Vector Competence of Italian Populations of Culicoides for Some Bluetongue Virus Strains Responsible for Recent Northern African and European Outbreaks
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Sites
2.2. Field Activities
2.3. Laboratory Activities
2.3.1. Virus Isolates
- BTV-2, strain 2000TE8341: It was isolated from the spleen of a Sardinian sheep that died because of BTV in 2000. The strain was passed once onto the Kc cell line and three times onto VERO cells (from African green monkey kidney) before use.
- BTV-4 ITA, strain 2014TE31172: It was isolated from the blood of a sheep infected with BTV in the Apulia region in 2014. The strain was passed once onto the Kc cell line and three times onto VERO cells before use.
- BTV-4 MOR, strain MOR2009-07: Kindly provided by the Pirbright Institute (Lara Harrup), it was isolated from a blood sample of a BTV-infected sheep in Morocco in 2009. This strain was passed three times onto the Kc cell line and twice onto VERO cells before use.
- BTV-8, strain UKG2007-82: Kindly provided by the Pirbright Institute (Lara Harrup), it was isolated from a blood sample of a bovine infected in the UK in 2007. The strain was passed three times onto the Kc cell line and twice onto VERO cells before use.
2.3.2. Culicoides Oral Infection
2.3.3. Species Identification
2.3.4. Virus Detection
2.4. Statistical Analysis
3. Results
3.1. Feeding Rate
3.2. Competence Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simón, F.; González-Miguel, J.; Diosdado, A.; Gómez, P.J.; Morchón, R.; Kartashev, V. The complexity of zoonotic filariasis episystem and its consequences: A multidisciplinary view. BioMed. Res. Int. 2017, 2017, 6436130. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Conte, A.; Cocciolito, R.; Meiswinkel, R. Distribuzione e abbondanza di Culicoides imicola in Italia. Vet. Ital. 2003, 39, 22–32. [Google Scholar]
- Goffredo, M.; Catalani, M.; Federici, V.; Portanti, O.; Marini, V.; Mancini, G.; Quaglia, M.; Santilli, A.; Teodori, L.; Savini, G. Vector species of Culicoides midges implicated in the 2012–2014 Bluetongue epidemics in Italy. Vet. Ital. 2015, 51, 131–138. [Google Scholar] [CrossRef]
- Goffredo, M.; Meiswinkel, R.; Federici, V.; Di Nicola, F.; Mancini, G.; Ippoliti, C.; Di Lorenzo, A.; Quaglia, M.; Santilli, A.; Conte, A.; et al. The “Culicoides obsoletus group” in Italy: Relative abundance, geographic range, and role as vector for Bluetongue virus. Vet. Ital. 2016, 52, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Culicoides variipennis and bluetongue-virus epidemiology in the United States. Annu. Rev. Entomol. 1996, 41, 23–43. [Google Scholar] [CrossRef]
- Conte, A.; Goffredo, M.; Ippoliti, C.; Meiswinkel, R. Influence of biotic and abiotic factors on the distribution and abundance of Culicoides imicola and the Obsoletus Complex in Italy. Vet. Parasitol. 2007, 150, 333–344. [Google Scholar] [CrossRef]
- Calistri, P.; Giovannini, A.; Conte, A.; Nannini, D.A.O.; Santucci, U.; Patta, C.; Rolesu, S.; Caporale, V. Bluetongue in Italy: Part I. Vet. Ital. 2004, 40, 243–251. [Google Scholar]
- Foxi, C.; Delrio, G.; Falchi, G.; Marche, M.G.; Satta, G.; Ruiu, L. Role of different Culicoides vectors (Diptera: Ceratopogonidae) in bluetongue virus transmission and overwintering in Sardinia (Italy). Parasites Vectors 2016, 9, 440. [Google Scholar] [CrossRef]
- Savini, G.; Goffredo, M.; Monaco, F.; Di Gennaro, A.; Cafiero, M.A.; Baldi, L.; De Santis, P.; Meiswinkel, R.; Caporale, V. Bluetongue virus isolations from midges belonging to the Obsoletus complex (Culicoides, Diptera: Ceratopogonidae) in Italy. Vet. Rec. 2005, 157, 133. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); More, S.; Bicout, D.; Bøtner, A.; Butterworth, A.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Gortázar, S.C.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Bluetongue. Efsa J. 2017, 15, e04957. [Google Scholar] [CrossRef]
- Lorusso, A.; Guercio, A.; Purpari, G.; Cammà, C.; Calistri, P.; D’Alterio, N.; Hammami, S.; Sghaier, S.; Savini, G. Bluetongue virus serotype 3 in Western Sicily, November 2017. Vet. Ital. 2017, 53, 273–275. [Google Scholar] [PubMed]
- Lhor, Y.; Kyriaki, N.; Khayli, M.; Bouslikhane, M.; Fassi Fihri, O.; El Harrak, M. Bluetongue in Morocco 2004 to 2015: An overview. J. Infect. Dis. Epidemiol. 2017, 3, 23. [Google Scholar] [CrossRef]
- Lhor, Y.; Khayli, M.; Bouslikhane, M.; El Harrak, M.; Fihri, O.F. Spatial and seasonal distribution of Culicoides species in Morocco in relation to the transmission of Bluetongue Viruses. Br. J. Virol. 2015, 2, 88–95. [Google Scholar] [CrossRef]
- Goffredo, M.; Romeo, G.; Monaco, F.; Di Gennaro, A.; Savini, G. Laboratory survival and blood feeding response of wild-caught Culicoides obsoletus Complex (Diptera: Ceratopogonidae) through natural and artificial membranes. Vet. Ital. 2004, 40, 282–285. [Google Scholar]
- Jennings, D.M.; Mellor, P.S. The vector potential of British Culicoides species for bluetongue virus. Vet. Microbiol. 1988, 17, 1–10. [Google Scholar] [CrossRef]
- Mellor, P.S. Culicoides as potential orbivirus vectors in Europe. In Bluetongue, African Horse Sickness, and Related Orbiviruses: Proceedings of the Second International Symposium; CRC Press: Boca Raton, FL, USA, 1992; pp. 278–283. [Google Scholar]
- Paslaru, A.I.; Mathis, A.; Torgerson, P.; Veronesi, E. Vector competence of pre-alpine Culicoides (Diptera: Ceratopogonidae) for bluetongue virus serotypes 1, 4 and 8. Parasites Vectors 2018, 11, 466. [Google Scholar] [CrossRef]
- Carpenter, S.; McArthur, C.; Selby, R.; Ward, R.; Nolan, D.V.; Luntz, A.J.; Dallas, J.F.; Tripet, F.; Mellor, P.S. Experimental infection studies of UK Culicoides species midges with bluetongue virus serotypes 8 and 9. Vet. Rec. 2008, 163, 589. [Google Scholar] [CrossRef]
- Venter, G.J.; Paweska, J.T.; Lunt, H.; Mellor, P.S.; Carpenter, S. An alternative method of blood-feeding Culicoides imicola and other haematophagous Culicoides species for vector competence studies. Vet. Parasitol. 2005, 131, 331–335. [Google Scholar] [CrossRef]
- Venter, G.J.; Paweska, J.T.; Van Dijk, A.A.; Mellor, P.S.; Tabachnick, W.J. Vector competence of Culicoides bolitinos and C. imicola for South African bluetongue virus serotypes 1, 3 and 4. Med. Vet. Entomol. 1998, 12, 378–385. [Google Scholar] [CrossRef]
- Venter, G.J.; Gerdes, G.H.; Mellor, P.S.; Paweska, J.T. Transmission potential of South African Culicoides species for live-attenuated bluetongue virus. Vet. Ital. 2004, 40, 199. [Google Scholar]
- Venter, G.J.; Mellor, P.S.; Paweska, J.T. Oral susceptibility of South African stock-associated Culicoides species to bluetongue virus. Med. Vet. Entomol. 2006, 20, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.J.; Wright, I.M.; Del Rio, R.; Lucientes, J.; Miranda, M.A. The susceptibility of Culicoides imicola and other South African livestock-associated Culicoides species to infection with bluetongue virus serotype 8. Med. Vet. Entomol. 2011, 25, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T.; Prinsloo, S.; Venter, G.J. Oral susceptibility of South African Culicoides species to live-attenuated serotype-specific vaccine strains of African horse sickness virus (AHSV). Med. Vet. Entomol. 2003, 17, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, E.; Antony, F.; Gubbins, S.; Golding, N.; Blackwell, A.; Mertens, P.P.; Brownlie, J.; Darpel, K.E.; Mellor, P.S.; Carpenter, S. Measurement of the infection and dissemination of bluetongue virus in Culicoides biting midges using a semi-quantitative rt-PCR assay and isolation of infectious virus. PLoS ONE 2013, 8, e70800. [Google Scholar] [CrossRef][Green Version]
- Veronesi, E.; Henstock, M.; Gubbins, S.; Batten, C.; Manley, R.; Barber, J.; Hoffmann, B.; Beer, M.; Attoui, H.; Mertens, P.P.; et al. Implicating Culicoides biting midges as vectors of Schmallenberg virus using semi-quantitative RT-PCR. PLoS ONE 2013, 8, e57747. [Google Scholar] [CrossRef]
- Barber, J.; Harrup, L.E.; Silk, R.; Veronesi, E.; Gubbins, S.; Bachanek-Bankowska, K.; Carpenter, S. Blood-feeding, susceptibility to infection with Schmallenberg virus and phylogenetics of Culicoides (Diptera: Ceratopogonidae) from the United Kingdom. Parasites Vectors 2018, 11, 116. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Delécolle, J.C. Nouvelle contribution à l’étude systématique et iconographique des espèces du genre Culicoides (Diptera: Ceratopogonidae) du Nord-Est de la France. Ph.D. Thesis, Université Louis Pasteur de Strasbourg, Strasbourg, France, 1985. [Google Scholar]
- Campbell, J.A.; Pelham-Clinton, E.C. X.—A taxonomic review of the British species of Culicoides Latreille (Diptera: Ceratopogonidae). Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1960, 67, 181–302. [Google Scholar] [CrossRef]
- Goffredo, M.; Meiswinkel, R. Entomological surveillance of bluetongue in Italy: Methods of capture, catch analysis and identification of Culicoides biting midges. Vet. Ital. 2004, 40, 260–265. [Google Scholar]
- Gomulski, L.M.; Meiswinkel, R.; Delécolle, J.C.; Goffredo, M.; Gasperi, G. Phylogenetic relationships of the subgenus Avaritia Fox, 1955 including Culicoides obsoletus (Diptera, Ceratopogonidae) in Italy based on internal transcribed spacer 2 ribosomal DNA sequences. Syst. Entomol. 2005, 30, 619–631. [Google Scholar] [CrossRef]
- Sarvašová, A.; Goffredo, M.; Sopoliga, I.; Savini, G.; Kočišová, A. Culicoides midges (Diptera: Ceratopogonidae) as vectors of orbiviruses in Slovakia. Vet. Ital. 2014, 50, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Griot, C.; Renzullo, S.; Mader, M.; Chaignat, V.; Worwa, G.; Thuer, B. Genetic characterization of toggenburg orbivirus, a new bluetongue virus, from goats, Switzerland. Emerg. Infect. Dis. 2008, 14, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Leake, C.J.; Mertens, P.P.C.; Mellor, P.S. The barriers to bluetongue virus infection, dissemination and transmission in the vector, Culicoides variipennis (Diptera: Ceratopogonidae). Arch. Virol. 1999, 144, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Sivia, D.S. Data Analysis: A Bayesian Tutorial; Clarendon Press: Oxford, UK, 1996; pp. 106–110. [Google Scholar]
- Carpenter, S.; Lunt, H.L.; Arav, D.; Venter, G.J.; Mellor, P.S. Oral susceptibility to bluetongue virus of Culicoides (Diptera: Ceratopogonidae) from the United Kingdom. J. Med. Entomol. 2006, 43, 73–78. [Google Scholar] [CrossRef]
- Paweska, J.T.; Venter, G.J.; Hamblin, C. A comparison of the susceptibility of Culicoides imicola and C. bolitinos to oral infection with eight serotypes of epizootic haemorrhagic disease virus. Med. Vet. Entomol. 2005, 19, 200–207. [Google Scholar] [CrossRef]
- Savini, G.; Monaco, F.; Facchinei, A.; Pinoni, C.; Salucci, S.; Cofini, F.; Di Ventura, M. Field vaccination of sheep with bivalent modified-live vaccine against bluetongue virus serotypes 2 and 9: Effect on milk production. Vet. Ital. 2004, 40, 627–630. [Google Scholar]
- Savini, G.; Monaco, F.; Citarella, R.; Calzetta, G.A.O.; Panichi, G.; Ruiu, A.; Caporale, V. Monovalent modified-live vaccine against bluetongue virus serotype 2: Immunity studies in cows. Vet. Ital. 2004, 40, 664–667. [Google Scholar]
- Mills, M.K.; Michel, K.; Pfannenstiel, R.S.; Ruder, M.G.; Veronesi, E.; Nayduch, D. Culicoides–virus interactions: Infection barriers and possible factors underlying vector competence. Curr. Opin. Insect. Sci. 2017, 22, 7–15. [Google Scholar] [CrossRef]
- Nayduch, D.; Erram, D.; Lee, M.B.; Zurek, L.; Saski, C.A. Impact of the blood meal on humoral immunity and microbiota in the gut of female Culicoides sonorensis. Vet. Ital. 2015, 51, 385–392. [Google Scholar] [CrossRef]
- Del Rio Lopez, R.; Miranda, M.A.; Paredes-Esquivel, C.; Lucientes, J.; Calvete, C.; Estrada, R.; Venter, G.J. Recovery rates of bluetongue virus serotypes 1, 2, 4 and 8 Spanish strains from orally infected Culicoides imicola in South Africa. Med. Vet. Entomol. 2012, 26, 162–167. [Google Scholar] [CrossRef]
- Savini, G.; MacLachlan, N.J.; Sanchez-Vizcaino, J.M.; Zientara, S. Vaccines against bluetongue in Europe. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 101–120. [Google Scholar] [CrossRef] [PubMed]

| BTV-4 ITA | Fed via Hemotek | Fed via Cotton Pledget | |||||||
| SPECIES | SITE | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi |
| Culicoides obsoletus/scoticus | Abruzzo | 5 | 9139 | 0.05 | 4 | 566 | 3923 | 14.43 | 190 |
| Culicoides obsoletus/scoticus | Lazio | 229 | 752 | 30.45 | 186 | ||||
| Culicoides imicola | Sardinia | 1107 | 54,471 | 2.03 | 359 | 330 | 2078 | 15.88 | 258 |
| Culicoides imicola | Calabria | 0 | 2586 | 0 | 0 | 400 | 2196 | 18.21 | 269 |
| BTV-2 | Fed via Hemotek | Fed via Cotton Pledget | |||||||
| SPECIES | SITE | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi |
| Culicoides obsoletus/scoticus | Abruzzo | 4 | 4131 | 0.1 | 1 | 447 | 4589 | 9.74 | 226 |
| Culicoides obsoletus/scoticus | Lazio | 173 | 516 | 33.53 | 75 | ||||
| Culicoides imicola | Sardinia | 719 | 71,843 | 1 | 257 | 351 | 3376 | 10.40 | 228 |
| Culicoides imicola | Calabria | 0 | 1213 | 0 | 0 | 337 | 2426 | 13.89 | 219 |
| BTV-8 | Fed via Hemotek | Fed via Cotton Pledget | |||||||
| SPECIES | SITE | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi |
| Culicoides obsoletus/scoticus | Abruzzo | 174 | 484 | 35.95 | 110 | ||||
| Culicoides obsoletus/scoticus | Lazio | 77 | 185 | 41.62 | 61 | ||||
| Culicoides imicola | Sardinia | 190 | 57,318 | 0.33 | 107 | 153 | 1593 | 9.6 | 125 |
| Culicoides imicola | Calabria | 165 | 402 | 41.04 | 117 | ||||
| BTV-4 MOR | Fed via Hemotek | Fed via Cotton Pledget | |||||||
| SPECIES | SITE | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi | ENGORGED | TOTAL NUMBER | FEEDING RATE (%) | 10 dpi |
| Culicoides obsoletus/scoticus | Abruzzo | 168 | 720 | 23.33 | 126 | ||||
| Culicoides obsoletus/scoticus | Lazio | 87 | 278 | 31.29 | 64 | ||||
| Culicoides imicola | Sardinia | 178 | 26,863 | 0.66 | 120 | ||||
| Culicoides imicola | Calabria | 153 | 471 | 32.48 | 116 | ||||
| Culicoides obsoletus/scoticus | SUBTOTAL | 9 | 13,270 | 0.07 | 5 | 1921 | 11,447 | 16.78 | 1038 |
| Culicoides imicola | SUBTOTAL | 2194 | 214,294 | 1.02 | 843 | 1889 | 12,542 | 15.06 | 1332 |
| TOTAL | 2203 | 227,564 | 0.97 | 848 | 3810 | 23,989 | 15.88 | 2370 | |
| Virus | Minimum–Maximum Virus Titer TCID50 mL (Number of Blood Meals) |
|---|---|
| BTV-4 ITA | 105.3–106.54 (20) |
| BTV-2 | 105.01–106.14 (22) |
| BTV-8 | 105–106.3 (16) |
| BTV-4 MOR | 105.68–106.39 (15) |
| Serogroup-Specific RT PCR (Positive Midges Ct < 50) | Serotype-Specific RT PCR (Positive Midges Ct < 40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feeding Method | BTV Strain | Population | Number of Tested | Number of Positive | Recovery Rate (%) | Ct Mean (Min–Max) | Number of Positive | Recovery Rate (%) | Ct Mean (Min–Max) |
| cotton | 4 ITA | C. obsoletus/scoticus Abruzzo | 16 | 14 | 87.5 | 36 (31–44) | / | / | / |
| cotton | 4 ITA | C. obsoletus/scoticus Lazio | 11 | 11 | 100 | 38 (36–39) | 11 | 100 | 33 (32–35) |
| Hemotek | 4 ITA | C. imicola Sardinia | 35 | 33 | 94.3 | 37 (35–42) | 33 | 94.3 | 33 (30–36) |
| cotton | 4 ITA | C. imicola Sardinia | 10 | 8 | 80 | 40 (38–42) | 8 | 80 | 35 (33–37) |
| cotton | 4 ITA | C. imicola Calabria | 15 | 15 | 100 | 39 (36–42) | 15 | 100 | 33 (31–36) |
| cotton | 2 | C. obsoletus/scoticus Abruzzo | 15 | 12 | 80 | 37 (33–42) | 1 | 6.7 | 39 (39–39) |
| cotton | 2 | C. obsoletus/scoticus Lazio | 11 | 11 | 100 | 38 (36–39) | 11 | 100 | 34 (33–35) |
| Hemotek | 2 | C. imicola Sardinia | 21 | 19 | 90.5 | 39 (36–43) | 18 | 85.7 | 34 (32–39) |
| cotton | 2 | C. imicola Sardinia | 14 | 7 | 50 | 43 (41–44) | 4 | 28.6 | 38 (36–39) |
| cotton | 2 | C. imicola Calabria | 12 | 12 | 100 | 40 (35–43) | 12 | 100 | 35 (32–38) |
| cotton | 8 | C. obsoletus/scoticus Abruzzo | 17 | 12 | 70.6 | 42 (39–47) | 7 | 41.2 | 35 (34–36) |
| cotton | 8 | C. obsoletus/scoticus Lazio | 7 | 4 | 57.1 | 41 (39–42) | 2 | 28.6 | 36 (34–37) |
| Hemotek | 8 | C. imicola Sardinia | 9 | / | / | / | 8 | 88.9 | 35 (32–37) |
| cotton | 8 | C. imicola Sardinia | 13 | / | / | / | 9 | 69.2 | 36 (35–37) |
| cotton | 8 | C. imicola Calabria | 15 | 13 | 86.7 | 39 (37–41) | 13 | 86.7 | 35 (33–37) |
| cotton | 4 MOR | C. obsoletus/scoticus Abruzzo | 13 | 10 | 76.9 | 42 (38–49) | 10 | 76.9 | 35 (33–38) |
| cotton | 4 MOR | C. obsoletus/scoticus Lazio | 8 | 7 | 87.5 | 40 (37–47) | 7 | 87.5 | 33 (31–35) |
| Hemotek | 4 MOR | C. imicola Sardinia | 6 | 6 | 100 | 36 (34–38) | 6 | 100 | 31 (29–32) |
| cotton | 4 MOR | C. imicola Calabria | 14 | 14 | 100 | 39 (36–41) | 13 | 92.9 | 34 (31–37) |
| Serogroup-Specific RT PCR (Positive Midges Ct < 50) | Serotype-Specific RT PCR (Positive Midges Ct < 40) | ||||||
|---|---|---|---|---|---|---|---|
| Feeding Method | BTV Strain | Population | Number of Tested | Number of Positive | Ct Mean (Min–Max) | Number of Positive | Ct Mean (Min–Max) |
| Hemotek | 4 ITA | C. obsoletus/scoticus Abruzzo | 4 | 0 | / | / | / |
| cotton | 4 ITA | C. obsoletus/scoticus Abruzzo | 190 | 9 | 40 (34–46) | 1 | 29 (29–29) |
| cotton | 4 ITA | C. obsoletus/scoticus Lazio | 186 | 18 | 41 (25–47) | 2 | 33 (27–39) |
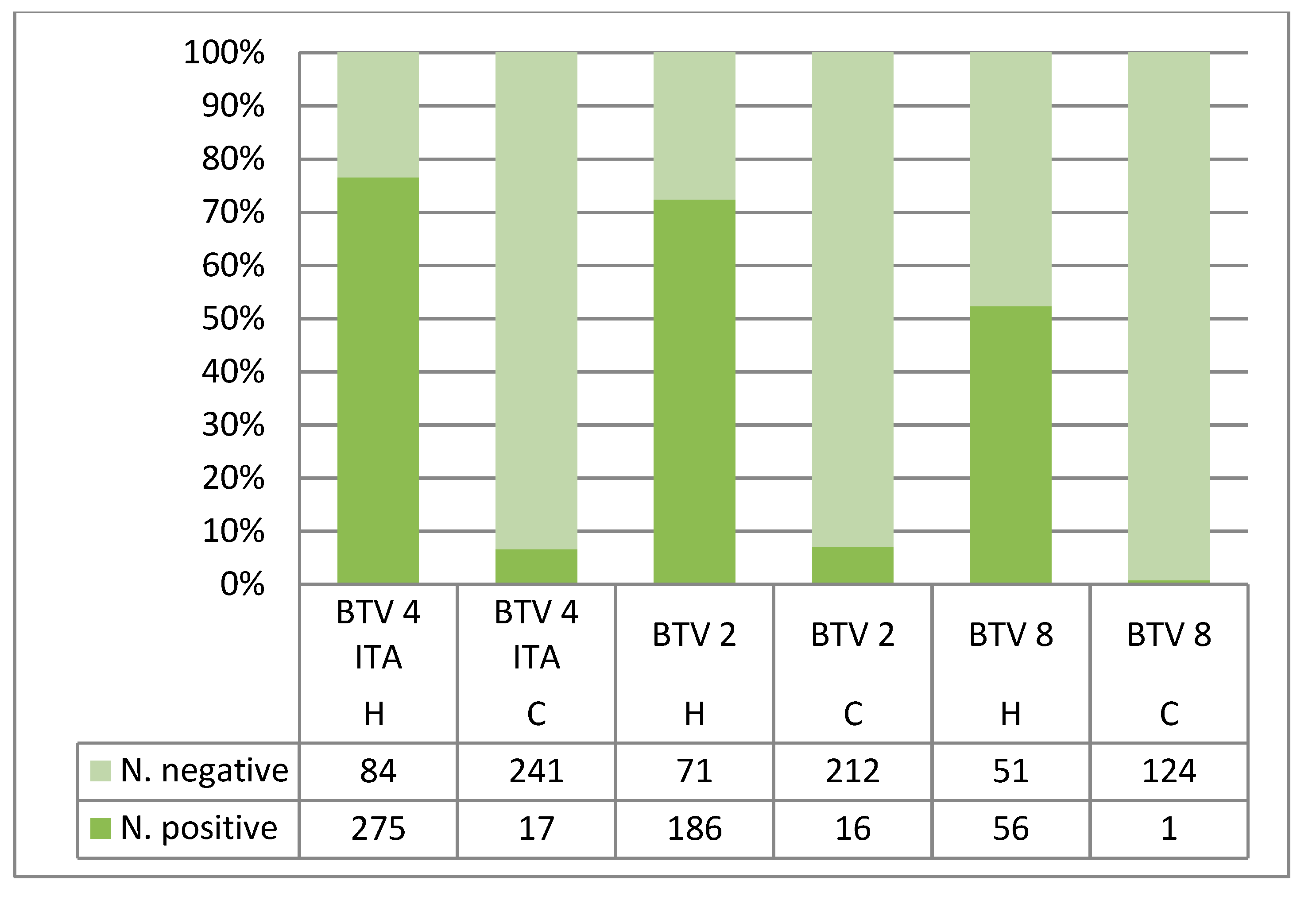
| Hemotek | 4 ITA | C. imicola Sardinia | 359 | 275 | 32 (24–48) | 253 | 30 (23–39) |
| cotton | 4 ITA | C. imicola Sardinia | 258 | 17 | 36 (27–41) | 9 | 33 (27–38) |
| cotton | 4 ITA | C. imicola Calabria | 269 | 15 | 40 (26–47) | 6 | 33 (24–37) |
| Hemotek | 2 | C. obsoletus/scoticus Abruzzo | 1 | 0 | / | / | / |
| cotton | 2 | C. obsoletus/scoticus Abruzzo | 226 | 6 | 38 (27–42) | 1 | 30 (30–30) |
| cotton | 2 | C. obsoletus/scoticus Lazio | 75 | 9 | 38 (33–40) | 2 | 36 (33–38) |
| Hemotek | 2 | C. imicola Sardinia | 257 | 186 | 32 (22–47) | 171 | 29 (23–39) |
| cotton | 2 | C. imicola Sardinia | 228 | 16 | 40 (32–45) | 12 | 35 (30–39) |
| cotton | 2 | C. imicola Calabria | 219 | 7 | 41 (28–45) | 2 | 32 (25–38) |
| cotton | 8 | C. obsoletus/scoticus Abruzzo | 110 | 4 | 43 (39–46) | 1 | 38 (38–38) |
| cotton | 8 | C. obsoletus/scoticus Lazio | 61 | 2 | 42 (39–44) | 0 | / |
| Hemotek | 8 | C. imicola Sardinia | 107 | / | / | 56 | 30 (27–38) |
| cotton | 8 | C. imicola Sardinia | 125 | / | / | 1 | 30 (30–30) |
| cotton | 8 | C. imicola Calabria | 117 | 12 | 40 (31–44) | 1 | 34 (34–34) |
| cotton | 4 MOR | C. obsoletus/scoticus Abruzzo | 126 | 0 | / | / | / |
| cotton | 4 MOR | C. obsoletus/scoticus Lazio | 64 | 4 | 40 (38–43) | 0 | / |
| Hemotek | 4 MOR | C. imicola Sardinia | 120 | 62 | 38 (31–45) | 59 | 32 (27–38) |
| cotton | 4 MOR | C. imicola Calabria | 116 | 8 | 38 (33–43) | 6 | 32 (29–39) |
| Serogroup-Specific RT-PCR | Serotype-Specific RT-PCR | ||||||
|---|---|---|---|---|---|---|---|
| Feeding Method | BTV Strain | Population | Number of Tested | Number of Positive with Ct < 50 (Recovery Rate%) | Number of Positive with Ct < Mean Ct at 0 dpi (Recovery Rate%) | Number of Positive with Ct < 40 (Recovery Rate%) | Number of Positive with Ct < Mean Ct at 0 dpi (Recovery Rate%) |
| cotton | 4 ITA | C. obsoletus/scoticus Abruzzo | 190 | 9 (4.7) | 1 (0.5) | 1 (0.5) | / |
| cotton | 4 ITA | C. obsoletus/scoticus Lazio | 186 | 18 (9.7) | 2 (1.1) | 2 (1.1) | 1 (0.5) |
| Hemotek | 4 ITA | C. imicola Sardinia | 359 | 275 (76.6) | 201 (56) | 253 (70.5) | 165 (46) |
| cotton | 4 ITA | C. imicola Sardinia | 258 | 17 (6.6) | 13 (5) | 9 (3.5) | 6 (2.3) |
| cotton | 4 ITA | C. imicola Calabria | 269 | 15 (5.6) | 4 (1.5) | 6 (2.2) | 1 (0.4) |
| cotton | 2 | C. obsoletus/scoticus Abruzzo | 226 | 6 (2.7) | 2 (0.9) | 1 (0.4) | 1 (0.4) |
| cotton | 2 | C. obsoletus/scoticus Lazio | 75 | 9 (12.0) | 2 (2.7) | 2 (2.7) | 1 (1.3) |
| Hemotek | 2 | C. imicola Sardinia | 257 | 186 (72.4) | 148 (57.6) | 171 (66.5) | 131 (51.4) |
| cotton | 2 | C. imicola Sardinia | 228 | 16 (7.0) | 11 (4.8) | 12 (5.3) | 10 (4.4) |
| cotton | 2 | C. imicola Calabria | 219 | 7 (3.2) | 2 (0.9) | 2 (0.9) | 1 (0.5) |
| cotton | 8 | C. obsoletus/scoticus Abruzzo | 110 | 4 (3.6) | 2 (1.8) | 1 (0.9) | 0 (0) |
| cotton | 8 | C. obsoletus/scoticus Lazio | 61 | 2 (3.3) | 1 (1.6) | 0 (0) | 0 (0) |
| Hemotek | 8 | C. imicola Sardinia | 107 | / | / | 56 (52.3) | 54 (50.5) |
| cotton | 8 | C. imicola Sardinia | 125 | / | / | 1 (0.8) | 1 (0.8) |
| cotton | 8 | C. imicola Calabria | 117 | 12 (10.3) | 2 (1.7) | 1 (0.9) | 1 (0.9) |
| cotton | 4 MOR | C. obsoletus/scoticus Abruzzo | 126 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| cotton | 4 MOR | C. obsoletus/scoticus Lazio | 64 | 4 (6.3) | 2 (3.1) | 0 (0) | 0 (0) |
| Hemotek | 4 MOR | C. imicola Sardinia | 120 | 62 (51.7) | 16 (13.3) | 59 (49.2) | 21 (17.5) |
| cotton | 4 MOR | C. imicola Calabria | 116 | 8 (6.9) | 5 (4.3) | 6 (5.2) | 4 (3.4) |
| Recovery Rate % (Confidence Intervals %) | ||||||
|---|---|---|---|---|---|---|
| RT-PCR | Population | Feeding Method | BTV-4 ITA | BTV-2 | BTV-8 | BTV-4 MOR |
| Serogroup-specific RT-PCR | C. obsoletus/scoticus Abruzzo | Cotton | 0.5 (0.1–2.9) | 0.9 (0.3–3.1) | 1.8 (0.6–6.4) | 0 (0–2.3) |
| C. obsoletus/scoticus Lazio | Cotton | 1.1 (0.3–3.8) | 2.7 (0.8–9.2) | 1.6 (0.4–8.7) | 3.1 (1–10.7) | |
| C. imicola Sardinia | Hemotek | 56 (50.8–61) | 57.6 (51.5–63.5) | / | 13.3 (8.4–20.6) | |
| Cotton | 5 (3–8.4) | 4.8 (2.7–8.4) | / | / | ||
| C. imicola Calabria | Cotton | 1.5 (0.6–3.7) | 0.9 (0.3–3.2) | 1.7 (0.5–6) | 4.3 (1.9–9.7) | |
| Serotype-specific RT-PCR | C. obsoletus/scoticus Abruzzo | Cotton | / | 0.4 (0.1–2.4) | 0 (0–2.7) | 0 (0–2.3) |
| C. obsoletus/scoticus Lazio | Cotton | 0.5 (0.1–2.9) | 1.3 (0.3–7.1) | 0 (0–4.7) | 0 (0–4.5) | |
| C. imicola Sardinia | Hemotek | 46 (40.9–51.1) | 51.4 (44.9–57) | 50.5 (41.1–59.8) | 17.5 (11.8–25.3) | |
| Cotton | 2.3 (1.1–5) | 4.4 (2.4–7.9) | 0.8 (0.2–4.3) | / | ||
| C. imicola Calabria | Cotton | 0.4 (0.1–2) | 0.5 (0.1–2.5) | 0.9 (0.2–4.6) | 3.4 (1.4–8.5) | |
| Dpi | BTV Strain | Site | Species | Number of Positive/Number of Tested | Recovery Rate % | Confidence Intervals % | CT Mean (Min–Max) |
|---|---|---|---|---|---|---|---|
| 0 | 4 ITA | Abruzzo | C. obsoletus | 5/6 | 83.3 | / | 37 (32–44) |
| C. scoticus | 8/9 | 88.9 | / | 34 (31–40) | |||
| C. obsoletus/scoticus * | 1/1 | 100 | / | 41 (41–41) | |||
| Lazio | C. obsoletus | 1/1 | 100 | / | 37 (37–37) | ||
| C. scoticus | 10/10 | 100 | / | 38 (36–39) | |||
| 2 | Abruzzo | C. obsoletus | 2/2 | 100 | / | 38 (33–42) | |
| C. scoticus | 10/12 | 83.3 | / | 37 (33–42) | |||
| Lazio | C. obsoletus | 2/2 | 100 | / | 38 (36–39) | ||
| C. scoticus | 9/9 | 100 | / | 38 (36–39) | |||
| 8 | Abruzzo | C. obsoletus | 6/8 | 75 | / | 42 (39–45) | |
| C. scoticus | 4/7 | 57.1 | / | 42 (39–47) | |||
| C. obsoletus/scoticus * | 2/2 | 100 | / | 41 (40–42) | |||
| Lazio | C. obsoletus | 6/6 | 100 | / | 41 (41–42) | ||
| C. scoticus | 1/1 | 100 | / | 39 (39–39) | |||
| 4 MOR | Abruzzo | C. obsoletus | 3/5 | 60 | / | 43 (40–48) | |
| C. scoticus | 7/8 | 87.5 | / | 42 (38–49) | |||
| Lazio | C. obsoletus | 3/4 | 75 | / | 39 (38–41) | ||
| C. scoticus | 4/4 | 100 | / | 40 (37–47) | |||
| 10 | 4 ITA | Abruzzo | C. obsoletus | 1/14 | 7.1 | 1.7–31.9 | 34 (34–34) |
| C. scoticus | 8/173 | 4.6 | 2.4–8.9 | 41 (37–46) | |||
| C. obsoletus/scoticus * | 0/3 | 0 | / | / | |||
| Lazio | C. obsoletus | 3/39 | 7.7 | 2.8–20.4 | 41 (38–43) | ||
| C. scoticus | 15/146 | 10.3 | 6.4–16.3 | 40 (25–47) | |||
| C. obsoletus/scoticus * | 0/1 | 0 | / | / | |||
| 2 | Abruzzo | C. obsoletus | 0/46 | 0 | 0.1–7.5 | / | |
| C. scoticus | 6/178 | 3.4 | 1.6–7.2 | 38 (27–42) | |||
| C. obsoletus/scoticus * | 0/2 | 0 | / | / | |||
| Lazio | C. obsoletus | 2/16 | 12.5 | 3.8–36.4 | 36 (33–39) | ||
| C. scoticus | 7/59 | 11.9 | 5.9–22.6 | 39 (37–40) | |||
| 8 | Abruzzo | C. obsoletus | 1/45 | 2.2 | 0.5–11.5 | 44 (44–44) | |
| C. scoticus | 3/63 | 4.8 | 1.7–13.1 | 42 (39–46) | |||
| C. obsoletus/scoticus * | 0/2 | 0 | / | / | |||
| Lazio | C. obsoletus | 2/46 | 4.3 | 1.3–14.5 | 42 (39–44) | ||
| C. scoticus | 0/15 | 0 | 0.2–20.6 | / | |||
| 4 MOR | Abruzzo | C. obsoletus | 0/22 | 0 | 0.1–14.8 | / | |
| C. scoticus | 0/103 | 0 | 0–3.5 | / | |||
| C. obsoletus/scoticus * | 0/1 | 0 | / | / | |||
| Lazio | C. obsoletus | 2/28 | 7.1 | 2.2–22.8 | 41 (38–43) | ||
| C. scoticus | 2/36 | 5.6 | 0.1–9.5 | 40 (39–41) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federici, V.; Goffredo, M.; Mancini, G.; Quaglia, M.; Santilli, A.; Di Nicola, F.; De Ascentis, M.; Cabras, P.; Volpicelli, C.; De Liberato, C.; et al. Vector Competence of Italian Populations of Culicoides for Some Bluetongue Virus Strains Responsible for Recent Northern African and European Outbreaks. Viruses 2019, 11, 941. https://doi.org/10.3390/v11100941
Federici V, Goffredo M, Mancini G, Quaglia M, Santilli A, Di Nicola F, De Ascentis M, Cabras P, Volpicelli C, De Liberato C, et al. Vector Competence of Italian Populations of Culicoides for Some Bluetongue Virus Strains Responsible for Recent Northern African and European Outbreaks. Viruses. 2019; 11(10):941. https://doi.org/10.3390/v11100941
Chicago/Turabian StyleFederici, Valentina, Maria Goffredo, Giuseppe Mancini, Michela Quaglia, Adriana Santilli, Francesca Di Nicola, Matteo De Ascentis, Pierangela Cabras, Carmela Volpicelli, Claudio De Liberato, and et al. 2019. "Vector Competence of Italian Populations of Culicoides for Some Bluetongue Virus Strains Responsible for Recent Northern African and European Outbreaks" Viruses 11, no. 10: 941. https://doi.org/10.3390/v11100941
APA StyleFederici, V., Goffredo, M., Mancini, G., Quaglia, M., Santilli, A., Di Nicola, F., De Ascentis, M., Cabras, P., Volpicelli, C., De Liberato, C., Satta, G., Federico, G., Leone, A., Pisciella, M., Portanti, O., Pizzurro, F., Teodori, L., & Savini, G. (2019). Vector Competence of Italian Populations of Culicoides for Some Bluetongue Virus Strains Responsible for Recent Northern African and European Outbreaks. Viruses, 11(10), 941. https://doi.org/10.3390/v11100941






