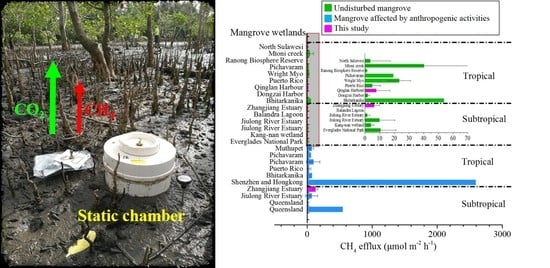
Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities
Abstract
:1. Introduction
2. Materials and Methods
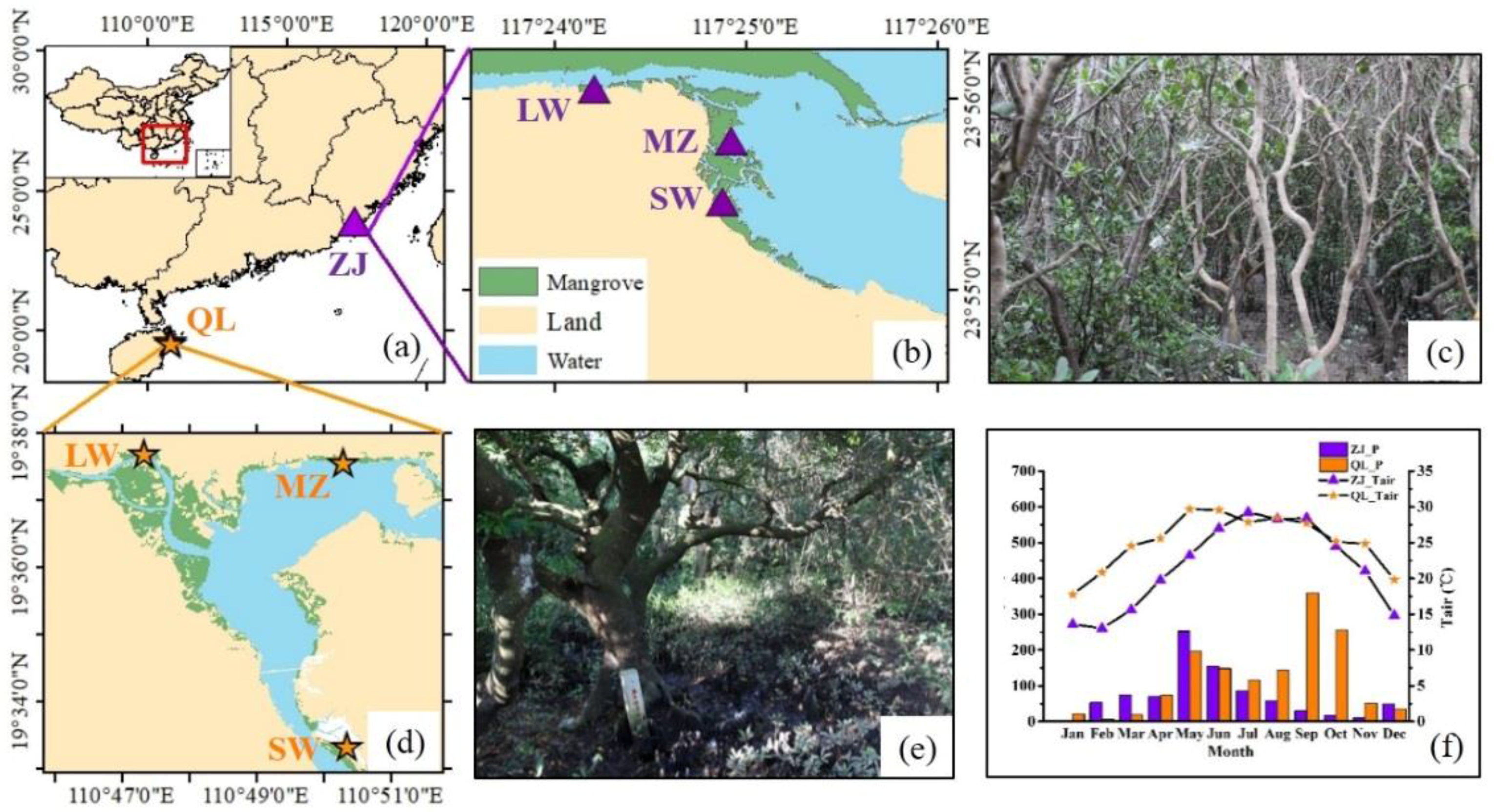
2.1. Study Site Description
2.2. Measurements of Soil CH4 and CO2 Effluxes
2.3. Measurements of Environmental Factors
2.4. Conversion to CO2—Equivalent Efflux
2.5. Collecting CH4 Efflux Data from Literature
2.6. Statistical Analysis
3. Results
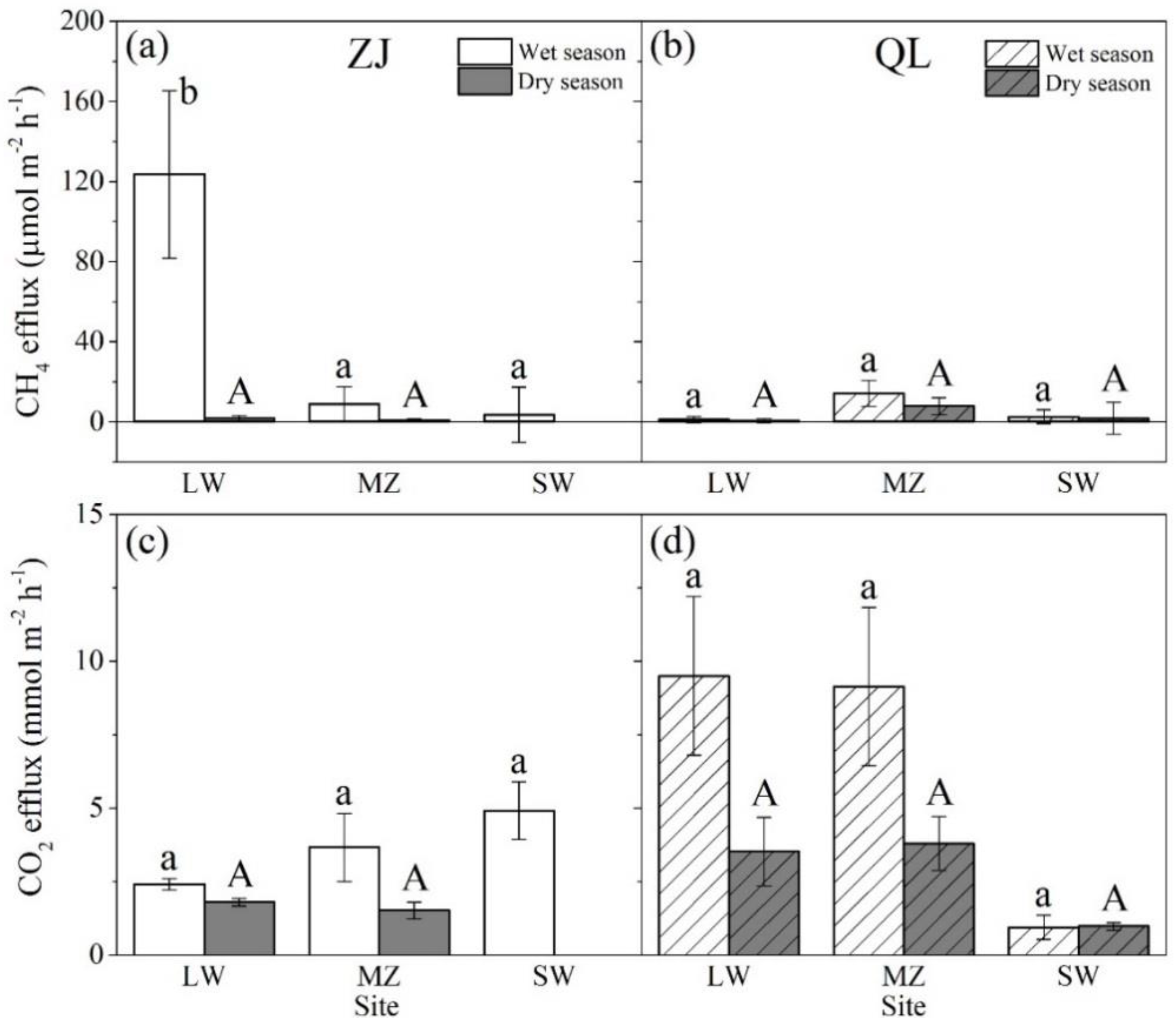
3.1. Soil CH4 and CO2 Effluxes
3.2. Soil Characteristics
3.3. The Relationship between Gas Effluxes and Soil Characteristics
4. Discussions
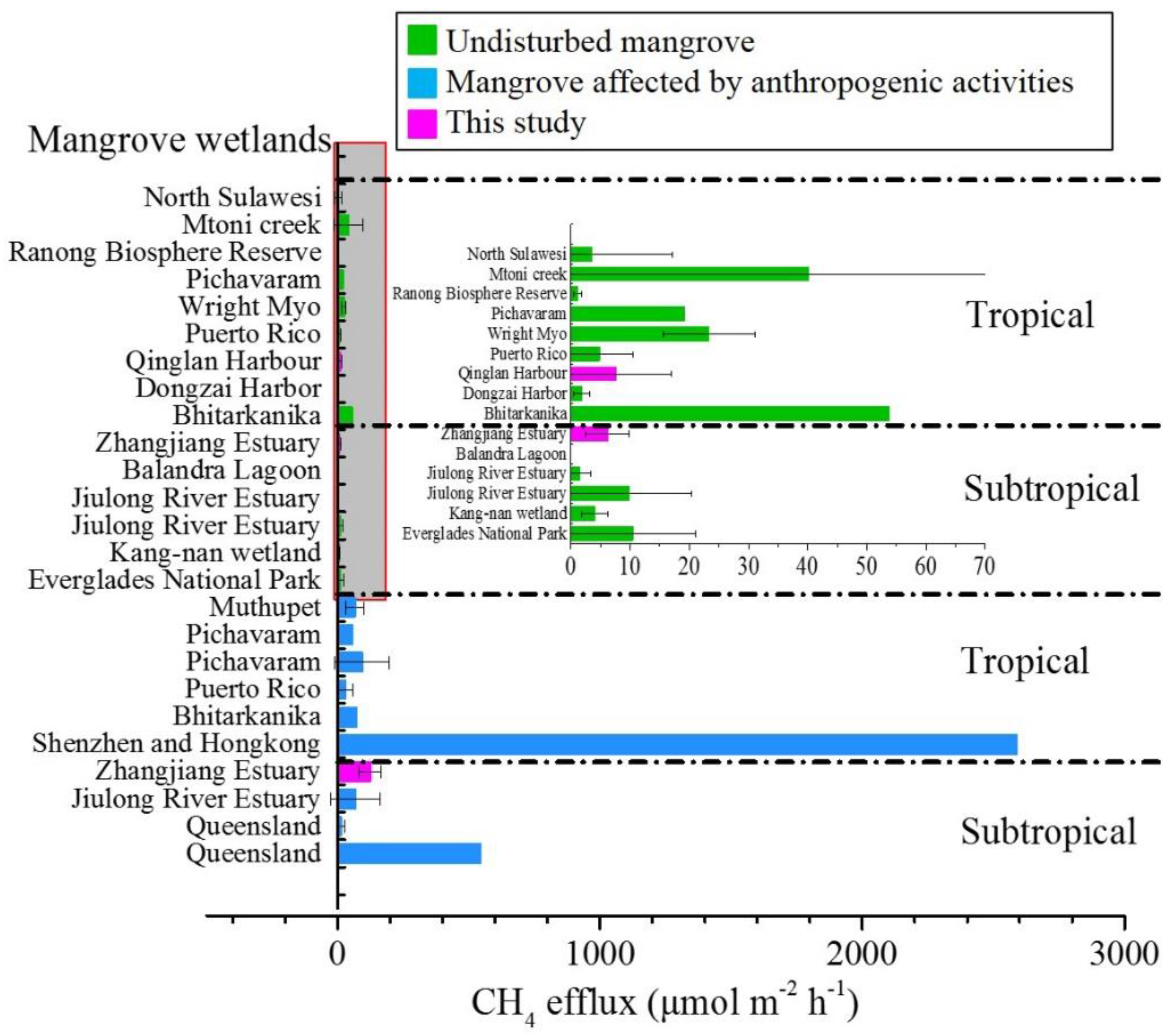
4.1. Magnitude of CH4 Efflux from Mangrove Wetland Soils
4.2. Contribution of CH4 Efflux from Mangrove Wetlands to Climate Warming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2012, 28, 583–597. [Google Scholar] [CrossRef]
- Sun, L.; Song, C.; Lafleur, P.M.; Miao, Y.; Wang, X.; Gong, C.; Qiao, T.; Yu, X.; Tan, W. Wetland-atmosphere methane exchange in Northeast China: A comparison of permafrost peatland and freshwater wetlands. Agric. For. Meteorol. 2017, 249, 239–249. [Google Scholar] [CrossRef]
- Mitra, S.; Wassmann, R.; Vlek, P.L.G. An appraisal of global wetland area and its organic carbon stock. Curr. Sci. 2005, 88, 25–35. [Google Scholar]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Sitoe, A.; Mandlate, L.; Guedes, B. Biomass and Carbon Stocks of Sofala Bay Mangrove Forests. Forests 2014, 5, 1967–1981. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Liu, F.; Zhang, Y.; Liu, C.; Lin, G. Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: A meta-analysis of eddy covariance data. Glob. Chang. Biol. 2017, 23, 1180–1198. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Ann. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Benson, L.; Glass, L.; Jones, T.; Ravaoarinorotsihoarana, L.; Rakotomahazo, C. Mangrove Carbon Stocks and Ecosystem Cover Dynamics in Southwest Madagascar and the Implications for Local Management. Forests 2017, 8, 190. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Manyothers, I.; Patra, P.; Ciais, P.; Sabine, C.; Manyothers, I.; Patra, P. Chapter 6: Carbon and Other Biogeochemical Cycles; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 465–570. [Google Scholar]
- Harriss, R.C.; Sebacher, D.I.; Bartlett, K.B.; Bartlett, D.S.; Crill, P.M. Sources of atmospheric methane in the south Florida environment. Glob. Biogeochem. Cycle 1988, 2, 231–243. [Google Scholar] [CrossRef]
- Sotomayor, D.; Corredor, J.E.; Morell, J.M. Methane flux from mangrove sediments along the southwestern coast of Puerto Rico. Estuaries 1994, 17, 140–147. [Google Scholar] [CrossRef]
- Kristensen, E. Carbon Balance in Mangrove Sediments: The Driving Processes and Their Controls; Gendai Tosho: Kanagawa, Japan, 2007; pp. 61–78. [Google Scholar]
- Chen, G.C.; Ulumuddin, Y.I.; Pramudji, S.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Biswas, H.; Mukhopadhyay, S.K.; Sen, S.; Jana, T.K. Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India. J. Mar. Syst. 2007, 68, 55–64. [Google Scholar] [CrossRef]
- Segarra, K.E.A.; Comerford, C.; Slaughter, J.; Joye, S.B. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta 2013, 115, 15–30. [Google Scholar] [CrossRef]
- Nobrega, G.N.; Ferreira, T.O.; Neto, M.S.; Queiroz, H.M.; Artur, A.G.; Mendonca, E.D.; Silva, E.D.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Biswas, H.; De, T.; Sen, B.; Sen, S.; Jana, T. Impact of Sundarban mangrove biosphere on the carbon dioxide and methane mixing ratios at the NE Coast of Bay of Bengal, India. Atmos. Environ. 2002, 36, 629–638. [Google Scholar] [CrossRef]
- Allen, D.E.; Dalal, R.C.; Rennenberg, H.; Meyer, R.L.; Reeves, S.; Schmidt, S. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol. Biochem. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Yu, D.; Tam, N.F.Y.; Ye, Y.; Chen, S. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ren, H.; Hui, D.; Wang, W.; Liao, B.; Cao, Q. Carbon stocks and potential carbon storage in the mangrove forests of China. J. Environ. Manag. 2014, 133, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Liao, G.S.; D’Souza, M.; Yu, X.Q.; Yang, J.; Yang, X.R.; Zheng, T.L. Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environ. Sci. Pollut. Res. 2016, 23, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Datta, A.; Ramanathan, A.L.; Adhya, T.K. Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 2015, 107, 95–106. [Google Scholar] [CrossRef]
- Walter, B.P.; Heimann, M. A process-based, climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Glob. Biogeochem. Cycle 2000, 14, 745–765. [Google Scholar] [CrossRef] [Green Version]
- Inubushi, K.; Furukawa, Y.; Hadi, A.; Purnomo, E.; Tsuruta, H. Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere 2003, 52, 603–608. [Google Scholar] [CrossRef]
- Zai-Wang, Z.; Xiang-Rong, X.; Yu-Xin, S.; Shen, Y.; Yong-Shan, C.; Jia-Xi, P. Heavy metal and organic contaminants in mangrove ecosystems of China: A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 11938–11950. [Google Scholar]
- Lin, P. The Comprehensive Report of Science Investigation on the Natural Reserve of Mangrove Wetland of Zhangjiang Estuary in Fujian; Xiamen University Press: Xiamen, China, 2001. [Google Scholar]
- Ding, H.; Yao, S.; Chen, J. Authigenic pyrite formation and re-oxidation as an indicator of an unsteady-state redox sedimentary environment: Evidence from the intertidal mangrove sediments of Hainan Island, China. Cont. Shelf Res. 2014, 78, 85–99. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Guo, Y.; Wen, W.; Cao, M.; Guo, J.; Li, Z. Soil carbon sequestration and its relationship with soil pH in Qinglangang mangrvoe wetlands in Hainan island (in Chinese). Sci. Silvae Sin. 2014, 50, 8–15. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B. Anthropogenic and natural radiative forcing. Clim. Chang. 2013, 423, 658–740. [Google Scholar]
- Lu, C.Y.; Wong, Y.S.; Tam, N.F.; Ye, Y.; Lin, P. Methane flux and production from sediments of a mangrove wetland on Hainan Island, China. Mangroves Salt Marshes 1999, 3, 41–49. [Google Scholar] [CrossRef]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, G.C.; Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total Environ. 2015, 526, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Buchholz, J.; Rennenberg, H. Emission of Methane and Nitrous Oxide by Australian Mangrove Ecosystems. Plant Biol. 2003, 5, 423–431. [Google Scholar] [CrossRef]
- Purvaja, R.; Ramesh, R. Natural and Anthropogenic Methane Emission from Coastal Wetlands of South India. Environ. Manag. 2001, 27, 547–557. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Truu, J.; Espenberg, M.; Mander, Ü.; Smith, P. Emissions of methane from northern peatlands: A review of management impacts and implications for future management options. Ecol. Evolut. 2016, 6, 7080–7102. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, Q.A.; Peng, C.H.; Wu, N.; Wang, Y.F.; Fang, X.Q.; Jiang, H.; Xiang, W.H.; Chang, J.; Deng, X.W.; et al. Methane emissions from rice paddies natural wetlands, lakes in China: Synthesis new estimate. Glob. Chang. Biol. 2013, 19, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, A.; Mitsch, W.J.; MacDonnell, C.; Zhang, L.; Bydałek, F.; Lasso, A. Methane emissions from mangrove soils in hydrologically disturbed and reference mangrove tidal creeks in southwest Florida. Ecol. Eng. 2018, 114, 57–65. [Google Scholar] [CrossRef]

| Mangrove Wetland | Season | Study Site | Species | Tsoil | Salinity (ppt) | pH | Soil Moisture Content | NH4+-N (ug g−1) | NO3--N (ug g−1) | TN (%) | TC (%) | C:N Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZJ | Wet season | LW | KO | 27.19 ± 0.19c | 2.07 ± 0.25a | 5.46 ± 0.31a | 0.85 ± 0.15a | 9.50 ± 0.92 | 0.14 ± 0.04 | 0.15 ± 0.03a | 1.82 ± 0.18a | 12.50 ± 1.07b |
| MZ | KO | 27.74 ± 0.05c | 5.70 ± 0.66ab | 7.11 ± 0.03c | 0.89 ± 0.23a | 9.09 ± 0.88 | 0.15 ± 0.05 | 0.15 ± 0.01a | 1.36 ± 0.01a | 9.25 ± 0.35a | ||
| SW | KO | 27.42 ± 0.09c | 7.70 ± 1.45ab | 6.82 ± 0.03ab | 1.04 ± 0.45a | 0.17 ± 0.01a | 1.96 ± 0.18a | 11.44 ± 0.65ab | ||||
| Dry season | LW | KO | 17.35 ± 0.19a | 9.00 ± 1.27b | 6.23 ± 0.10b | 0.75 ± 0.07a | 0.16 ± 0.02a | 1.98 ± 0.16a | 12.29 ± 0.47b | |||
| MZ | KO | 18.47 ± 0.09b | 15.55 ± 2.56c | 7.01 ± 0.14c | 0.81 ± 0.50a | 0.14 ± 0.01a | 1.49 ± 0.09a | 10.90 ± 0.31ab | ||||
| SW | KO | |||||||||||
| QL | Wet season | LW | BS, HL | 29.00D | 12.09 ± 1.37A | 6.81 ± 0.05AB | 1.85 ± 0.25B | 8.02 ± 0.90B | 4.31 ± 0.45B | 0.73 ± 0.10B | 11.48 ± 2.00AB | 15.44 ± 0.93A |
| MZ | BS, RA | 28.90D | 17.84 ± 5.91AB | 6.62 ± 0.16A | 0.56 ± 0.12A | 3.11 ± 0.55A | 1.28 ± 0.13A | 0.24 ± 0.05A | 4.01 ± 0.66AB | 17.03 ± 0.61A | ||
| SW | Mixed | 30.50E | 25.91 ± 4.22B | 6.85 ± 0.02AB | 0.51 ± 0.01A | 3.17 ± 0.13A | 1.63 ± 0.05A | 0.08 ± 0.01A | 4.48 ± 0.12AB | 57.81 ± 7.33B | ||
| Dry season | LW | KO | 25.18 ± 0.07B | 11.12 ± 1.21A | 7.30 ± 0.11BC | 2.08 ± 0.39B | 8.14 ± 1.10B | 3.55 ± 0.35B | 0.79 ± 0.11B | 12.41 ± 2.58B | 15.10 ± 0.89A | |
| MZ | KO | 23.34 ± 0.03A | 15.60 ± 0.67AB | 6.76 ± 0.16A | 0.60 ± 0.14A | 2.26 ± 0.75A | 1.42 ± 0.30A | 0.19 ± 0.05A | 3.52 ± 0.88A | 19.47 ± 0.74B | ||
| SW | KO | 25.45 ± 0.01C | 19.90 ± 0.30AB | 7.43 ± 0.14C | 0.44 ± 0.06A | 2.26 ± 0.33A | 1.31 ± 0.01A | 0.07 ± 0.01A | 4.75 ± 0.16AB | 74.26 ± 7.84C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Guo, J.; Song, W.; Feng, J.; Lin, G. Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities. Forests 2018, 9, 738. https://doi.org/10.3390/f9120738
Zheng X, Guo J, Song W, Feng J, Lin G. Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities. Forests. 2018; 9(12):738. https://doi.org/10.3390/f9120738
Chicago/Turabian StyleZheng, Xiawan, Jiemin Guo, Weimin Song, Jianxiang Feng, and Guanghui Lin. 2018. "Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities" Forests 9, no. 12: 738. https://doi.org/10.3390/f9120738
APA StyleZheng, X., Guo, J., Song, W., Feng, J., & Lin, G. (2018). Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities. Forests, 9(12), 738. https://doi.org/10.3390/f9120738