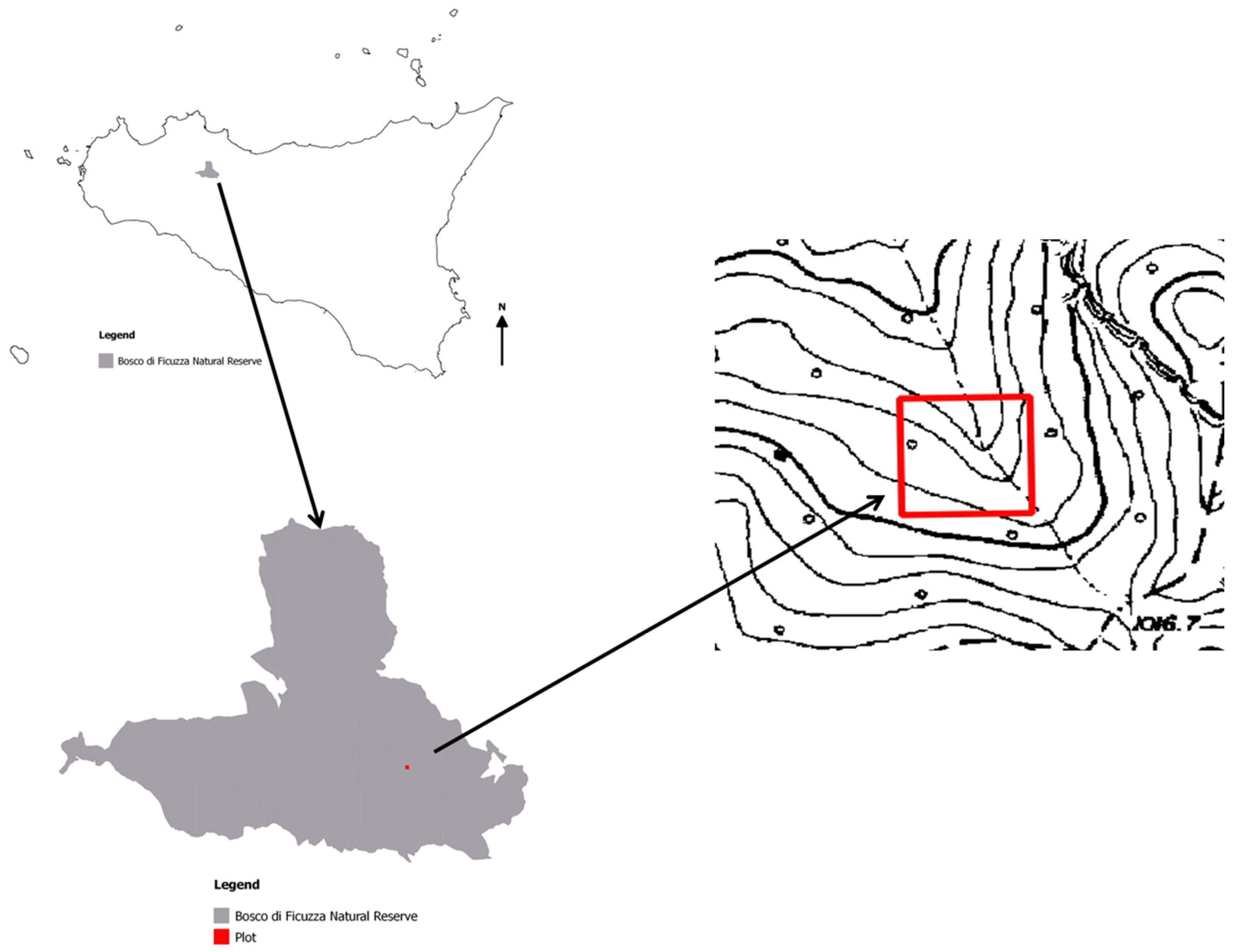
2.1. Study Area and Vegetation
2.2. Sampling Protocol
2.3. Field Surveys
3. Results
3.1. Living Biomass (Dendrometric and Structure Data)
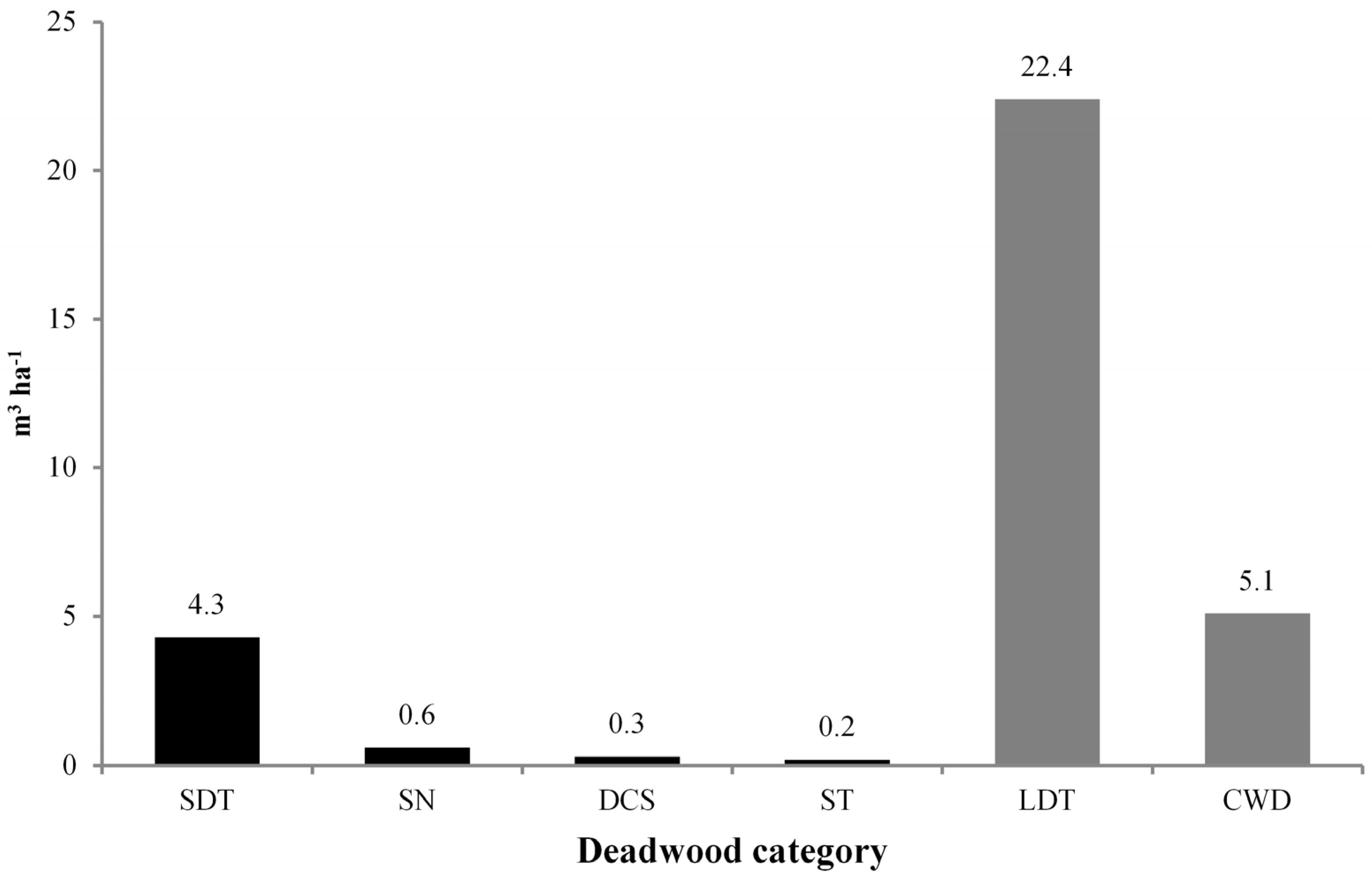
3.2. Deadwood Characterisation
4. Discussion
- Dead to live wood ratio should approach 10%;
- Lying deadwood should be prevalent;
- There should be a good diversification of deadwood both in terms of size and decay classes.
5. Conclusions
- Completely fence off the study area, at least the inner and more preserved area, in order to protect it from livestock grazing, enhancing the chance of seedling establishment by native tree species and also reducing soil erosion processes. Such a restriction measure appears to be urgently needed because abusive grazing still occurs in the Nature Reserve [70];
- Preserve the remnant nuclei of other woody species, different from the dominant tree species, which significantly contribute to the biological and structural diversification of the forest stand;
- Deepen the overall knowledge of the forest ecosystem, including ad hoc investigations of all the biological communities (animals, plants, insects, and fungi) and their competitive and symbiotic relationships, including the role of seed dispersers, mycorrhizae, phytopathogens, etc. [71];
- Establish a permanent monitoring area in which to assess the natural dynamics of deadwood and woody vegetation in a Mediterranean forest stand left unmanaged for about 50 years. It could be known, for instance, the average annual accumulation rate of deadwood [60], the average time needed for the transition from standing to lying on the ground deadwood [72], or the mean degradation time of Quercus spp. wood in similar Mediterranean ecological contexts [73].
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nilsson, S.G.; Niklasson, M.; Hedin, J.; Aronsson, G.; Gutowski, J.M.; Linder, P.; Ljungberg, H.; Mikusìnski, G.; Ranius, T. Densities of large and dead trees in old-growth temperate and boreal forests. For. Ecol. Manag. 2002, 161, 189–204. [Google Scholar] [CrossRef]
- Forest Europe. State of Europe’s Forests 2015. Available online: http://www.foresteurope.org/docs/fullsoef2015.pdf (accessed on 20 February 2017).
- Park, P.S.; Oliver, C.D. Variability of stand structures and development in old-growth forests in the Pacific Northwest, USA. Forests 2015, 6, 3177–3196. [Google Scholar] [CrossRef]
- Hilbert, J.; Wiensczyk, A. Old-growth definitions and management: A literature review. BC J. Ecosyst. Manag. 2007, 8, 15–31. [Google Scholar]
- Torras, O.; Saura, S. Effects of silvicultural treatments on forest biodiversity indicators in the Mediterranean. For. Ecol. Manag. 2008, 255, 3322–3330. [Google Scholar] [CrossRef]
- Spies, T.A. Ecological concepts and diversity of old-growth forests. J. For. 2004, 102, 14–20. [Google Scholar]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 211–213. [Google Scholar] [CrossRef] [PubMed]
- IPCC (Intergovernmental Panel on Climate Change). Good Practice Guidance for Land Use, Land-Use Change and Forestry; IPCC National Greenhouse Gas Inventories Programme: Hayama, Japan, 2003; pp. 1–590. [Google Scholar]
- Burrascano, S.; Keeton, W.S.; Sabatini, F.M.; Blasi, C. Commonality and variability in the structural attributes of moist temperate old-growth forests: A global review. For. Ecol. Manag. 2013, 291, 458–479. [Google Scholar] [CrossRef]
- Green, P.; Peterken, G.F. Variation in the amount of dead wood in the woodlands of the Lower Wye Valley UK, in the relation to the intensity of management. For. Ecol. Manag. 1997, 98, 229–238. [Google Scholar] [CrossRef]
- Marchetti, M.; Lombardi, F. Analisi quali-quantitativa del legno morto in un soprassuolo non gestito: Il caso “Bosco Pennataro”, Alto Molise. L’Italia For. Mont. 2006, 275–302. [Google Scholar] [CrossRef]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Odor, P.; Standovar, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Mayer, P.; Winter, S.; et al. Dead wood in European beech (Fagus sylvatica) forest reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Ryberg, N.; Götmark, F.; Olausson, B. Relative importance of coarse and fine woody debris for the diversity of wood-inhabiting fungi in temperate broadleaf forests. Biol. Conserv. 2004, 117, 1–10. [Google Scholar]
- La Mantia, T.; Spoto, M.; Massa, B. The colonisation of the Great Spotted Woodpecker (Picoides major L.) in Eucalypt woods and popular cultivations in Sicily. Ecol. Mediterr. 2002, 28, 65–73. [Google Scholar]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Müller, J.; Hothorn, T.; Pretzsch, H. Long-term effects of logging intensity on structures, birds, saproxylic beetles and wood-inhabiting fungi in stands of European beech Fagus sylvatica L. For. Ecol. Manag. 2007, 242, 297–305. [Google Scholar] [CrossRef]
- Nilsson, S.G. Effect of forest management on the breeding bird community in southern Sweden. Biol. Conserv. 1979, 16, 135–143. [Google Scholar] [CrossRef]
- La Mantia, T.; Lo Duca, R.; Massa, B.; Nocentini, S.; Rühl, J. La biodiversità dei boschi siciliani. Part I: L’avifauna. L’Italia For. Mont. 2014, 69, 173–193. [Google Scholar] [CrossRef]
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Pignatti, G.; De Natale, F.; Gasperini, P.; Paletto, A. Il legno morto nei boschi italiani secondo l’Inventario Forestale Nazionale. Forest@ 2009, 6, 365–375. [Google Scholar] [CrossRef]
- MCPFE (Ministerial Conference on the Protection of Forests in Europe). State of Europe’s Forests 2007; The MCPFE Report on Sustainable Forest Management in Europe; MCPFE: Warsaw, Poland, 2007; pp. 1–263. [Google Scholar]
- Marchetti, M.; Lombardi, F.; Tognetti, R.; Chirici, G. Verso una rete di connessione dei boschi vetusti. Gazz. Ambient. 2012, 3, 39–50. [Google Scholar]
- Bertini, G.; Fabbio, G.; Piovosi, M.; Calderisi, M. Densità di biomassa e necromassa legnosa in cedui di cerro in evoluzione naturale in Toscana. Forest@ 2010, 7, 88–103. [Google Scholar] [CrossRef]
- Paletto, A.; De Meo, I.; Cantiani, P.; Ferretti, F. Effects of forest management on the amount of deadwood in Mediterranean oak ecosystems. Ann. For. Sci. 2014, 71, 791–800. [Google Scholar] [CrossRef]
- Badalamenti, E.; Pasta, S.; La Mantia, T.; La Mela Veca, D.S. Old-growth forests in Mediterranean ecosystems: Preliminary investigations and management proposals in a Sicilian study case. Nat. Areas J. (under review).
- Rivas-Martínez, S. Global Bioclimatics (Clasificación Bioclimática de la Tierra). Available online: http://www.globalbioclimatics.org/book/bioc/global_bioclimatics-2008_00.htm (accessed on 20 February 2017).
- Servizio Idrografico. Bacini Con Foce Al Litorale Della Sicilia. Annali Idrologici, Sezione di Palermo. 2012. Available online: http://www.osservatorioacque.it/dati/ANNALI/annale_2012_parte_I.pdf (accessed on 12 February 2017).
- Fierotti, G. Carta dei Suoli Della Sicilia (Scala 1:250,000); Regione Siciliana; Assessorato Territorio e Ambiente: Palermo, Italy, 1988. [Google Scholar]
- Gianguzzi, L. Il Paesaggio Vegetale Della Riserva Naturale Orientata “Bosco Della Ficuzza, Rocca Busambra, Bosco del Cappelliere, Gorgo del Drago”; Collana Sicilia Foreste; Regione Siciliana; AFDRS: Palermo, Italy, 2004; Volume 22, pp. 1–160. [Google Scholar]
- Sala, G.; Giardina, G.; La Mantia, T. I fattori di rischio per la biodiversità forestale in Sicilia: Il caso studio del cerro di Gussone. L’Italia For. Mont. 2011, 66, 71–80. [Google Scholar] [CrossRef]
- Pasta, S.; de Rigo, D.; Caudullo, G. Quercus pubescens in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 156–157. [Google Scholar]
- Gianguzzi, L.; La Mantia, A. Le serie di vegetazione della Riserva Naturale Orientata “Bosco Ficuzza, Rocca Busambra, Bosco del Cappelliere e Gorgo del Drago” con allegata carta della vegetazione (scala 1:20,000). Nat. Sicil 2004, 28, 205–242. [Google Scholar]
- Giardina, G.; La Mantia, T.; Sala, G.; Di Leo, C.; Pasta, S. Possibile origine e consistenza di un popolamento di Quercus trojana Webb subsp. trojana (Fagaceae) nel Bosco della Ficuzza (Palermo, Sicilia). Nat. Sicil 2014, 38, 265–289. [Google Scholar]
- Giardina, G.; La Mantia, T.; Sala, G.; Pasta, S. Ostrya carpinifolia Scop. (fam. Betulaceae) a Ficuzza (Monti Sicani, provincia di Palermo): Note ecologiche e demografiche. Nat. Sicil 2015, 39, 73–75. [Google Scholar]
- Bianchetto, E.; Buscemi, I.; Corona, P.; Giardina, G.; La Mantia, T.; Pasta, S. Fitting the stocking rate with pastoral resources to manage and preserve Mediterranean forestlands: A case study. Sustainability 2015, 7, 7232–7244. [Google Scholar] [CrossRef]
- Calamini, G.; Maltoni, A.; Travaglini, D.; Iovino, F.; Nicolaci, A.; Menguzzato, G.; Corona, P.; Ferrari, B.; Di Santo, D.; Chirici, G.; et al. Stand structure attributes in potential old-growth forests in the Apennines, Italy. L’Italia For. Mont. 2011, 66, 365–381. [Google Scholar] [CrossRef]
- Lombardi, F.; Marchetti, M.; Corona, P.; Merlini, P.; Chirici, G.; Tognetti, R.; Puletti, N. Quantifying the effect of sampling plot size on the estimation of structural indicators in old-growth forest stands. For. Ecol. Manag. 2015, 346, 89–97. [Google Scholar] [CrossRef]
- Tabacchi, G.; Di Cosmo, L.; Gasparini, P.; Morelli, S. Stima del Volume e Della Fitomassa Delle Principali Specie Forestali Italiane. Equazioni di Previsione, Tavole del Volume e Tavole Della Fitomassa Arborea Epigea; Consiglio per la Ricerca e la Sperimentazione in Agricoltura, Unità di Ricerca per il Monitoraggio e la Pianificazione Forestale: Trento, Italy, 2011; pp. 1–412. [Google Scholar]
- INFC. Le stime di superficie 2005–Prima parte. In Inventario Nazionale Delle Foreste e dei Serbatoi Forestali di Carbonio, MiPAAF–Corpo Forestale dello Stato; Tabacchi, G., De Natale, F., Di Cosmo, L., Floris, A., Gagliano, C., Gasparini, P., Genchi, L., Scrinzi, G., Tosi, V., Eds.; Ispettorato Generale, CRA–ISAFA: Trento, Italy, 2007. [Google Scholar]
- Hunter, M.L., Jr. Wildlife, Forests and Forestry: Principles for Managing Forests for Biological Diversity; Prentice Hall: Englewood Cliffs, NJ, USA, 1990. [Google Scholar]
- Chiavetta, U.; Sallustio, L.; Garfì, V.; Maesano, M.; Marchetti, M. Classification of the oldgrowthness of forest inventory plots with dissimilarity metrics in Italian national parks. Eur. J. For. Res. 2012, 131, 1473–1483. [Google Scholar] [CrossRef]
- Motta, R.; Garbarino, M.; Berretti, R.; Meloni, F.; Nosenzo, A.; Vacchiano, G. Development of old-growth characteristics in uneven-aged forests of the Italian Alps. Eur. J. For. Res. 2015, 134, 19–31. [Google Scholar] [CrossRef]
- Lombardi, F.; Chirici, G.; Marchetti, M.; Tognetti, R.; Lasserre, B.; Corona, P.; Barbati, A.; Ferrari, B.; Di Paolo, S.; Giuliarelli, D.; et al. Deadwood in forest stands close to old-growthness under Mediterranean conditions in the Italian Peninsula. L’Italia For. Mont. 2010, 65, 481–504. [Google Scholar] [CrossRef]
- La Mela Veca, D.S.; Cullotta, S.; Sferlazza, S.; Maetzke, F.G. Anthropogenic influences in land use/land cover changes in Mediterranean forest landscapes in Sicily. Land 2016, 5, 3. [Google Scholar] [CrossRef]
- Vizzarri, M.; Sallustio, L.; Travaglini, D.; Bottalico, F.; Chirici, G.; Garfì, V.; Lafortezza, R.; La Mela Veca, D.S.; Lombardi, F.; Federico Maetzke, F.; et al. The MIMOSE approach to support sustainable forest management planning at regional scale in Mediterranean contexts. Sustainability 2017, 9, 316. [Google Scholar] [CrossRef]
- Richard, F.; Millot, S.; Gardes, M. Diversity and specificity of ectomycorrhizal fungi retrieved from an old-growth Mediterranean forest dominated by Quercus ilex. New Phytol. 2005, 166, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, L.; Lancellotti, E.; Franceschini, A.; Montecchio, L. The ectomycorrhizal community in Mediterranean old-growth Quercus ilex forests along an altitudinal gradient. Plant Biosyst. 2014, 148, 74–82. [Google Scholar] [CrossRef]
- Marziliano, P.A. Analisi quali-quantitativa della necromassa in cedui invecchiati di leccio (Quercus ilex L.) del Gargano. Forest@ 2009, 6, 19–28. [Google Scholar] [CrossRef]
- Bertini, G.; Fabbio, G.; Piovosi, M.; Calderisi, M. Densità di biomassa e necromassa legnosa in cedui invecchiati di leccio in Sardegna e di faggio in Toscana. Forest@ 2012, 9, 108–129. [Google Scholar] [CrossRef]
- Della Rocca, F.; Stefanelli, S.; Pasquaretta, C.; Campanaro, A.; Bogliani, G. Effect of deadwood management on saproxylic beetle richness in the floodplain forests of northern Italy: Some measures for deadwood sustainable use. J. Insect Conserv. 2014, 18, 121–136. [Google Scholar] [CrossRef]
- Hofmann, A.; Cibella, R.; Bertani, R.; Miozzo, M.; Fantoni, I.; Luppi, S. Strumenti Conoscitivi per la Gestione Delle Risorse Forestali Della Sicilia. Sistema Informativo Forestale; Assessorato Territorio e Ambiente Regione Siciliana: Perugia, Italy, 2011; pp. 1–208. [Google Scholar]
- Lombardi, F.; Lasserre, B.; Tognetti, R.; Marchetti, M. Deadwood in relation to stand management and forest type in Central Apennines (Molise, Italy). Ecosystems 2008, 11, 882–894. [Google Scholar] [CrossRef]
- Bagnato, S.; Merlino, A.; Mercurio, R.; Solano, F.; Scarfò, F.; Spampinato, G. Le basi conoscitive per il restauro forestale: Il caso di Bosco Pomieri (Parco Regionale delle Madonie, Sicilia). Forest@ 2012, 9, 8–19. [Google Scholar] [CrossRef]
- Barreca, L.; Cutini, A.; Mercurio, R. Caratterizzazione della necromassa in boschi di farnetto (Quercus frainetto Ten.) della Calabria. Forest@ 2008, 5, 187–194. [Google Scholar] [CrossRef]
- Persiani, A.M.; Lombardi, F.; Lunghini, D.; Granito, V.M.; Tognetti, R.; Maggi, O.; Pioli, S.; Marchetti, M. Stand structure and deadwood amount influences saproxylic fungal biodiversity in Mediterranean mountain unmanaged forests. iForest 2015, 9, 115–124. [Google Scholar] [CrossRef]
- Nordén, B.; Götmark, F.; Tönnberg, M.; Ryberg, M. Dead wood in semi-natural temperate broadleaved woodland: Contribution of coarse and fine dead wood, attached dead wood and stumps. For. Ecol. Manag. 2004, 194, 235–248. [Google Scholar] [CrossRef]
- Paletto, A.; Ferretti, F.; De Meo, I.; Cantiani, P.; Focacci, M. Ecological and environmental role of deadwood in managed and unmanaged forests. In Sustainable Forest Management; Diez, J.J., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 219–238. [Google Scholar]
- Lombardi, F.; Lasserre, B.; Chirici, G.; Tognetti, R.; Marchetti, M. Deadwood occurrence and forest structure as indicators of old-growth forest conditions in Mediterranean mountainous ecosystems. Ecoscience 2012, 19, 344–355. [Google Scholar] [CrossRef]
- Vandekerkhove, K.; De Keersmaeker, L.; Menke, N.; Meyer, P.; Verscheide, P. When nature takes over from man: Dead wood accumulation in previously managed oak and beech woodlands in North-western and Central Europe. For. Ecol. Manag. 2009, 258, 425–435. [Google Scholar] [CrossRef]
- Mason, F.; Nardi, G.; Whitmore, D. Recherches sur la restauration des habitats du bois mort: L’exemple du LIFE “Bosco della Fontana” (Italie). In Bois Mort et à Cavités, Une clé Pour Des Forêts Vivantes; Vallauri, D., André, J., Dodelin, B., Eynard-Machet, R., Rambaud, D., Eds.; Éditions Tec & Doc: Paris, France, 2005; pp. 285–291. [Google Scholar]
- Kirby, K.J.; Reid, C.M.; Thomas, R.C.; Goldsmith, F.B. Preliminary estimates of fallen dead wood and standing dead trees in managed and unmanaged forests in Britain. J. Appl. Ecol. 1998, 35, 148–155. [Google Scholar] [CrossRef]
- Dittrich, S.; Jacob, M.; Bade, C.; Leuschner, C.; Hauck, M. The significance of deadwood for total bryophyte, lichen, and vascular plant diversity in an old-growth spruce forest. Plant Ecol. 2014, 215, 1123–1137. [Google Scholar] [CrossRef]
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Müller, J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv. Biol. 2015, 29, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Citterio, G.; Puxeddu, M.; Giannini, R. La foresta relitta di roverella dei Monti del Gennargentu, Sardegna. Forest@ 2007, 4, 11–18. [Google Scholar] [CrossRef]
- Tiscar, P.; Lucas-Borja, M. Structure of old-growth and managed stands and growth of old trees in a Mediterranean Pinus nigra forest in southern Spain. Forestry 2016, 89, 201–207. [Google Scholar] [CrossRef]
- Paillet, Y.; Pernot, C.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F. Quantifying the recovery of old-growth attributes in forest reserves: A first reference for France. For. Ecol. Manag. 2015, 346, 51–64. [Google Scholar] [CrossRef]
- Bianchi, L.; Brovelli, M.; Maltoni, A.; Calamini, G. Confronto tra metodologie di stima della necromassa legnosa in un ceduo invecchiato di leccio. Forest@ 2013, 10, 34–42. [Google Scholar] [CrossRef]
- La Mantia, T.; Pasta, S. The Sicilian Phanerophytes: Still a Noteworthy Patrimony, Soon a Lost Resource? IUFRO Conference “Monitoring and Indicators of Forest Biodiversity in Europe—From Ideas to Operationality”, Firenze, Italy, 15 November 2003. [Google Scholar]
- La Mantia, T.; Pasta, S.; Giardina, G.; Marchetti, M. The Effect of Grazing in Forests: The Case Study of Ficuzza (W Sicily). In Proceedings of the International Congress: “Silvopastoralism and Sustainable Management”, Lugo, Spain, 18–24 April 2004. [Google Scholar]
- La Mantia, T.; Bellavista, M.; Giardina, G.; Sparacio, I. Longhorn beetles of the Ficuzza woods (W Sicily, Italy) and their relationship with plant diversity (Coleoptera, Cerambycidae). Biodivers. J. 2010, 1, 15–44. [Google Scholar]
- Corace, R.G., III; Seefelt, N.E.; Goebel, P.C.; Shaw, H.L. Snag longevity and decay class development in a recent jack pine clearcut in Michigan. North. J. Appl. For. 2010, 27, 125–131. [Google Scholar]
- Schowalter, T.D.; Zhang, Y.L.; Sabin, T.E. Decomposition and nutrient dynamics of oak Quercus spp. logs after five years of decomposition. Ecography 1998, 21, 3–10. [Google Scholar] [CrossRef]








| Deadwood Category | Size Threshold (cm) | Collected Attribute |
|---|---|---|
| Standing Deadwood | ||
| Standing Dead Trees (SDT) | H ≥ 130 | D1.30m |
| Snags (SN) | H ≥ 130 | Dbase, Dtop |
| Dead Coppice Shoots (DCS) | H ≥ 100 | D1.30m |
| Stumps (ST) | H < 130 | Dbase, Dtop, origin (natural or artificial) |
| Lying Deadwood | ||
| Lying Dead Trees (LDT) | L ≥ 130 | D1.30m, length |
| Coarse Woody Debris (CWD) | L ≥ 100 | Dmin, Dmax |
| Species | Tree Density | Mean Diameter | Mean Height | Volume | Basal Area | |||
|---|---|---|---|---|---|---|---|---|
| N ha−1 | % | cm | m | m3 ha−1 | % | m2 ha−1 | % | |
| Quercus pubescens | 178.2 | 44.3 | 42.0 ± 13.1 | 19.9 ± 5.9 | 297.38 | 81.7 | 24.75 | 71.9 |
| Quercus ilex | 113.6 | 28.2 | 18.6 ± 7.8 | 10.2 ± 3.9 | 18.98 | 5.2 | 3.08 | 9.0 |
| Acer campestre | 78.3 | 19.5 | 28.0 ± 15.2 | 12.9 ± 4.2 | 35.07 | 9.6 | 4.84 | 14.1 |
| Castanea sativa | 7.8 | 1.9 | 43.3 ± 8.6 | 18.7 ± 5.1 | 10.59 | 2.9 | 1.19 | 3.5 |
| Other woody species | 24.5 | 6.1 | 15.0 ± 7.7 | 6.0 ± 2.2 | 1.79 | 0.5 | 0.54 | 1.6 |
| Total | 402.4 | 363.8 | 34.4 | |||||
| Deadwood Category | Density | Volume | ||
|---|---|---|---|---|
| N ha−1 | % | m3 ha−1 | % | |
| Standing deadwood | ||||
| Standing Dead Trees (SDT) | 14.7 | 45.5 (6.4) | 4.3 | 79.6 (13.1) |
| Snags (SN) | 7.8 | 24.1 (3.4) | 0.6 | 11.1 (1.8) |
| Dead Coppice Shoots (DCS) | 3.9 | 12.1 (1.7) | 0.3 | 5.6 (0.9) |
| Stumps (ST) | 5.9 | 18.3 (2.6) | 0.2 | 3.7 (0.6) |
| Total | 32.3 | 100 (14.1) | 5.4 | 100 (16.4) |
| Lying deadwood | ||||
| Lying Dead Trees (LDT) | 66.6 | 33.8 (29.1) | 22.4 | 81.5 (68.1) |
| Coarse Woody Debris (CWD) | 130.2 | 66.2 (56.8) | 5.1 | 18.5 (15.5) |
| Total | 196.8 | 100 (85.9) | 27.5 | 100 (83.6) |
| Total deadwood | 229.1 | 32.9 | ||
| Deadwood Category | Density | Volume | ||
|---|---|---|---|---|
| N ha−1 | % | m3 ha−1 | % | |
| Standing deadwood | ||||
| Standing Dead Trees (SDT30) | 3.9 | 26.7 | 3.2 | 18.4 # (74.4 §) |
| Snags (SN30) | 2.0 | 25.0 | 0.5 | 2.9 # (83.3 §) |
| Dead Coppice Shoots (DCS30) | - | - | - | - |
| Stumps (ST30) | 2.9 | 50.0 | 0.17 | 1.1 # (77.3 §) |
| Total | 8.8 | 27.3 | 3.9 | 22.4 # (72.2 §) |
| Lying deadwood | ||||
| Lying Dead Trees (LDT30) | 16.6 | 25.0 | 13.5 | 77.6 # (60.3) |
| Coarse Woody Debris (CWD30) | - | - | - | - |
| Total | 16.6 | 8.5 | 13.5 | 100 (77.6) |
| Total coarse deadwood | 25.4 | 11.1 * | 17.4 | 52.9 * |
| Parameter | Range 1 | The Fanuso Wood |
|---|---|---|
| Dead to live wood ratio (%) | 9–89 | 9 |
| Basal area (m2 ha−1) | 24–57 | 34.4 |
| Stem density (N ha−1) | 124–1835 | 402.4 |
| Large living trees * (N ha−1) | 36.5–122 | 38.2 |
| Living aboveground biomass (m3 ha−1) | 255.5–510 | 363.9 |
| Quadratic mean diameter (cm) | 14.6–64.3 | 33.0 |
| Coarse woody debris (m3 ha−1) | 45–469 | 32.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, E.; La Mantia, T.; La Mantia, G.; Cairone, A.; La Mela Veca, D.S. Living and Dead Aboveground Biomass in Mediterranean Forests: Evidence of Old-Growth Traits in a Quercus pubescens Willd. s.l. Stand. Forests 2017, 8, 187. https://doi.org/10.3390/f8060187
Badalamenti E, La Mantia T, La Mantia G, Cairone A, La Mela Veca DS. Living and Dead Aboveground Biomass in Mediterranean Forests: Evidence of Old-Growth Traits in a Quercus pubescens Willd. s.l. Stand. Forests. 2017; 8(6):187. https://doi.org/10.3390/f8060187
Chicago/Turabian StyleBadalamenti, Emilio, Tommaso La Mantia, Giovanni La Mantia, Antonino Cairone, and Donato Salvatore La Mela Veca. 2017. "Living and Dead Aboveground Biomass in Mediterranean Forests: Evidence of Old-Growth Traits in a Quercus pubescens Willd. s.l. Stand" Forests 8, no. 6: 187. https://doi.org/10.3390/f8060187
APA StyleBadalamenti, E., La Mantia, T., La Mantia, G., Cairone, A., & La Mela Veca, D. S. (2017). Living and Dead Aboveground Biomass in Mediterranean Forests: Evidence of Old-Growth Traits in a Quercus pubescens Willd. s.l. Stand. Forests, 8(6), 187. https://doi.org/10.3390/f8060187







