Tree-Ring Growth of Larch (Larix decidua Mill.) in the Polish Sudetes—The Influence of Altitude and Site-Related Factors on the Climate–Growth Relationship
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Characteristics and Site Locations
2.2. Construction and Characteristics of Site Chronologies
2.3. Climate Data
2.4. Regional Patterns of Common Variation among Site Chronologies
2.5. Climate Influence on Tree-Ring Growth
3. Results
3.1. Characteristics of the Chronologies
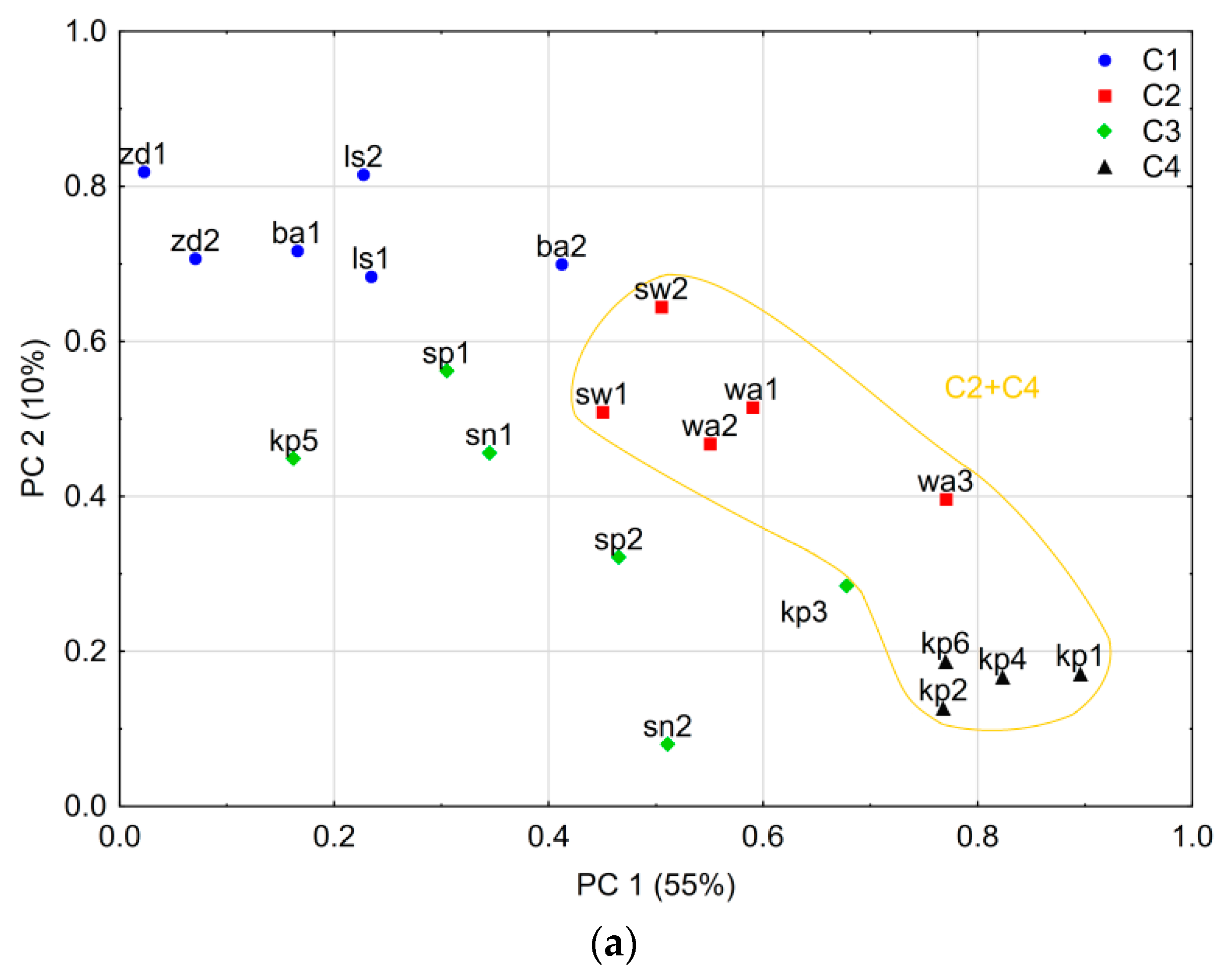
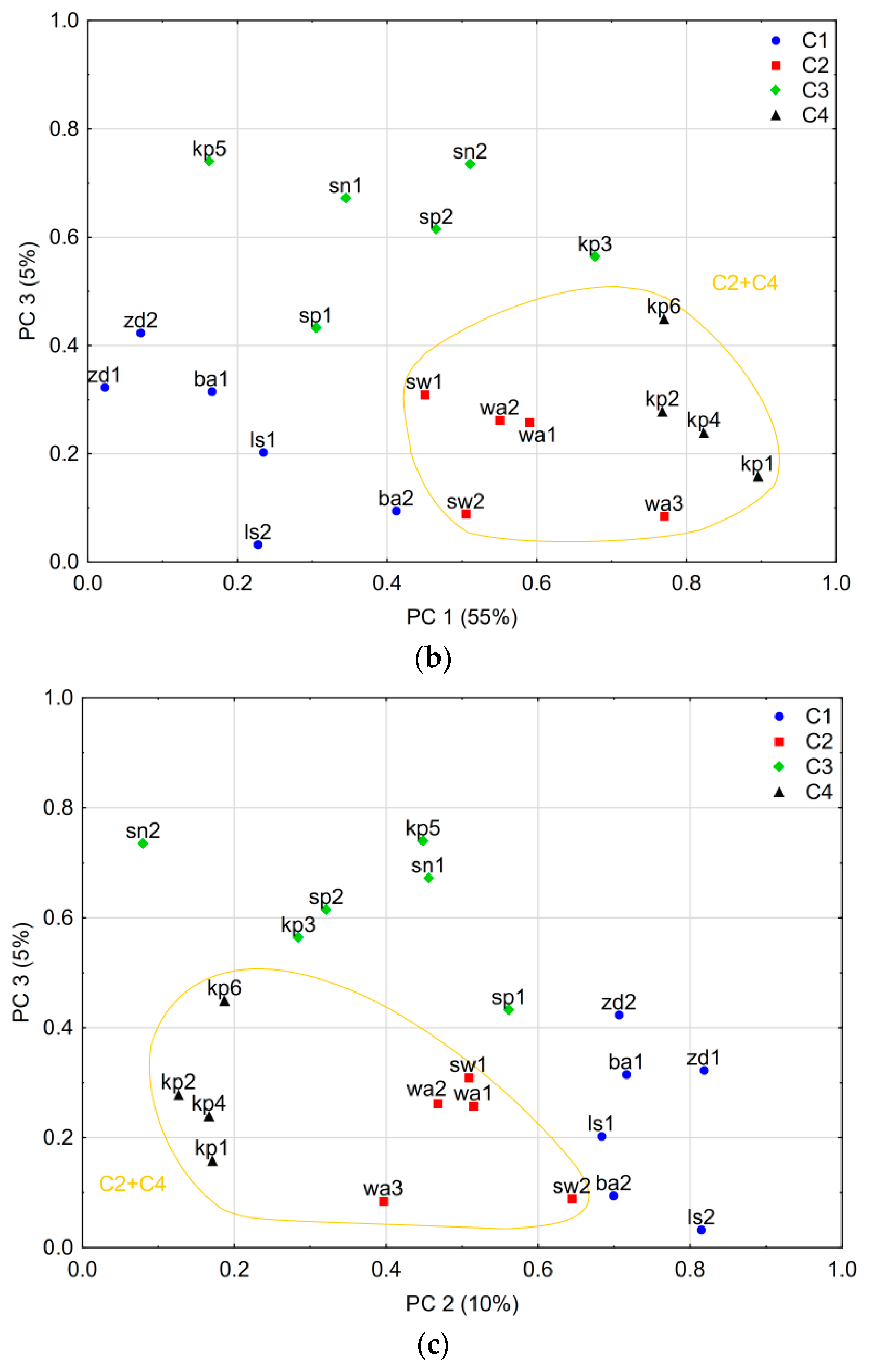
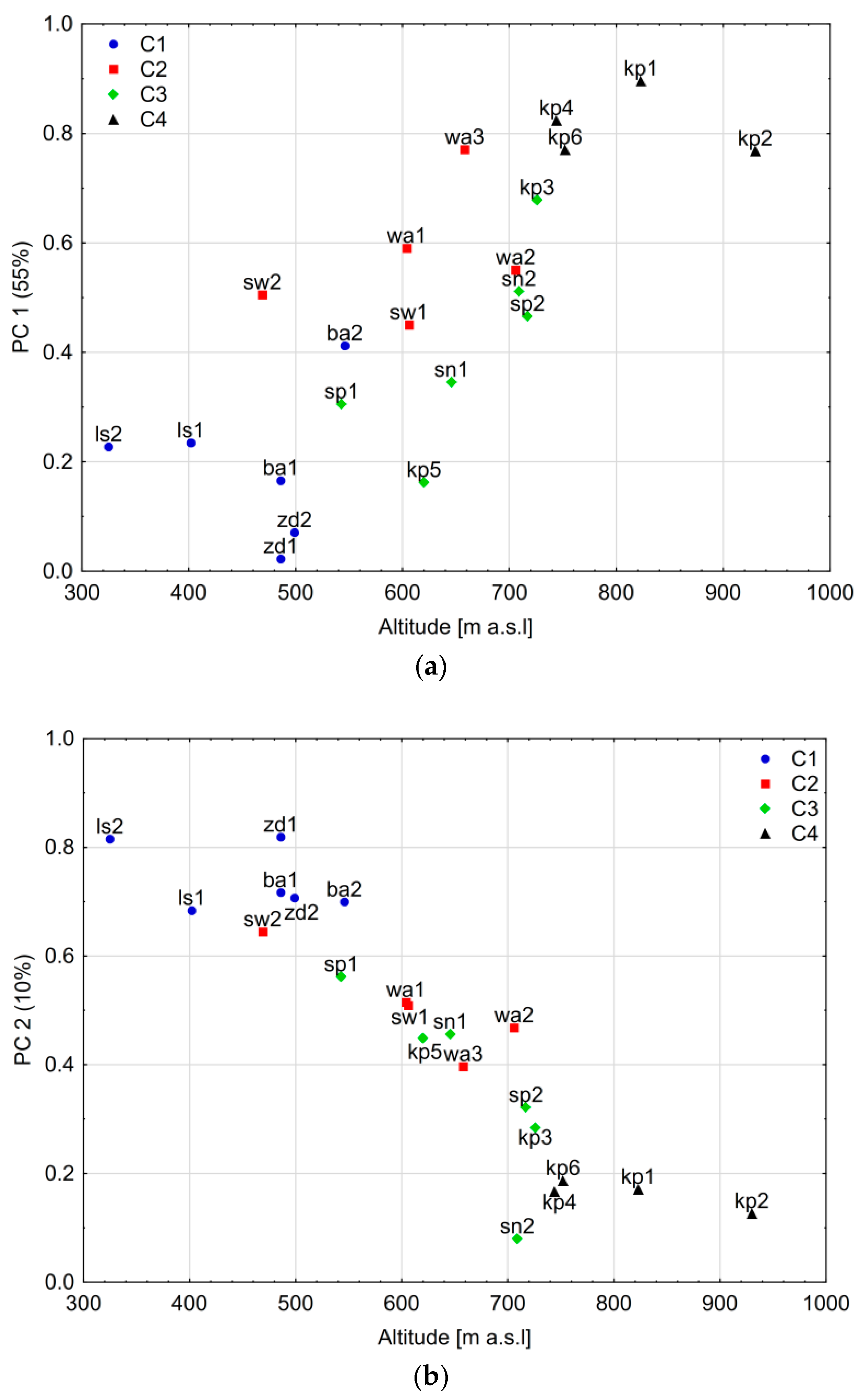
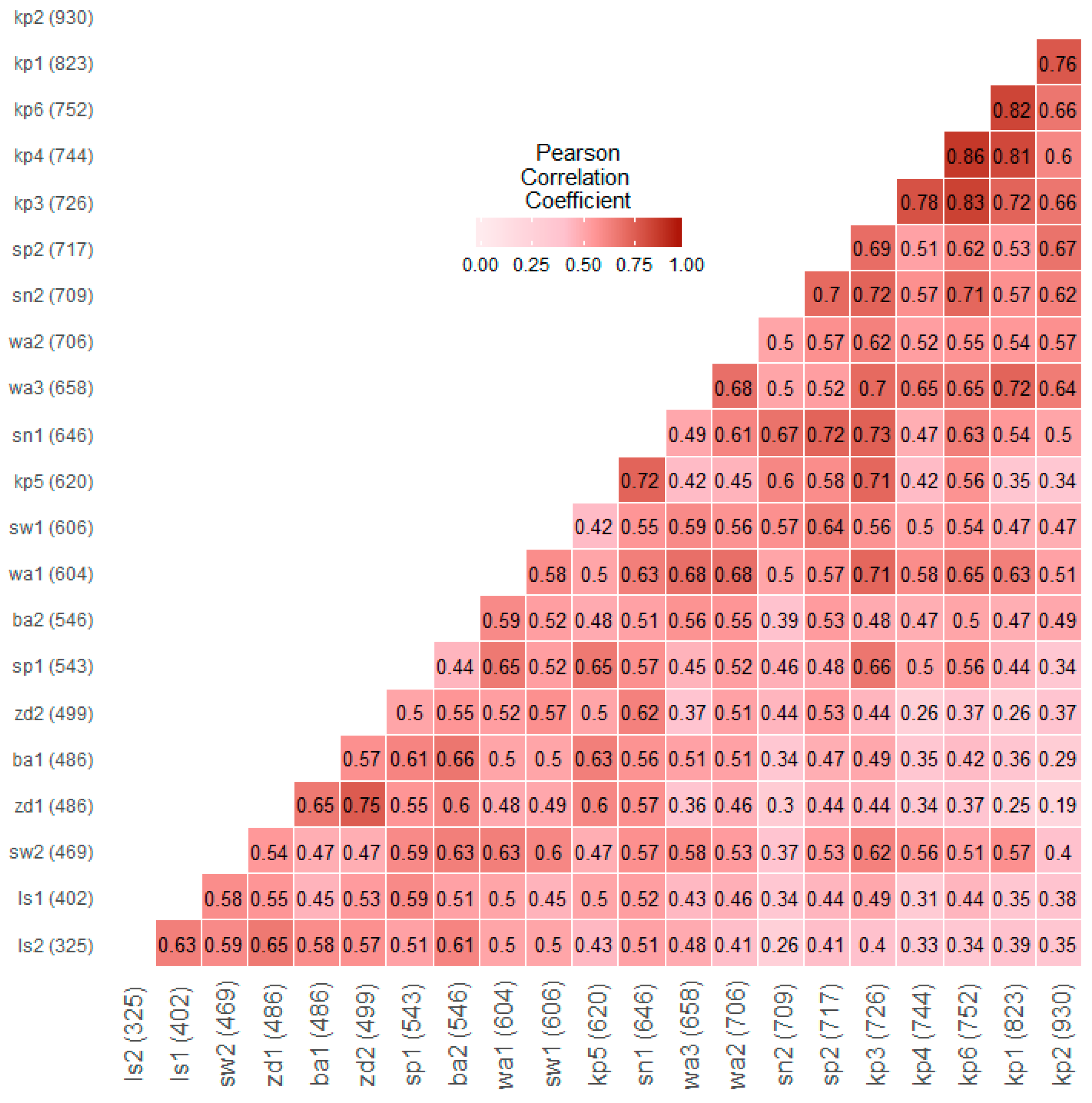
3.2. Spatial Variability of Larch Tree-Ring Growth
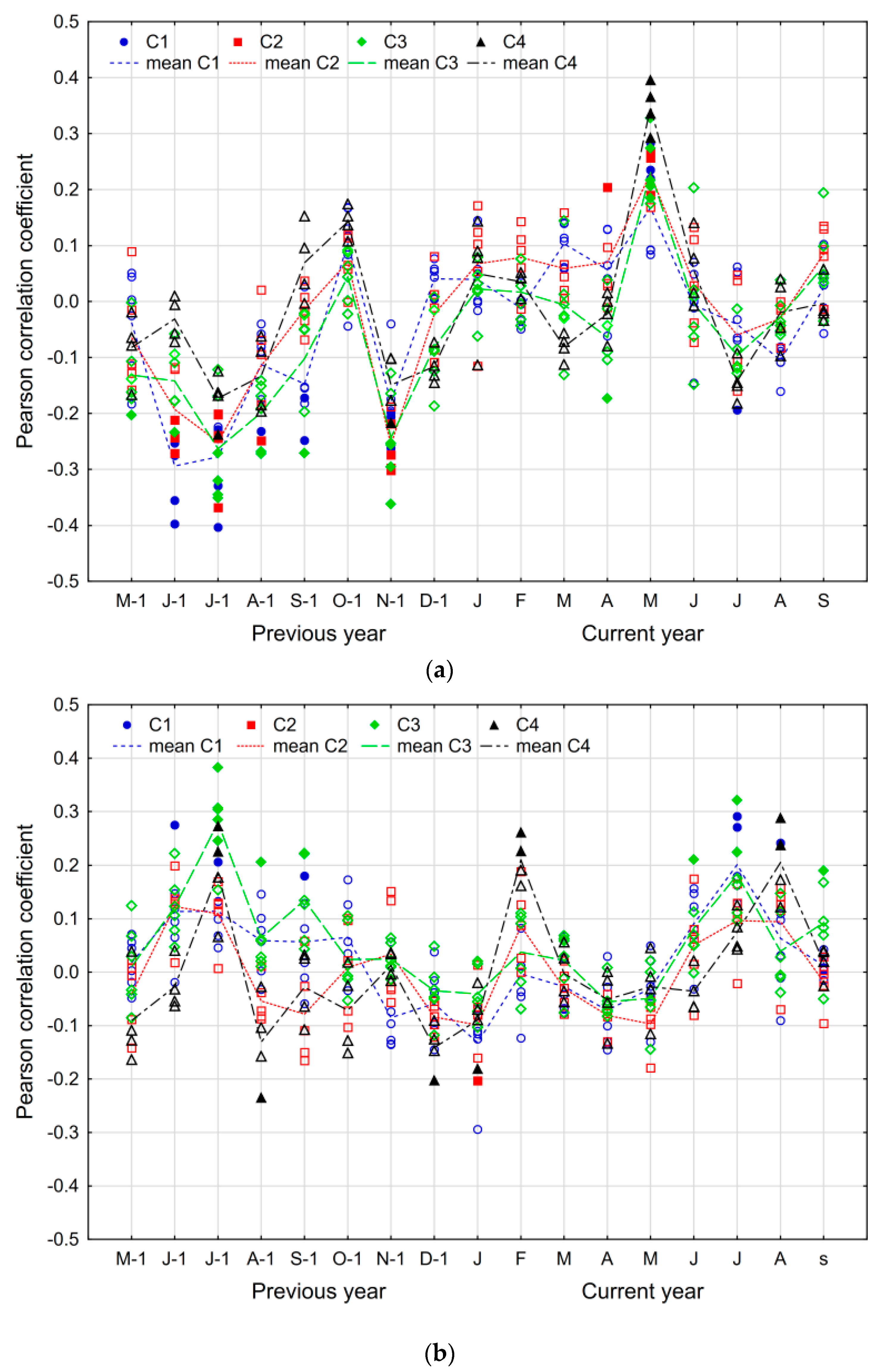
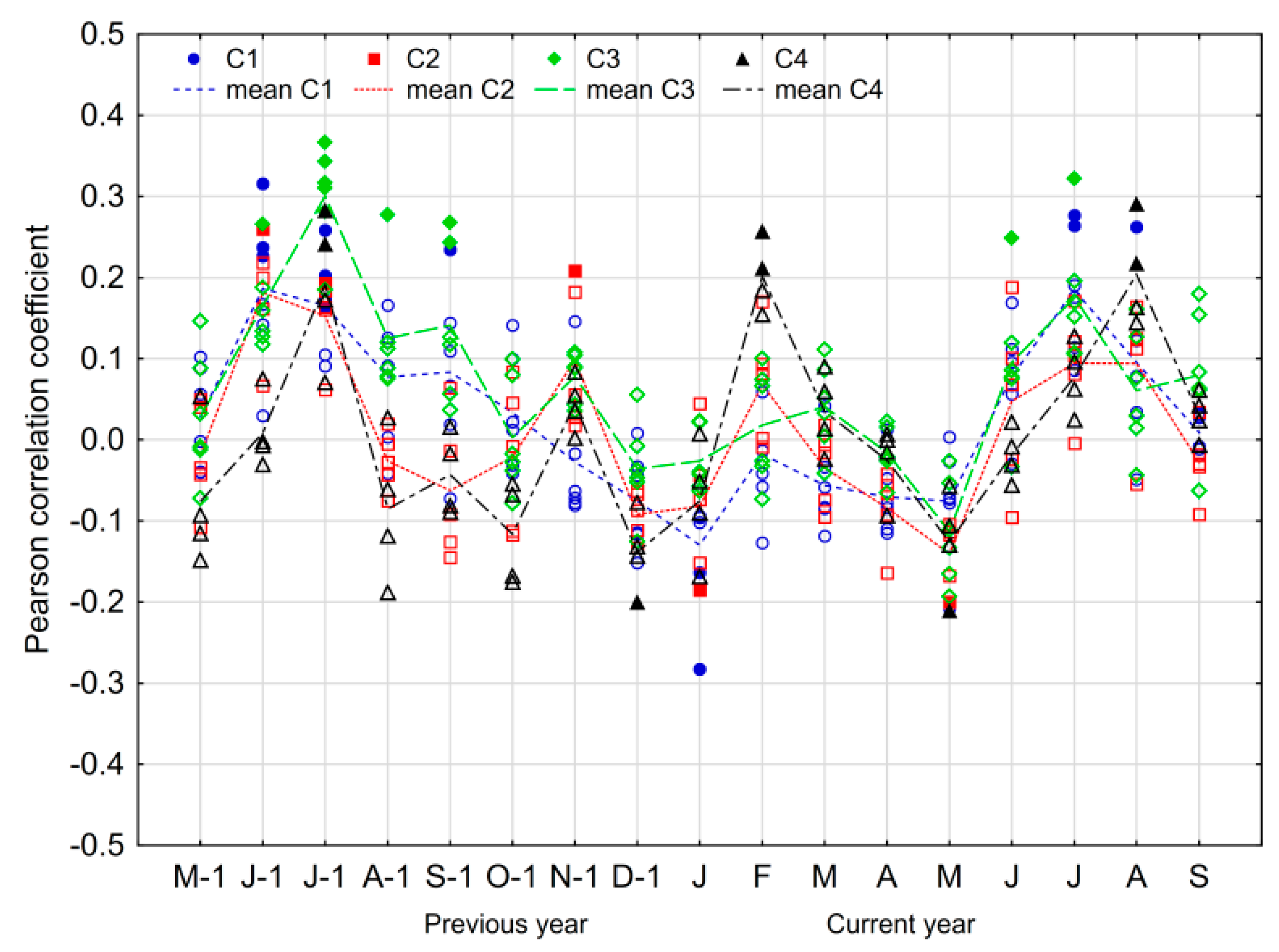
3.3. The Climate–Growth Relationship
4. Discussion
4.1. Spatial Variability of the Tree-Ring Signal
4.2. The Climate–Growth Relationship
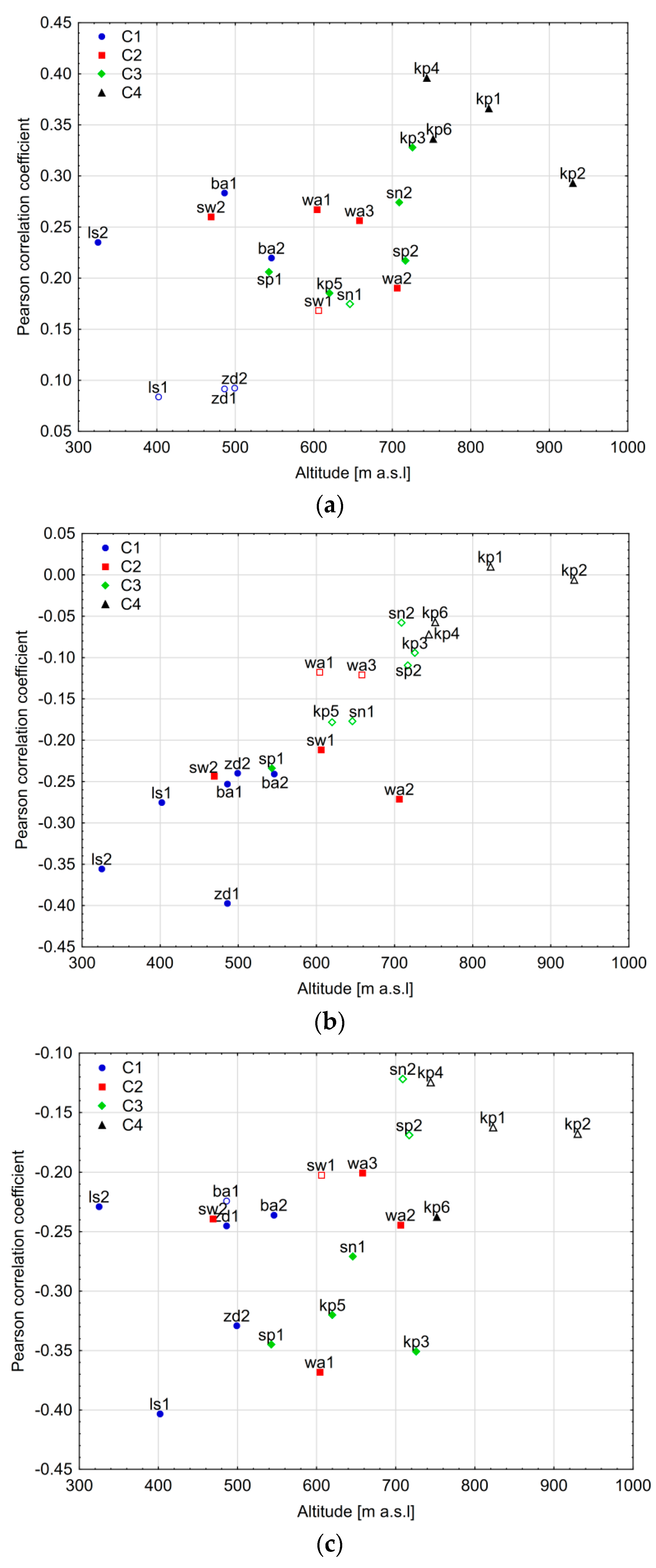
4.3. The Influence of Other Site-Related Factors on the Climate–Growth Relationship
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Spiecker, H. Overview of recent growth trends in European forests. Water Air Soil Pollut. 1999, 116, 33–46. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.-J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienast, F.; Wildi, O.; Brzeziecki, B. Potential impacts of climate change on species richness in mountain forests—An ecological risk assessment. Biol. Conserv. 1998, 83, 291–305. [Google Scholar] [CrossRef]
- Beniston, M. Climatic change in mountain regions: A review of possible impacts. Clim. Chang. 2003, 59, 5–31. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Sidor, C.G.; Popa, I.; Vlad, R.; Cherubini, P. Different tree-ring responses of Norway spruce to air temperature across an altitudinal gradient in the Eastern Carpathians (Romania). Trees 2015, 29, 985–997. [Google Scholar] [CrossRef]
- Babst, F.; Poulter, B.; Trouet, V.; Tan, K.; Neuwirth, B.; Wilson, R.; Carrer, M.; Grabner, M.; Tegel, W.; Levanic, T.; et al. Site- and species-specific responses of forest growth to climate across the European continent. Glob. Ecol. Biogeogr. 2013, 22, 706–717. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol. 2006, 170, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Büntgen, U.; Frank, D.; Wilson, R.; Carrer, M.; Urbinati, C.; Esper, J. Testing for tree-ring divergence in the European Alps. Glob. Chang. Biol. 2008, 14, 2443–2453. [Google Scholar] [CrossRef] [Green Version]
- Büntgen, U.; Frank, D.; Neuenschwander, T.; Esper, J. Fading temperature sensitivity of Alpine tree growth at its Mediterranean margin and associated effects on large-scale climate reconstructions. Clim. Chang. 2012, 114, 651–666. [Google Scholar] [CrossRef]
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Rolland, C. Decreasing teleconnections with inter-site distance in monthly climatic data and tree-ring width networks in a mountainous Alpine area. Theor. Appl. Clim. 2002, 71, 63–75. [Google Scholar] [CrossRef]
- Frank, D.; Esper, J. Characterization and climate response patterns of a high-elevation, multi-species tree-ring network in the European Alps. Dendrochronologia 2005, 22, 107–121. [Google Scholar] [CrossRef]
- Leal, S.; Melvin, T.M.; Grabner, M.; Wimmer, R.; Briffa, K.R. Tree ring-growth variability in the Austrian Alps: The influence of site, altitude, tree species and climate. Boreas 2007, 36, 426–440. [Google Scholar] [CrossRef]
- Danek, M.; Chuchro, M.; Walanus, A. Variability in larch (Larix decidua Mill.) tree-ring growth response to climate in the Polish Carpathian Mountains. Forests 2017, 8, 354. [Google Scholar] [CrossRef]
- Korzybski, D.; Mionskowski, M.; Dmyterko, E.; Bruchwald, A. Stopień uszkodzenia świerka, jodły i modrzewia w Sudetach Zachodnich. Sylwan 2013, 157, 104–112. [Google Scholar]
- Krzysik, F. Nauka o Drewnie; Wyd. Naukowe PWN: Warszawa, Poland, 1978. [Google Scholar]
- Einspahr, D.W.; Wyckoff, G.W.; Fiscus, M.H. Larch—A fast-growing fiber source for the Lake States and Northeast. J. For. 1984, 82, 104–106. [Google Scholar]
- Karolewski, P.; Białobok, S. Wpływ dwutlenku siarki, ozonu, mieszaniny tych gazów i fluorowodoru na uszkodzenie igieł modrzewia europejskiego. Arbor. Kórn. 1979, 24, 297–305. [Google Scholar]
- Feliksik, E. Studies on sensitivity of the more important forest trees to industrial pollution. Zesz. Naukowe AKad. Rol. Kraków Leśni. 1991, 254, 353–391. [Google Scholar]
- Migoń, P.; Placek, A. Litologiczno-strukturalne uwarunkowania rzeźby Sudetów. Prz. Geol. 2014, 64, 36–43. [Google Scholar]
- Walczak, W. Dolny Śląsk. Cz. I—Sudety; Wyd. Naukowe PWN: Warszawa, Poland, 1968. [Google Scholar]
- Błaś, M.; Sobik, M. Mgła w Karkonoszach i wybranych masywach górskich Europy. Opera Corcon. 2000, 37, 35–46. [Google Scholar]
- Sarosiek, J.; Sembrat, K.; Wiktor, A. Sudety; Wiedza Powszechna: Warszawa, Poland, 1967. [Google Scholar]
- Boratyński, A. Systematyka i geograficzne rozmieszczenie. In Modrzewie Larix Mill.; Białobok, S., Ed.; PWN: Warszawa/Poznań, Poland, 1986; pp. 63–108. [Google Scholar]
- Maciejowski, K. Modrzew w lasach polskich. Sylwan 1956, 100, 6–57. [Google Scholar]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled SRTM for the Globe; Version 4. Available online: http://srtm.csi.cgiar.org (accessed on 13 August 2018).
- Walanus, A. Program Quercus, Instrukcja Obsługi; AGH-UST: Kraków, Poland, 2005. [Google Scholar]
- Cook, E.R.; Peters, K. Calculating unbiased tree-ring indices for the study of climatic and environmental change. Holocene 1997, 361–370. [Google Scholar] [CrossRef]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985; p. 171. [Google Scholar]
- Holmes, R.L.; Adams, R.K.; Fritts, H.C. Tree-Ring Chronologies of Western North America: California, Eastern Oregon and Northern Great Basin with Procedures Used in the Chronology Development Work Including Users Manuals for the COFECHA and ARSTAN Computer Programs; Chronology Series VI; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1986; p. 182. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Academic Press: London, UK, 1976; p. 567. ISBN 978-0-12-268450-0. [Google Scholar]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees 2006, 20, 735–746. [Google Scholar] [CrossRef]
- Cook, E.R.; Kariukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer Science + Business Media: New York, NY, USA, 1990; p. 288. ISBN 978-90-481-4060-2. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.; Osborn, T.; Lister, D. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Chuchro, M.; Danek, M. Selection of optimal gridded dataset for application in Polish Sudetes Mountains. In Proceedings of the World Multidisciplinary Earth Sciences Symposium, Prague, Czech Republic, 3–7 September 2018. in press. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2002; ISBN 0-387-95442-2. [Google Scholar]
- Richman, M.B. Rotation of principal components. J. Climatol. 1986, 6, 293–335. [Google Scholar] [CrossRef]
- Peterson, D.W.; Peterson, D.L.; Ettl, G.J. Growth responses of subalpine fir to climatic variability in the Pacific Northwest. Can. J. For. Res. 2002, 32, 1503–1517. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Eduard, J.L.; Motta, R.; Urbinati, C. Regional variability of climate–growth relationships in Pinus cembra high elevation forests in the Alps. J. Ecol. 2007, 95, 1072–1083. [Google Scholar] [CrossRef]
- Büntgen, U.; Frank, D.C.; Kaczka, R.J.; Verstege, A.; Zwijacz-Kozica, T.; Esper, J. Growth responses to climate in a multi-species tree-ring network in the Western Carpathian Tatra Mountains, Poland and Slovakia. Tree Physiol. 2007, 27, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuszkiewicz, W. Fitosocjologiczne podstawy typologii lasów Polski. Pr. Inst. Badaw. Leśn. 1978, 558, 3–39. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM 2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- LeBlanc, D.C.; Stahle, D.W. Radial growth responses of four oak species to climate in eastern and central North America. Can. J. For. Res. 2015, 45, 793–804. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index–SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Pawlak, W. Atlas Śląska Dolnego i Opolskiego; Uniwersytet Wrocławski, Pracownia Atlasu Dolnego Śląska: Wrocław, Poland, 1997; p. 125. [Google Scholar]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.; Fonti, P.; Büntgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2009, 30, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Swidrak, I.; Schuster, R.; Oberhuber, W. Comparing growth phenology of co-occurring deciduous and evergreen conifers exposed to drought. Flora 2013, 208. [Google Scholar] [CrossRef]
- Chałupka, W.; Kosiński, G. Fizjologia wzrostu i rozwoju. In Modrzewie Larix Mill.; Białobok, S., Ed.; PWN: Warsaw/Poznan, Poland, 1986; pp. 173–213. [Google Scholar]
- Oribe, Y.; Kubo, T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiol. 1997, 17, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gričar, J.; Zupančič, M.; Čufar, K.; Koch, G.; Schmitt, U.; Oven, P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Ann. Bot. 2006, 97, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.-O.; et al. Climate change and the regulation of wood formation in trees by temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Ermich, K. Zależność przyrostu drzew w Tatrach od wahań klimatycznych. Acta Soc. Bot. Pol. 1955, 24, 245–273. [Google Scholar]
- Koprowski, M. Long-term increase of March temperature has no negative impact on tree rings of European larch (Larix decidua) in lowland Poland. Trees 2012, 26, 1895–1903. [Google Scholar] [CrossRef]
- Vitas, A. A dendroclimatological analysis of European larch (Larix decidua Mill.) from Lithuania. Balt. For. 2015, 20, 65–72. [Google Scholar]
- Oleksyn, J.; Fritts, H. Influence of climatic factors upon tree rings of Larix decidua and L. decidua × L. kaempferi from Puławy, Poland. Trees-Struct. Funct. 1991, 5, 75–82. [Google Scholar] [CrossRef]
- Woś, A. Klimat Polski; Wydawn, Naukowe PWN: Warszawa, Poland, 1999; p. 301. [Google Scholar]
- Oleksyn, J.; Lorenc-Plucińska, G. Wymiana gazowa i gospodarka wodna. In Modrzewie Larix Mill.; Białobok, S., Ed.; PWN: Warsaw/Poznan, Poland, 1986; pp. 137–171. [Google Scholar]
- Olaczek, R. Zarys ekologii i fitocenologii. In Modrzewie Larix Mill.; Białobok, S., Ed.; PWN: Warsaw/Poznan, Poland, 1986; pp. 381–440. [Google Scholar]
- Anfodillo, T.; Rento, S.; Carraro, V.; Furlanetto, L.; Urbinati, C.; Carrer, M. Tree water relations and climatic variations at the alpine timberline: Seasonal changes of sap flux and xylem water potential in Larix decidua Miller, Picea abies (L.) Krast and Pinus cembra L. Ann. For. Sci. 1998, 55, 159–172. [Google Scholar] [CrossRef]
- Feliksik, E. Wpływ warunków klimatycznych na wielkość przyrostów radialnych modrzewia europejskiego (Larix decidua Mill.) występującego w Karpatach. Sylwan 1992, 136, 61–67. [Google Scholar]
- Feliksik, E.; Wilczyński, S. Wpływ warunków termicznych i pluwialnych na przyrost roczny modrzewi (Larix decidua Mill.). Sylwan 1998, 152, 85–90. [Google Scholar]
- Grudd, H.; Briffa, K.R.; Karlén, W.; Bartholin, T.S.; Jones, P.D.; Kromer, B. A 7400-year tree-ring chronology in northern Swedish Lapland: Natural climatic variability expressed on annual to millennial timescales. Holocene 2002, 12, 657–665. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Simak, M. Photo- and thermoperiodic responses of different larch provenances (Larix decidua Mill.). Stud. For. Suec. 1970, 86, 1–31. [Google Scholar]
- Heide, O.M. High autumn temperature delays spring bud burst in boreal trees, counterbalancing the effect of climatic warming. Tree Physiol. 2003, 23, 931–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häkkinen, R.; Linkosalo, T.; Hari, P. Effects of dormancy and environmental factors on timing of bud burst in Betula pendula. Tree Physiol. 1998, 18, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, R.; Miętus, M. Niektóre cechy wieloletniej zmienności temperatury powietrza w Polsce (1951–2010). Prz. Geogr. 2014, 86, 339–364. [Google Scholar] [CrossRef]
- Łapińska, M.; Bednorz, E. Występowanie wiatrów fenowych w Karpatach i Sudetach na przykładzie dwóch stacji meteorologicznych—Kasprowego Wierchu i Śnieżki. Badania Fizjogr. Ser. A. Geogr. Fiz. 2013, 64, 121–135. [Google Scholar]
- Kwiatkowski, J. Zasięg fenów sudeckich i ich wpływ na mezoklimat regionów południowo-zachodniej i środkowej Polski. Prz. Geofiz. 1975, 20, 15–30. [Google Scholar]
- Service of Institute of Meteorology and Water Management, National Research Institute (IMGW-PIB), Poland. Available online: http://klimat.pogodynka.pl/pl/climate-maps (accessed on 14 June 2018).
- Blazejczyk, K. Climate and bioclimate of Poland. In Natural and Human Environment of Poland—A Geographical Overview; Degórski, M., Ed.; Polish Academy of Sciences, Institute of Geography and Spatial Organization Polish Geographical Society: Warsaw, Poland, 2006; pp. 31–48. [Google Scholar]
- Szwed, M. Variability of precipitation in Poland under climate change. Theor. Appl. Climatol. 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Okołowicz, W.; Martyn, D. Regiony klimatyczne. In Atlas Geograficzny Polski; Górski, H., Melnicka, P., Eds.; PPWK: Warszawa, Poland, 1984; p. 11. [Google Scholar]
- Błaś, M.; Sobik, M. Osobliwości klimatu Karkonoszy i Gór Izerskich. In Rola Stacji Terenowych w Badaniach Geograficznych; Krzemień, K., Trepińska, J., Bokwa, A., Eds.; Instytut Geografii i Gospodarki Przestrzennej UJ: Kraków, Poland, 2005; pp. 109–122. [Google Scholar]
- Degirmendžić, J.; Kożuchowski, K.; Żmudzka, E. Changes of air temperature and precipitation in Poland in the period 1951–2000 and their relationship to atmospheric circulation. Int. J. Climatol. 2004, 24, 291–310. [Google Scholar] [CrossRef] [Green Version]
- Michalska, B. Tendencje zmian temperatury powietrza w Polsce. Pr. Stud. Geogr. 2011, 47, 67–75. [Google Scholar]
- Wypych, A.; Sulikowska, A.; Ustrnul, Z.; Czekierda, D. Temporal variability of summer temperature extremes in Poland. Atmosphere 2017, 8, 51. [Google Scholar] [CrossRef]
- Leonelli, G.; Pelfini, M.; Battipaglia, G.; Cherubini, P. Site-aspect influence on climate sensitivity over time of a high-altitude Pinus cembra tree-ring network. Clim. Chang. 2009, 96, 185–201. [Google Scholar] [CrossRef]
- Montwé, D.; Isaac-Renton, M.; Hamann, A.; Spiecker, H. Cold adaptation recorded in tree rings highlights risks associated with climate change and assisted migration. Nat. Commun. 2018, 9, 1574. [Google Scholar] [CrossRef] [PubMed]

| No. | Site | Longitude | Latitude | Altitude [m a.s.l.] | Slope Aspect | Forest Type | Soil Type | No. of Trees |
|---|---|---|---|---|---|---|---|---|
| 1 | ls1 | 15°28′40.80″ E | 51°03′25.88″ N | 402 | flat | UMFF | cambisols | 16 |
| 2 | ls2 | 15°41′02.00″ E | 51°02′32.00″ N | 325 | N | UMFF | cambisols | 14 |
| 3 | sn1 | 15°41′05.99″ E | 50°47′36.53″ N | 646 | W | MCMFF | podzols | 15 |
| 4 | sn2 | 15°40′43.81″ E | 50°47′24.64″ N | 709 | NE, near summit | MMFF | cambisols | 17 |
| 5 | zd1 | 16°29′23.10″ E | 50°25′02.00″ N | 486 | NE, slope flattering | MFF | cambisols | 15 |
| 6 | zd2 | 16°27′53.50″ E | 50°23′55.00″ N | 499 | NW | MFF | podzols | 15 |
| 7 | ba1 | 16°43′07.70″ E | 50°26′39.60″ N | 486 | N | MFF | cambisols | 15 |
| 8 | ba2 | 16°45′59.90″ E | 50°26′31.60″ N | 546 | S | MFF | cambisols | 15 |
| 9 | kp1 | 15°44′48.90″ E | 50°45′41.30″ N | 823 | E-EN | MMFF | arenosols | 20 |
| 10 | kp2 | 15°43′34.10″ E | 50°45′37.20″ N | 930 | N-NE | MMFF | arenosols | 20 |
| 11 | kp3 | 15°36′29.90″ E | 50°48′25.60″ N | 726 | SW | MMFF | arenosols | 20 |
| 12 | kp4 | 15°35′12.70″ E | 50°48′32.80″ N | 744 | N-NW | MMFF | arenosols | 20 |
| 13 | kp5 | 15°36′38.80″ E | 50°48′45.90″ N | 620 | N | MMFF | arenosols | 20 |
| 14 | kp6 | 15°45′40.20″ E | 50°45′33.70″ N | 752 | NE | MMFF | cambisols | 20 |
| 15 | wa1 | 16°07′57.60″ E | 50°40′22.70″ N | 604 | SE-SEE | MMFF | cambisols | 20 |
| 16 | wa2 | 16°22′00.60″ E | 50°38′44.40″ N | 706 | S-SSE | MCMFF | podzols | 20 |
| 17 | wa3 | 16°27′56.50″ E | 50°41′49.80″ N | 658 | SW | MMFF | cambisols | 20 |
| 18 | sp1 | 15°37′13.80″ E | 50°53′43.30″ N | 543 | W | MFF | cambisols | 20 |
| 19 | sp2 | 15°30′54.20″ E | 50°49′08.50″ N | 717 | N-NW | MMFF | planosols | 20 |
| 20 | sw1 | 15°21′31.90″ E | 50°53′30.20″ N | 606 | N-NW | MMFF | cambisols | 20 |
| 21 | sw2 | 15°29′09.20″ E | 50°57′36.90″ N | 469 | flat | MMFF | planosols | 20 |
| No. | Site Code | Cluster | Full Period Covered | Number of Years | No. of Trees in Chronology | MSL | AGR [mm] | AC | MS | mRbar (30_15) | mEPS (30_15) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ls1 | C1 | 1851–2010 | 160 | 16 | 125 | 1.604 | 0.70 | 0.29 | 0.53 | 0.94 |
| 2 | ls2 | C1 | 1884–2010 | 127 | 12 | 110 | 1.811 | 0.73 | 0.30 | 0.57 | 0.96 |
| 3 | sn1 | C3 | 1857–2010 | 154 | 15 | 125 | 1.284 | 0.66 | 0.20 | 0.39 | 0.93 |
| 4 | sn2 | C3 | 1870–2010 | 141 | 13 | 127 | 1.090 | 0.75 | 0.19 | 0.53 | 0.96 |
| 5 | zd1 | C1 | 1850–2010 | 161 | 14 | 148 | 1.783 | 0.77 | 0.23 | 0.49 | 0.94 |
| 6 | zd2 | C1 | 1834–2010 | 177 | 12 | 146 | 1.811 | 0.76 | 0.24 | 0.48 | 0.94 |
| 7 | ba1 | C1 | 1897–2010 | 114 | 14 | 92 | 2.194 | 0.63 | 0.29 | 0.51 | 0.96 |
| 8 | ba2 | C1 | 1912–2010 | 99 | 14 | 89 | 2.567 | 0.70 | 0.20 | 0.44 | 0.95 |
| 9 | kp1 | C4 | 1893–2014 | 122 | 18 | 109 | 1.602 | 0.69 | 0.23 | 0.56 | 0.97 |
| 10 | kp2 | C4 | 1871–2014 | 144 | 19 | 129 | 1.363 | 0.77 | 0.24 | 0.55 | 0.98 |
| 11 | kp3 | C3 | 1881–2014 | 134 | 19 | 111 | 1.506 | 0.70 | 0.20 | 0.49 | 0.97 |
| 12 | kp4 | C4 | 1885–2014 | 130 | 15 | 117 | 1.658 | 0.65 | 0.22 | 0.53 | 0.97 |
| 13 | kp5 | C3 | 1866–2014 | 149 | 18 | 133 | 1.264 | 0.70 | 0.21 | 0.50 | 0.97 |
| 14 | kp6 | C4 | 1889–2014 | 126 | 20 | 106 | 1.798 | 0.61 | 0.24 | 0.57 | 0.98 |
| 15 | wa1 | C2 | 1915–2015 | 101 | 19 | 88 | 2.009 | 0.66 | 0.23 | 0.53 | 0.97 |
| 16 | wa2 | C2 | 1894–2015 | 122 | 20 | 109 | 1.854 | 0.61 | 0.27 | 0.55 | 0.97 |
| 17 | wa3 | C2 | 1898–2015 | 118 | 20 | 105 | 1.742 | 0.65 | 0.27 | 0.61 | 0.98 |
| 18 | sp1 | C3 | 1919–2016 | 98 | 20 | 88 | 2.283 | 0.57 | 0.27 | 0.53 | 0.98 |
| 19 | sp2 | C3 | 1866–2016 | 151 | 19 | 134 | 1.372 | 0.73 | 0.19 | 0.41 | 0.96 |
| 20 | sw1 | C2 | 1863–2016 | 154 | 17 | 138 | 1.309 | 0.62 | 0.20 | 0.42 | 0.95 |
| 21 | sw2 | C2 | 1925–2016 | 92 | 19 | 83 | 2.547 | 0.65 | 0.23 | 0.46 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danek, M.; Chuchro, M.; Walanus, A. Tree-Ring Growth of Larch (Larix decidua Mill.) in the Polish Sudetes—The Influence of Altitude and Site-Related Factors on the Climate–Growth Relationship. Forests 2018, 9, 663. https://doi.org/10.3390/f9110663
Danek M, Chuchro M, Walanus A. Tree-Ring Growth of Larch (Larix decidua Mill.) in the Polish Sudetes—The Influence of Altitude and Site-Related Factors on the Climate–Growth Relationship. Forests. 2018; 9(11):663. https://doi.org/10.3390/f9110663
Chicago/Turabian StyleDanek, Małgorzata, Monika Chuchro, and Adam Walanus. 2018. "Tree-Ring Growth of Larch (Larix decidua Mill.) in the Polish Sudetes—The Influence of Altitude and Site-Related Factors on the Climate–Growth Relationship" Forests 9, no. 11: 663. https://doi.org/10.3390/f9110663
APA StyleDanek, M., Chuchro, M., & Walanus, A. (2018). Tree-Ring Growth of Larch (Larix decidua Mill.) in the Polish Sudetes—The Influence of Altitude and Site-Related Factors on the Climate–Growth Relationship. Forests, 9(11), 663. https://doi.org/10.3390/f9110663





