Orthopoxvirus Seroprevalence in Cats and Veterinary Personnel in North-Eastern Italy in 2011
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Challenge Virus Stock Preparation
2.3. Serologic Tests
2.4. Statistical Analysis
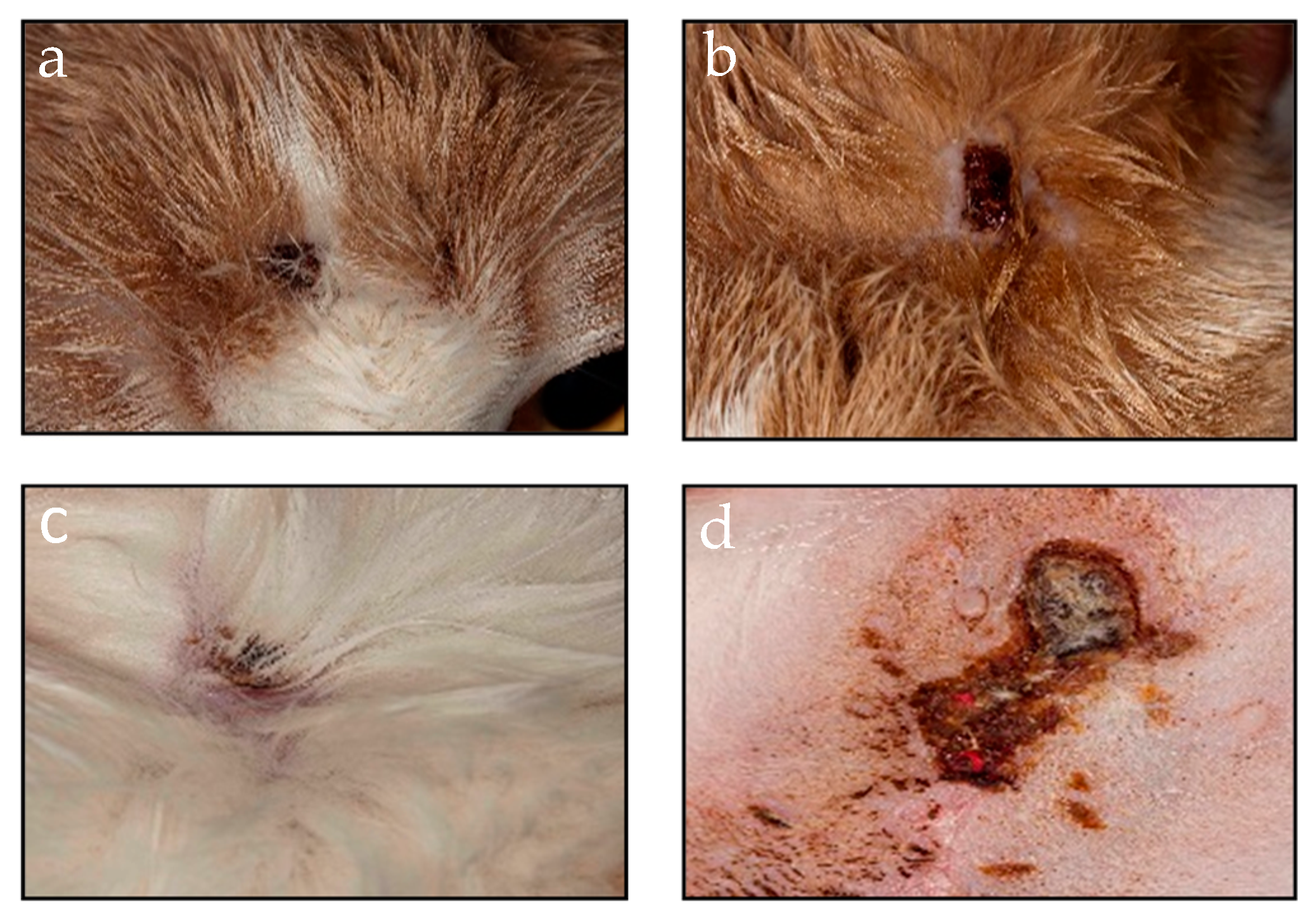
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Puro, V.; Fusco, F.M.; Castilletti, C.; Carletti, F.; Colavita, F.; Agrati, C.; Di Caro, A.; Capobianchi, M.R.; Ippolito, G. Occupational transmission of an Orthopoxvirus infection during an outbreak in a colony of Macaca tonkeana in Lazio Region, Italy, 2015. Zoonoses Public Health 2018, 65, 578–583. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Emergencies Preparedness, Response Monkeypox—Nigeria. Disease Outbreak News. 5 October 2018. Available online: http://www.who.int/csr/don/05-october-2018-monkeypox-nigeria/en/ (accessed on 12 November 2018).
- Public Health England. Monkeypox Case in England. Available online: https://www.gov.uk/government/news/monkeypox-case-in-england (accessed on 12 November 2018).
- Center for Infectious Disease Research and Policy (CIDRAP). News Scan for Oct 12, 2018. Israel Confirms First Imported Monkeypox Case. Available online: http://www.cidrap.umn.edu/news-perspective/2018/10/news-scan-oct-12-2018 (accessed on 12 November 2018).
- Glatz, M.; Richter, S.; Ginter-Hanselmayer, G.; Aberer, W.; Müllegger, R.R. Human cowpox in a veterinary student. Lancet Infect. Dis. 2010, 10, 288. [Google Scholar] [CrossRef]
- Kinnunen, P.M.; Henttonen, H.; Hoffmann, B.; Kallio, E.R.; Korthase, C.; Laakkonen, J.; Niemimaa, J.; Palva, A.; Schlegel, M.; Ali, H.S.; et al. Orthopox virus infections in Eurasian wild rodents. Vector Borne Zoonotic Dis. 2011, 11, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Vorou, R.M.; Papavassiliou, V.G.; Pierroutsakos, I.N. Cowpox virus infection: An emerging health threat. Curr. Opin. Infect. Dis. 2008, 21, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Duraffour, S.; Mertens, B.; Meyer, H.; van den Oord, J.J.; Mitera, T.; Matthys, P.; Snoeck, R.; Andrei, G. Emergence of cowpox: Study of the virulence of clinical strains and evaluation of antivirals. PLoS ONE 2013, 8, e55808. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed]
- Von Bomhard, W.; Mauldin, E.A.; Breuer, W.; Pfleghaar, S.; Nitsche, A. Localized cowpox infection in a 5-month-old Rottweiler. Vet. Dermatol. 2011, 22, 111–114. [Google Scholar] [CrossRef]
- Ninove, L.; Domart, Y.; Vervel, C.; Voinot, C.; Salez, N.; Raoult, D.; Meyer, H.; Capek, I.; Zandotti, C.; Charrel, R.N. Cowpox virus transmission from pet rats to humans, France. Emerg. Infect. Dis. 2009, 15, 781–784. [Google Scholar] [CrossRef]
- Kurth, A.; Wibbelt, G.; Gerber, H.P.; Petschaelis, A.; Pauli, G.; Nitsche, A. Rat-to-elephant-to-human transmission of cowpox virus. Emerg. Infect. Dis. 2008, 14, 670–671. [Google Scholar] [CrossRef]
- Campe, H.; Zimmermann, P.; Glos, K.; Bayer, M.; Bergemann, H.; Dreweck, C.; Graf, P.; Weber, B.K.; Meyer, H.; Büttner, M.; et al. Cowpox virus transmission from pet rats to humans, Germany. Emerg. Infect. Dis. 2009, 15, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Kurth, A.; Hessler, F.; Kramp, H.; Gokel, M.; Hoffmann, R.; Kuczka, A.; Nitsche, A. Cowpox virus infection in pet rat owners: Not always immediately recognized. Dtsch. Arztebl. Int. 2009, 106, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Sárdy, M.; Glos, K.; Korting, H.C.; Ruzicka, T.; Wollenberg, A. The Munich outbreak of cutaneous cowpox infection: Transmission by infected pet rats. Acta Derm. Venereol. 2012, 92, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Tack, D.M.; Reynolds, M.G. Zoonotic Poxviruses Associated with Companion Animals. Animals 2011, 1, 377–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantrey, J.; Meyer, H.; Baxby, D.; Begon, M.; Bown, K.J.; Hazel, S.M.; Jones, T.; Montgomery, W.I.; Bennett, M. Cowpox: Reservoir hosts and geographic range. Epidemiol. Infect. 1999, 122, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Gaskell, R.M.; Gaskell, C.J.; Baxby, D.; Kelly, D.F. Studies on poxvirus infection in cats. Arch. Virol. 1989, 104, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, J.; Kallio-Kokko, H.; Oktem, M.A.; Blasdell, K.; Plyusnina, A.; Niemimaa, J.; Karataş, A.; Plyusnin, A.; Vaheri, A.; Henttonen, H. Serological survey for viral pathogens in Turkish rodents. J. Wild Dis. 2006, 42, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Essbauer, S.; Hartnack, S.; Misztela, K.; Kiessling-Tsalos, J.; Bäumler, W.; Pfeffer, M. Patterns of orthopox virus wild rodent hosts in South Germany. Vector Borne Zoonotic Dis. 2009, 9, 301–311. [Google Scholar] [CrossRef]
- Oldal, M.; Sironen, T.; Henttonen, H.; Vapalahti, O.; Madai, M.; Horváth, G.; Dallos, B.; Kutas, A.; Földes, F.; Kemenesi, G.; et al. Serologic survey of orthopoxvirus infection among rodents in hungary. Vector Borne Zoonotic Dis. 2015, 15, 317–322. [Google Scholar] [CrossRef]
- Thomsett, L.R.; Baxby, D.; Denham, E.M. Cowpox in the domestic cat. Vet. Rec. 1978, 103, 567. [Google Scholar] [CrossRef]
- Appl, C.; von Bomhard, W.; Hanczaruk, M.; Meyer, H.; Bettenay, S.; Mueller, R. Feline cowpoxvirus infections in Germany: Clinical and epidemiological aspects. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 55–61. [Google Scholar] [PubMed]
- Carletti, F.; Bordi, L.; Castilletti, C.; Di Caro, A.; Falasca, L.; Gioia, C.; Ippolito, G.; Zaniratti, S.; Beltrame, A.; Viale, P.; et al. Cat-to-human orthopoxvirus transmission, northeastern Italy. Emerg. Infect. Dis. 2009, 15, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Lanave, G.; Dowgier, G.; Decaro, N.; Albanese, F.; Brogi, E.; Parisi, A.; Losurdo, M.; Lavazza, A.; Martella, V.; Buonavoglia, C.; et al. Novel Orthopoxvirus and Lethal Disease in Cat, Italy. Emerg. Infect. Dis. 2018, 24, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Pfaff, F.; Jenckel, M.; Hoffmann, B.; Höper, D.; Antwerpen, M.; Meyer, H.; Beer, M.; Hoffmann, D. Classification of Cowpox Viruses into Several Distinct Clades and Identification of a Novel Lineage. Viruses 2017, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, M.R.; Antwerpen, M.; Emerson, G.L.; Li, Y.; Zoeller, G.; Carroll, D.S.; Meyer, H. Cowpox virus: What’s in a Name? Viruses 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Pfleghaar, S.; von, B.D.; Kaaden, O.R.; Meyer, H. Retrospective investigation of feline cowpox in Germany. Vet. Rec. 2002, 150, 50–51. [Google Scholar] [CrossRef]
- Czerny, C.P.; Wagner, K.; Gessler, K.; Mayr, A.; Kaaden, O.R. A monoclonal blocking-ELISA for detection of orthopoxvirus antibodies in feline sera. Vet. Microbiol. 1996, 52, 185–200. [Google Scholar] [CrossRef]
- Nowotny, N. Serologic studies of domestic cats for potential human pathogenic virus infections from wild rodens [in German]. Zentralbl. Hyg. Umweltmed. 1996, 198, 452–461. [Google Scholar]
- Zimmer, K.; Bogantes, J.C.; Herbst, W.; Räther, W. Poxvirus infections in a cat and its owner. Tierarztl. Prax. 1991, 19, 423–427. (In German) [Google Scholar]
- Tryland, M.; Sandvik, T.; Holtet, L.; Nilsen, H.; Olsvik, O.; Traavik, T. Antibodies to orthopoxvirus in domestic cats in Norway. Vet. Rec. 1998, 143, 105–109. [Google Scholar] [CrossRef]
- Cardeti, G.; Gruber, C.E.M.; Eleni, C.; Carletti, F.; Castilletti, C.; Manna, G.; Rosone, F.; Giombini, E.; Selleri, M.; Lapa, D.; et al. Fatal Outbreak in Tonkean Macaques Caused by Possibly Novel Orthopoxvirus, Italy. Emerg. Infect. Dis. 2017, 23, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.E.M.; Giombini, E.; Selleri, M.; Tausch, S.H.; Andrusch, A.; Tyshaieva, A.; Cardeti, G.; Lorenzetti, R.; De Marco, L.; Carletti, F.; et al. Whole Genome Characterization of Orthopoxvirus (OPV) Abatino, a Zoonotic Virus Representing a Putative Novel Clade of Old World Orthopoxviruses. Viruses 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Fevola, C.; Forbes, K.M.; Mäkelä, S.; Putkuri, N.; Hauffe, H.C.; Kallio-Kokko, H.; Mustonen, J.; Jääskeläinen, A.J.; Vaheri, A. Lymphocytic choriomeningitis, Ljungan and orthopoxvirus seroconversions in patients hospitalized due to acute Puumala hantavirus infection. J. Clin. Virol. 2016, 84, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutchins, E.; Warren, J.; Jones, P.W. The antibody response to smallpox vaccination as measured by tissue-culture plaque method. J. Immunol. 1960, 85, 275–283. [Google Scholar] [PubMed]
- Newman, F.K.; Frey, S.E.; Blevins, T.P.; Mandava, M.; Bonifacio, A., Jr.; Yan, L.; Belshe, R.B. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 2003, 41, 3154–3157. [Google Scholar] [CrossRef] [PubMed]

| Veterinarians (Years) | n | Positive | Prevalence (%) * |
|---|---|---|---|
| ≤30 | 5 | 0 | 0 |
| 31–40 | 13 | 2 | 15.4 |
| 41–50 | 10 | 5 | 50 |
| >50 | 8 | 5 | 62.5 |
| Area of Residence | Positive/Analyzed Cats | Seroprevalence |
|---|---|---|
| A (blue) | 7/19 | 36.8% |
| B (red) | 10/25 | 40% |
| C (turquoise) | 11/72 | 15.3% |
| D (yellow) | 1/8 | 12.5% |
| E (purple) | 1/10 | 10% |
| F (pink) | 2/40 | 5% |
| G (brown) | 4/22 | 18.2% |
| H (green) | 2/7 | 28.6% |
| I (grey) | 1/3 | 33.3% |
| Not known | 5/20 | 25% |
| Overall | 44/226 | 19.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapa, D.; Beltrame, A.; Arzese, A.; Carletti, F.; Di Caro, A.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Orthopoxvirus Seroprevalence in Cats and Veterinary Personnel in North-Eastern Italy in 2011. Viruses 2019, 11, 101. https://doi.org/10.3390/v11020101
Lapa D, Beltrame A, Arzese A, Carletti F, Di Caro A, Ippolito G, Capobianchi MR, Castilletti C. Orthopoxvirus Seroprevalence in Cats and Veterinary Personnel in North-Eastern Italy in 2011. Viruses. 2019; 11(2):101. https://doi.org/10.3390/v11020101
Chicago/Turabian StyleLapa, Daniele, Anna Beltrame, Alessandra Arzese, Fabrizio Carletti, Antonino Di Caro, Giuseppe Ippolito, Maria Rosaria Capobianchi, and Concetta Castilletti. 2019. "Orthopoxvirus Seroprevalence in Cats and Veterinary Personnel in North-Eastern Italy in 2011" Viruses 11, no. 2: 101. https://doi.org/10.3390/v11020101
APA StyleLapa, D., Beltrame, A., Arzese, A., Carletti, F., Di Caro, A., Ippolito, G., Capobianchi, M. R., & Castilletti, C. (2019). Orthopoxvirus Seroprevalence in Cats and Veterinary Personnel in North-Eastern Italy in 2011. Viruses, 11(2), 101. https://doi.org/10.3390/v11020101






