Responses of Sap Flow of Deciduous and Conifer Trees to Soil Drying in a Subalpine Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sap Flow Measurement
2.3. Meteorological and Soil-Water Content Measurements
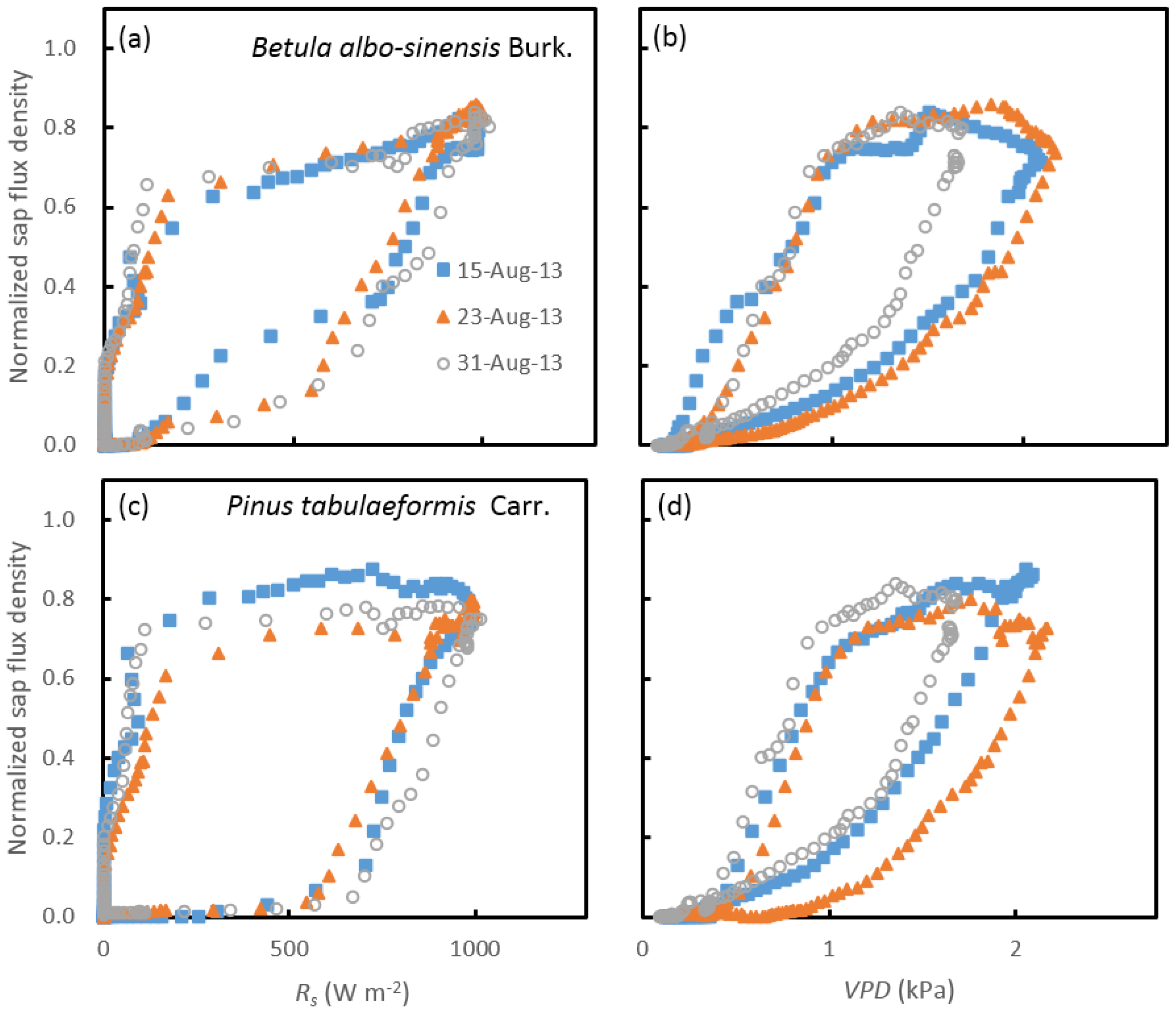
2.4. Data Analyses and Parameters Calculation
3. Results and Discussion
3.1. Meteorological and Soil-Water Conditions
3.2. Variations in the Diurnal Course of Sap Flow
3.3. Response of Sap Flow to Meteorological Factors under Different Soil-Water Conditions
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Hao, Y.; Wang, J.; Hong, J. The dynamics of land cover change pattern and landscape fragmentation in Jiuzhaigou Nature Reserve, China. Proc. SPIE 2009, 7498, 74983P. [Google Scholar] [CrossRef]
- Bossard, C.C.; Cao, Y.; Wang, J.; Rose, A.; Tang, Y. New patterns of establishment and growth of Picea, Abies and Betula tree species in subalpine forest gaps of Jiuzhaigou National Nature Reserve, Sichuan, southwestern China in a changing environment. For. Ecol. Manag. 2015, 356, 84–92. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Dolanc, C.R.; Gao, H.; Strauss, S.Y.; Schwartz, A.C.; Williams, J.N.; Tang, Y. Forest Structure, Stand Composition, and Climate-Growth Response in Montane Forests of Jiuzhaigou National Nature Reserve, China. PLoS ONE 2013, 8, e715598. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Tanaka, N.; Kume, T. Do coniferous forests evaporate more water than broad-leaved forests in Japan? J. Hydrol. 2007, 336, 361–375. [Google Scholar] [CrossRef]
- Swank, W.T.; Douglass, J.E. Streamflow greatly reduced by converting deciduous hardwood stands to pine. Science 1974, 185, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Di, B.F.; Zhang, K.S.; Luo, H. Analysis on the Dynamic changes of Wetland Monitoring Based on Remote Sensing in Jiuzhaigou. In Proceedings of the 2011 Meeting Environmental Scientific Society of Sichuan Province, Chengdu, China; 2011; pp. 138–145. [Google Scholar]
- Gan, J.J.; Zheng, L.M. A Study of Landscape Geological Environment and Water Cyclic System in the Central Scenic Spot in the Jiuzhai Valley-Huanglong Scenic Spots. Acta Geol. Sichuan 2007, 27, 53–55. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Wang, J. Landscape pattern and stability analysis of Jiuzhaigou Nature Reserve in Sichuan China. In Proceedings of the 2011 19th International Conference on Geoinformatics, Shanghai, China, 24–26 June 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Wu, N.; Bao, W.; Wu, Y.; Yu, S.; Ma, J.; Wang, L.; Wang, C.; Wang, J.; Wang, Q.; Wang, Y.; et al. The Ecological Environment and Sustainable Development of the World Natural Heritage Sites-Jiuzhaigou and Huanglong; Science Press: Beijing, China, 2012; p. 9. ISBN 978-70-3033-960-7. [Google Scholar]
- Chang, X.; Zhao, W.; He, Z. Radial pattern of sap flow and response to microclimate and soil moisture in Qinghai spruce (Picea crassifolia) in the upper Heihe River Basin of arid northwestern China. Agric. For. Meteorol. 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.; Kume, T.; Zhang, J.; Otsuki, K.; Yamanaka, N.; Liu, G. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Hogg, E.H.; Hurdle, P.A. Sap flow in trembling aspen: Implications for stomatal responses to vapor pressure deficit. Tree Physiol. 1997, 17, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, D.; Koch, O.; Korn, S.; Leuschner, C. Sap flux of five co-occurring tree species in a temperate broad-leaved forest during seasonal soil drought. Trees 2005, 19, 628–637. [Google Scholar] [CrossRef]
- McCarthy, H.R.; Pataki, D.E. Drivers of variability in water use of native and non-native urban trees in the greater Los Angeles area. Urban Ecosyst. 2010, 13, 393–414. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Oberbauer, S.F.; Clark, D.B. Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant Cell Environ. 2004, 27, 551–567. [Google Scholar] [CrossRef]
- Oren, R.; Pataki, D.E. Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia 2001, 127, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Zha, T.; Mo, K.; Zhang, Y.; Fang, X. Soil water affects transpiration response to rainfall and vapor pressure deficit in poplar plantation. New For. 2014, 45, 235–250. [Google Scholar] [CrossRef]
- Lagergren, F.; Lindroth, A. Transpiration response to soil moisture in pine and spruce trees in Sweden. Agric. For. Meteorol. 2002, 112, 67–85. [Google Scholar] [CrossRef]
- Köcher, P.; Gebauer, T.; Horna, V.; Leuschner, C. Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann. For. Sci. 2009, 66, 101. [Google Scholar] [CrossRef]
- Pataki, D.E.; Oren, R.; Smith, W.K. Sap flux of co-occurring species in a western subalpine forest during seasonal soil drought. Ecology 2000, 81, 2557–2566. [Google Scholar] [CrossRef]
- Yan, C.; Zhao, W.; Wang, Y.; Yang, Q.; Zhang, Q.; Qiu, G.Y. Effects of forest evapotranspiration on soil water budget and energy flux partitioning in a subalpine valley of china. Agric. For. Meteorol. 2017, 246, 207–217. [Google Scholar] [CrossRef]
- Dai, X.A.; Yang, W.N.; Tang, C. Analysis of landscape spatial pattern changes using remote sensing in Jiuzhaigou Valley, Northwestern Sichuan Province, China. In Proceedings of the 2010 International Conference on Multimedia Technology (ICMT), Ningbo, China, 29–31 October 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Winkler, D. The Forests of the Eastern Part of the Tibetan Plateau—A Case Study from Jiuzhaigou (Zitsa Degu; NNW Sichuan). Plant Res. Dev. 1998, 47, 184–212. [Google Scholar]
- Chinese Academy of Science Flora of China Editorial Board. Flora of China; Science Press: Beijing, China, 2004; Volume 7, p. 251, Volume 21, p. 121; ISBN 978-7-0302-7166-2. [Google Scholar]
- Wu, G.; Feng, Z. Study on the social characteristics and biomass of the Pinus tabulaeformis forest systems in China. Acta Ecol. Sin. 1994, 14, 415–422. [Google Scholar]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer: New York, NY, USA, 1998; ISBN 978-0-387-94937-6. [Google Scholar]
- Chen, D.; Wang, Y.; Liu, S.; Wei, X.; Wang, X. Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid Northwest China. Agric. Water Manag. 2014, 136, 23–33. [Google Scholar] [CrossRef]
- Iida, S.; Nakatani, S.; Tanaka, T. Evaluation of Transpiration from a Natural Deciduous Broad-leaved Forest Located at a Headwater Catchment Based on Measurement of Sap Flux Density. J. Jpn. Soc. Hydrol. Water Resour. 2006, 19, 7–16. [Google Scholar] [CrossRef]
- Kakubari, Y.; Hosokawa, K. Estimation of stand transpiration of a beech forest based on an eco-physiological computer simulation model and superporometer. J. Jpn. For. Soc. 1992, 74, 263–272. [Google Scholar]
- Gartner, K.; Nadezhdina, N.; Englisch, M.; Čermak, J.; Leitgeb, E. Sap flow of birch and Norway spruce during the European heat and drought in summer 2003. For. Ecol. Manag. 2009, 258, 590–599. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: Review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, C.; Jian, S. Canopy transpiration of Pinus tabulaeformis plantation forest in the Loess Plateau region of China. Environ. Earth Sci. 2016, 75, 376. [Google Scholar] [CrossRef]
- She, D.; Xia, Y.; Shao, M.; Peng, S.; Yu, S. Transpiration and canopy conductance of Caragana korshinskii trees in response to soil moisture in sand land of China. Agrofor. Syst. 2013, 87, 667–678. [Google Scholar] [CrossRef]
- Ewers, B.E.; Mackay, D.S.; Samanta, S. Interannual consistency in canopy stomatal conductance control of leaf water potential across seven tree species. Tree Physiol. 2007, 27, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Ewers, B.E.; Mackay, D.S.; Gower, S.T.; Ahl, D.E.; Burrows, S.N.; Samanta, S.S. Tree species effects on stand transpiration in northern Wisconsin. Water Resour. Res. 2002, 38, 8-1–8-11. [Google Scholar] [CrossRef]
- Kumagai, T.O.; Tateishi, M.; Shimizu, T.; Otsuki, K. Transpiration and canopy conductance at two slope positions in a Japanese cedar forest watershed. Agric. For. Meteorol. 2008, 148, 1444–1455. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Ewers, B.E. Urban tree species show the same hydraulic response to vapor pressure deficit across varying tree size and environmental conditions. PLoS ONE 2012, 7, e47882. [Google Scholar] [CrossRef] [PubMed]
- Raz-Yaseef, N.; Yakir, D.; Schiller, G.; Cohen, S. Dynamics of evapotranspiration partitioning in a semi-arid forest as affected by temporal rainfall patterns. Agric. For. Meteorol. 2012, 157, 77–85. [Google Scholar] [CrossRef]
- Sun, X.; Onda, Y.; Kato, H.; Otsuki, K.; Gomi, T. Partitioning of the total evapotranspiration in a Japanese cypress plantation during the growing season. Ecohydrology 2014, 7, 1042–1053. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Morales, D.; Wieser, G.; Jiménez, M.S. Canopy transpiration of a semi-arid Pinus canariensis forest at a treeline ecotone in two hydrologically contrasting years. Agric. For. Meteorol. 2015, 201, 120–127. [Google Scholar] [CrossRef]
- O’Grady, A.P.; Eamus, D.; Hutley, L.B. Transpiration increases during the dry season: Patterns of tree water use in eucalypt open-forests of northern Australia. Tree Physiol. 1999, 19, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Manzoni, S.; Katul, G.; Porporato, A.; Yang, D. The hysteretic evapotranspiration—Vapor pressure deficit relation. J. Geophys. Res. G Biogeosci. 2014, 119, 125–140. [Google Scholar] [CrossRef]
- Tabacchi, E.; Lambs, L.; Guilloy, H.; Planty-Tabacchi, A.M.; Muller, E.; Decamps, H. Impacts of riparian vegetation on hydrological processes. Hydrol. Process. 2000, 14, 2959–2976. [Google Scholar] [CrossRef]
- Arve, L.E.; Terfa, M.T.; Gislerød, H.R.; Olsen, J.E.; Torre, S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013, 36, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Wang, Q.; Li, H.; Ru, H.; Guo, B.; Huang, M. Photosynthesis and Transpiration Characteristics of Pinus tabulaeformis and Platycladus orientalis and Their Relationship with Environmental Factors. J. Northeast For. Univ. 2011, 39, 15–18. [Google Scholar] [CrossRef]
- Jarvis, P.G.; Mcnaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Meinzer, F.C.; Vertessy, R.A. A review of whole-plant water use studies in tree. Tree Physiol. 1998, 18, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Jin, S.; Cui, D. Comparative Analysis of Brown Soil Water Characteristic Curve Fitting. Agric. Sci. Technol. Equip. 2014, 8, 017. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; pp. 146–161. ISBN 0-521-42524-7. [Google Scholar]
- Zheng, C.; Wang, Q. Water-use response to climate factors at whole tree and branch scale for a dominant desert species in central Asia: Haloxylon ammodendron. Ecohydrology 2014, 7, 56–63. [Google Scholar] [CrossRef]
- Zeppel, M.J.; Murray, B.R.; Barton, C.; Eamus, D. Seasonal responses of xylem sap velocity to VPD and solar radiation during drought in a stand of native trees in temperate Australia. Funct. Plant Biol. 2004, 31, 461–470. [Google Scholar] [CrossRef]
- Oren, R.; Zimmermann, R.; Terbough, J. Transpiration in upper Amazonia floodplain and upland forests in response to drought-breaking rains. Ecology 1996, 77, 968–973. [Google Scholar] [CrossRef]
- Bréda, N.; Cochard, H.; Dreyer, E.; Granier, A. Water transfer in a mature oak stand (Quercus petraea): Seasonal evolution and effects of a severe drought. Can. J. For. Res. 1993, 23, 1136–1143. [Google Scholar] [CrossRef]
- Goulden, M.L. Carbon assimilation and water-use efficiency by neighboring mediterranean-climate oaks that differ in water access. Tree Physiol. 1996, 16, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Granier, A.; Loustau, D. Measuring and modelling the transpiration of a maritime pine canopy from sap-flow data. Agric. For. Meteorol. 1994, 71, 61–81. [Google Scholar] [CrossRef]
- Kume, T.; Takizawa, H.; Yoshifuji, N.; Tanaka, K.; Tantasirin, C.; Tanaka, N.; Suzuki, M. Impact of soil drought on sap flow and water status of evergreen trees in a tropical monsoon forest in northern Thailand. For. Ecol. Manag. 2007, 238, 220–230. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F.; Zhu, S.D. Growth–climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Liang, E.; Eckstein, D.; Liu, H. Climate-growth relationships of relict Pinus tabulaeformis at the northern limit of its natural distribution in northern China. J. Veg. Sci. 2009, 19, 393–406. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Vaganov, E.A.; Li, J.; Cai, Q. Statistical and process-based modeling analyses of tree growth response to climate in semi-arid area of north central China: A case study of Pinus tabulaeformis. J. Geophys. Res. D Atmos. 2008, 113, 341–356. [Google Scholar] [CrossRef]






| Tree Species | n | Diameter at Breast Height (DBH, cm) | Tree Height (H, m) | Sapwood Area at DBH (AS, cm2) |
|---|---|---|---|---|
| B. albosinensis | 10 | 5.9–20.6 | 6–11 | 75.24 (19.60–284.16) |
| P. tabulaeformis | 10 | 5.6–26.8 | 6–15 | 461.47 (23.30–479.53) |
| Species | Parameter | Before 14:00 | After 14:00 | ||||
|---|---|---|---|---|---|---|---|
| REW <0.4 | REW ≥0.4 | Parameter Difference | REW <0.4 | REW ≥0.4 | Parameter Difference | ||
| B. albosinensis | A2 | 0.85 | 0.86 | p = 0.46 | 0.98 | 0.89 | p < 0.001 |
| b | 0.79 | 0.89 | p < 0.001 | 1.71 | 1.52 | p = 0.04 p < 0.001 | |
| q | 2.48 | 2.57 | p = 0.9 | 1.52 | 1.40 | ||
| R2 | 0.9468 | 0.9688 | 0.7881 | 0.9383 | |||
| P. tabulaeformis | A2 | 0.80 | 0.87 | p < 0.001 | 0.79 | 0.84 | p < 0.001 |
| b | 0.85 | 0.92 | p < 0.001 | 1.57 | 1.36 | p < 0.001 | |
| q | 2.75 | 2.89 | p = 0.8 | 2.37 | 1.98 | p = 0.03 | |
| R2 | 0.9437 | 0.8784 | 0.7954 | 0.9540 | |||
| Species | Parameter | Before 14:00 | After 14:00 | ||||
|---|---|---|---|---|---|---|---|
| REW <0.4 | REW ≥0.4 | Parameter Difference | REW <0.4 | REW ≥0.4 | Parameter Difference | ||
| B. albosinensis | A2 | 0.85 | 0.861 | p = 0.6 | 0.82 | 0.75 | p < 0.001 |
| b | 21.52 | 24.63 | p < 0.001 | 15.02 | 12.37 | p < 0.001 | |
| q | 0.07 | 0.07 | p = 0.9 | 0.04 | 0.07 | P < 0.001 | |
| R2 | 0.9570 | 0.9758 | 0.9562 | 0.9134 | |||
| P. tabulaeformis | A2 | 0.80 | 0.86 | p < 0.001 | 0.79 | 0.83 | p < 0.001 |
| b | 23.69 | 25.71 | p < 0.001 | 12.75 | 9.50 | p < 0.001 | |
| q | 0.08 | 0.09 | p = 0.9 | 0.06 | 0.010 | p < 0.001 | |
| R2 | 0.9584 | 0.9837 | 0.9050 | 0.9610 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Wang, B.; Zhang, Y.; Zhang, X.; Takeuchi, S.; Qiu, G.Y. Responses of Sap Flow of Deciduous and Conifer Trees to Soil Drying in a Subalpine Forest. Forests 2018, 9, 32. https://doi.org/10.3390/f9010032
Yan C, Wang B, Zhang Y, Zhang X, Takeuchi S, Qiu GY. Responses of Sap Flow of Deciduous and Conifer Trees to Soil Drying in a Subalpine Forest. Forests. 2018; 9(1):32. https://doi.org/10.3390/f9010032
Chicago/Turabian StyleYan, Chunhua, Bei Wang, Yang Zhang, Xiaonan Zhang, Shinichi Takeuchi, and Guo Yu Qiu. 2018. "Responses of Sap Flow of Deciduous and Conifer Trees to Soil Drying in a Subalpine Forest" Forests 9, no. 1: 32. https://doi.org/10.3390/f9010032
APA StyleYan, C., Wang, B., Zhang, Y., Zhang, X., Takeuchi, S., & Qiu, G. Y. (2018). Responses of Sap Flow of Deciduous and Conifer Trees to Soil Drying in a Subalpine Forest. Forests, 9(1), 32. https://doi.org/10.3390/f9010032






