Effects of Drought and Salinity on European Larch (Larix decidua Mill.) Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Stress Treatments
2.2. Substrate Analysis
2.3. Analysis of Plant Growth and Dehydration of Samples
2.4. Photosynthetic Pigments
- Chl a (µg mL−1) = 12.21 × (A663) − 2.81 × (A646);
- Chl b (µg mL−1) = 20.13 × (A646) − 5.03 × (A663);
- Caro (µg mL−1) = (1000 × A470 − 3.27 × [Chl a] − 10 × [Chl b])/229
- Chlorophyll and carotenoid contents were finally expressed in mg g−1 DW.
2.5. Monovalent Ions
2.6. Osmolyte Quantification
2.7. Malondialdehyde (MDA) and Non-Enzymatic Antioxidants
2.8. Antioxidant Enzyme Activities
2.9. Data Analysis
3. Results
3.1. Substrate Analysis
3.2. Plant Growth Analysis
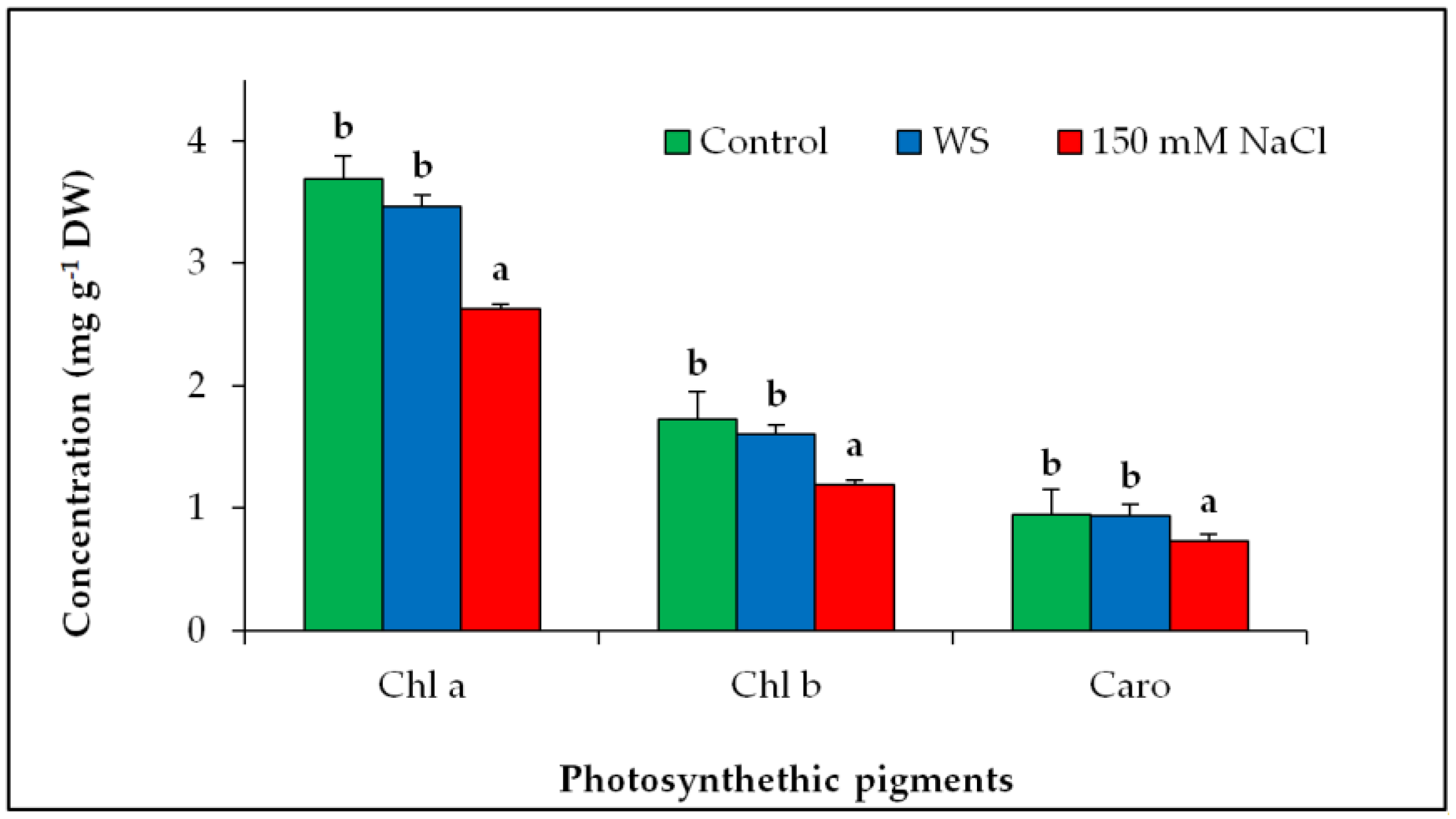
3.3. Degradation of Photosynthetic Pigments
3.4. Monovalent Ions Levels
3.5. Osmolytes Levels
3.6. Oxidative Stress
4. Discussion
4.1. Seedling Growth and Photosynthetic Pigments
4.2. Ion Homeostasis
4.3. Osmolyte Synthesis
4.4. Oxidative Stress and Antioxidant Mechanisms
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Koskela, J.; Buck, A.; du Cros, E.T. EUFORGEN Climate Change and Forest Genetic Diversity: Implications for Sustainable Forest Management in Europe; Biodiversity International: Rome, Italy, 2007; Available online: http://www.euforgen.org/fileadmin/bioversity/publications/pdfs/1216 (accessed on 15 November 2017).
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; Lexer, M.J. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Savolainen, O.; Bokma, F.; Knürr, T.; Kärkkäinen, K.; Pyhäjärvi, T.; Wachowiak, W. Adaptation of forest trees to climate change. In Proceedings of the Climate Change and Forest Genetic Diversity: Implications for Sustainable Forest Management in Europe, Paris, France, 15–16 March 2006; Biodiversity International: Rome, Italy, 2007; pp. 19–29. Available online: http://www.bioversityinternational.org/publications/Pdf/1216.pdf (accessed on 15 November 2017).
- Vilcan, A.; Mihalte, L.; Sestras, A.F.; Holonec, L.; Sestras, R.E. Genetic variation and potential genetic resources of several Romanian larch populations. Turk. J. Agric. For. 2017, 41, 82–91. [Google Scholar] [CrossRef]
- Masson, G. Autécologie des Essences Forestières; Editions TEC & DOC; Lavoisier: Paris, France, 2005; Volume 2, pp. 141–156. [Google Scholar]
- Matras, J.; Pâques, L. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Larch (Larix decidua); Biodiversity International: Rome, Italy, 2008; p. 6. [Google Scholar]
- Larcher, W. Physiological Plant Ecology; Springer: Berlin, Germany, 2003. [Google Scholar]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants, Volume 1. Targeting Sensory, Transport and Signaling Mechanisms; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.-S.P., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 85–136. [Google Scholar]
- Sun, Z.W.; Ren, L.K.; Fan, J.W.; Li, Q.; Wang, K.J.; Guo, M.M.; Wang, L.; Li, J.; Zhang, G.X.; Yang, Z.Y.; et al. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ. 2016, 62, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Cruz, E.; Zavala-García, F.; Picón-Rubio, F.; Urías-Orona, V.; Rodríguez-Fuentes, H.; Vidales-Contreras, J.; Carranza-De La Rosa, R.; Niño-Medina, G. Water stress effect on cell wall components of maize (Zea mays) Bran. Not. Sci. Biol. 2016, 8, 81–84. [Google Scholar] [CrossRef]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: a new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tong, J.; He, X.; Xu, Z.; Xu, L.; Wei, P.; Huang, Y.; Brestic, M.; Ma, H.; Shao, H. A novel soybean intrinsic protein gene, GmTIP2;3, involved in responding to osmotic stress. Front. Plant Sci. 2016, 6, 1237. [Google Scholar] [CrossRef] [PubMed]
- Fardus, J.; Matin, M.; Hasanuzzaman, M.; Hossain, M.; Nath, S.; Hossain, M.; Rohman, M.; Hasanuzzaman, M. Exogenous salicylic acid-mediated physiological responses and improvement in yield by modulating antioxidant defense system of wheat under salinity. Not. Sci. Biol. 2017, 9, 219–232. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.H.; Murata, N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.M.; Chandrasekhar, T.; Hazara, M.; Sultan, Z.; Saleh, B.K.; Gopal, G.R. Recent advances in salt stress biology—A review. Biotechnol. Mol. Biol. Rev. 2008, 3, 8–13. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef] [Green Version]
- Esfandiari, E.; Gohari, G. Response of ROS-scavenging systems to salinity stress in two different wheat (Triticum aestivum L.) cultivars. Not. Bot. Horti Agrobo. 2017, 45, 287–291. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; California Agricultural Experiment Station Publications Series; College of Agriculture, University of California: Davis, CA, USA, 1950. [Google Scholar]
- Shiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Identification of salt stress biomarkers in Romanian Carpathian populations of Picea abies (L.) Karst. PLoS ONE 2015, 10, e0135419. [Google Scholar] [CrossRef] [PubMed]
- Lichenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Palazzodemello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Conell, J.P.; Mullet, J.E. Pea chloroplast glutathione reductase: Purification and characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hand, M.; Taffouo, V.; Nouck, A.; Nyemene, K.; Tonfack, B.; Meguekam, T.; Youmbi, E. Effects of salt stress on plant growth, nutrient partitioning, chlorophyll content, leaf relative water content, accumulation of osmolytes and antioxidant compounds in pepper (Capsicum annuum L.) cultivars. Not. Bot. Horti Agrobot. 2017, 45, 481–490. [Google Scholar] [CrossRef]
- Badalotti, A.; Anfodillo, T.; Grace, J. Evidence of osmoregulation in Larix decidua at Alpine treeline and comparative responses to water availability of two co-occurring evergreen species. Ann. Forest Sci. 2000, 57, 623–633. [Google Scholar] [CrossRef]
- Gower, S.T.; Richards, J.H. Larches: Deciduous conifers in an evergreen world. Bioscience 1990, 40, 818–826. [Google Scholar] [CrossRef]
- Schuster, R.; Oberhuber, W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees 2013, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Schiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, E.; Vicente, O. Biochemical responses to drought, at the seedling stage, of several Romanian Carpathian populations of Norway spruce (Picea abies L. Karst). Trees 2017, 31, 1479–1490. [Google Scholar] [CrossRef]
- Renault, S. Tamarack response to salinity: Effects of sodium chloride on growth and ion, pigment, and soluble carbohydrate levels. Can. J. For. Res. 2005, 35, 2806–2812. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hort. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Alonso, R.; Elvira, S.; Castillo, F.J.; Gimeno, B.S. Interactive effects of ozone and drought stress on pigments and activities of antioxidative enzymes in Pinus halepensis. Plant Cell Environ. 2001, 24, 905–916. [Google Scholar] [CrossRef]
- Croser, C.; Renault, S.; Franklin, J.; Zwiazek, J. The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca and Pinus banksiana. Environ. Pollut. 2001, 115, 9–16. [Google Scholar] [CrossRef]
- Miron, M.S.; Sumalan, R.L. Physiological responses of Norway spruce (Picea abies [L.] Karst) seedlings to drought and overheating stress conditions. J. Hortic. For. Biotechnol. 2015, 19, 146–151. [Google Scholar]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Eker, S.; Cömertpay, G.; Konuşkan, Ö.; Ülger, A.C.; Öztürk, L.; Çarmak, İ. Effect of salinity stress on dry matter production and ion accumulation in hybrid maize varieties. Turk. J. Agric. For. 2006, 30, 365–373. [Google Scholar]
- Gu, M.F.; Li, N.; Shao, T.Y.; Long, X.H.; Brestič, M.; Shao, H.B.; Li, J.B.; Mbarki, S. Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil Environ. 2016, 62, 314–320. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Sun, J.; Pan, Z.; Gong, W.; Lu, Y.; Du, X. Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci. Rep. 2016, 6, 34548. [Google Scholar] [CrossRef] [PubMed]
- Bogemans, J.; Neirinckx, L.; Stassart, J.M. Effect of de-icing chloride salts on ion accumulation in spruce (Picea abies (L.) sp.). Plant Soil 1989, 113, 3–11. [Google Scholar] [CrossRef]
- Maynard, D.G.; Mallett, K.I.; Myrholm, C.L. Sodium carbonate inhibits emergence and growth of greenhouse-grown white spruce. Can. J. Soil Sci. 1996, 77, 99–105. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.P.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-induced variation in biochemical markers provides insight into the mechanisms of salt tolerance in common (Phaseolus vulgaris) and runner (P. coccineus) beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; López-Gresa, M.P.; Boscaiu, M.; Vicente, O. Stress tolerance mechanisms in Juncus: Responses to salinity and drought in three Juncus species adapted to different natural environments. Funct. Plant Biol. 2016, 43, 949–960. [Google Scholar] [CrossRef]
- Flowers, T.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in non-halophytes. Annu. Rev. Plant Biol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Lauchli, A.; Wieneke, J. Studies on growth and distribution of Na+, K+ and Cl- in soybean varieties differing in salt tolerance. Z. Pflanzenernaehr. Bodenk. 1979, 142, 3–13. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; Sen, S.; Puryear, J.D. Free proline changes in Pinus taeda L. callus in response to drought stress. Tree Physiol. 1986, 1, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Hakimi, M.H.; Mosleh Arany, A.; Kiani, B.; Rashtian, A. Comparing the effects of SNP and SA under salinity stress on proline, sugar, Na, K and chlorophyll of leaves of Pinus eldarica and Cupressus sempervirens in Iran. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 91–95. [Google Scholar]
- Taïbi, K.; del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought Tolerance in Pinus halepensis seed sources as identified by distinctive physiological and molecular markers. Front. Plant Sci. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Ditmarová, L.; Kurjak, D.; Palmroth, S.; Kmet, J.; Strelcová, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2010, 30, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bonet, A.; Lelu-Walter, M.A.; Faugeron, C.; Gloaguen, V.; Saladin, G. Physiological responses of the hybrid larch (Larix × eurolepis Henry) to cadmium exposure and distribution of cadmium in plantlets. Environ. Sci. Pollut. Res. Int. 2016, 23, 8617–8626. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, D.; Lelu-Walter, M.A.; Parkinson, M. Overproduction of proline in transgenic hybrid larch (Larix × leptoeuropaea (Dengler)) cultures renders them tolerant to cold, salt and frost. Mol. Breed. 2005, 15, 21–29. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher-plants. Annu. Rev. Plant Physiol. Mol. Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. The effect of the salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J. Arid Environ. 2000, 45, 73–84. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Pardo-Domènech, L.L.; Tifrea, A.; Grigore, M.N.; Boscaiu, M.; Vicente, O. Proline and glycine betaine accumulation in two succulent halophytes under natural and experimental conditions. Plant Biosyst. 2016, 150, 904–915. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.M.; Wagner, M.R.; Reich, P.B. Ecophysiology and insect herbivory. In Ecophysiology of Coniferous Forests; Smith, W.K., Hinckley, T.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 125–180. [Google Scholar]
- Rinne, K.T.; Saurer, M.; Kirdyanov, A.V.; Loader, N.J.; Bryukhanova, M.V.; Werner, R.A.; Siegwolf, R.T.W. The relationship between needle sugar carbon isotope ratios and tree rings of larch in Siberia. Tree Physiol. 2015, 35, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Boscaiu, M.; Lull, C.; Bautista, I.; Lidón, A.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805–818. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Patussi Brammer, S. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants. Recent Advances and Future Perspectives; Shanker, A., Ed.; InTechOpen: London, UK, 2016; pp. 463–480. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Biochemical Parameter | Control | WS | 150 mM NaCl |
|---|---|---|---|
| MDA (nmol g−1 DW) | 205.50 ± 10.68 a | 259.13 ± 6.39 b | 284.06 ± 11.70 b |
| TPC (mg eq. GA g−1 DW) | 4.29 ± 0.25 ab | 4.55 ± 0.15 b | 3.84 ± 0.13 a |
| TF (mg eq. C g−1 DW) | 3.18 ± 0.36 a | 3.54 ± 0.27 a | 3.16 ± 0.31 a |
| SOD (U mg−1 protein) | 56.72 ± 4.87 a | 81.59 ± 5.68 b | 131.81 ± 13.92 c |
| CAT (U mg−1 protein) | 32.32 ± 5.15 a | 43.03 ± 3.95 b | 87,27 ± 6.24 c |
| APX (U mg−1 protein) | 918.50 ± 96.18 a | 2419.61 ± 282.58 b | 3521.70 ± 225.17 c |
| GR (U mg−1 protein) | 45.64 ± 3.61 a | 59.86 ± 6.68 b | 86.34 ± 4.63 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plesa, I.M.; González-Orenga, S.; Al Hassan, M.; Sestras, A.F.; Vicente, O.; Prohens, J.; Sestras, R.E.; Boscaiu, M. Effects of Drought and Salinity on European Larch (Larix decidua Mill.) Seedlings. Forests 2018, 9, 320. https://doi.org/10.3390/f9060320
Plesa IM, González-Orenga S, Al Hassan M, Sestras AF, Vicente O, Prohens J, Sestras RE, Boscaiu M. Effects of Drought and Salinity on European Larch (Larix decidua Mill.) Seedlings. Forests. 2018; 9(6):320. https://doi.org/10.3390/f9060320
Chicago/Turabian StylePlesa, Ioana M., Sara González-Orenga, Mohamad Al Hassan, Adriana F. Sestras, Oscar Vicente, Jaime Prohens, Radu E. Sestras, and Monica Boscaiu. 2018. "Effects of Drought and Salinity on European Larch (Larix decidua Mill.) Seedlings" Forests 9, no. 6: 320. https://doi.org/10.3390/f9060320
APA StylePlesa, I. M., González-Orenga, S., Al Hassan, M., Sestras, A. F., Vicente, O., Prohens, J., Sestras, R. E., & Boscaiu, M. (2018). Effects of Drought and Salinity on European Larch (Larix decidua Mill.) Seedlings. Forests, 9(6), 320. https://doi.org/10.3390/f9060320










