Hepatitis C Virus Affects Tuberculosis-Specific T Cells in HIV-Negative Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design and Setting
2.3. Peripheral Blood Mononuclear Cell Isolation and Stimulation
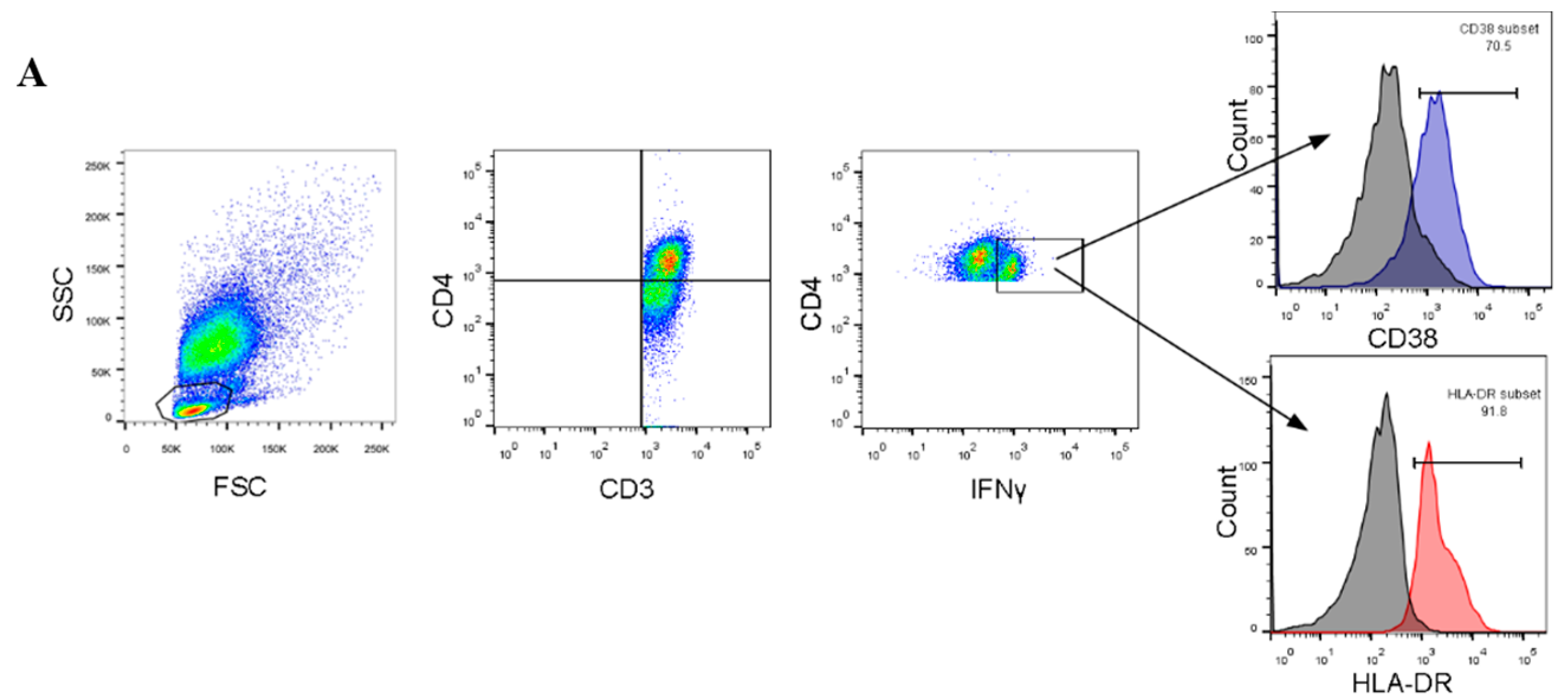
2.4. Flow Cytometry and Staining
2.5. Data Collection and Statistical Analysis
3. Results
3.1. Characteristics of Study Subjects
3.2. Expression of T-Cell Activation Markers
3.3. Correlation between TB-Specific T Cells and Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Ali, M.E.; El-Badawy, O.; Afifi, N.A.; Eldin, A.S.; Hassan, E.A.; Halby, H.M.; El-Mokhtar, M.A. Role of t-helper 9 cells in chronic hepatitis c-infected patients. Viruses 2018, 10, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandeel, A.; Genedy, M.; El-Refai, S.; Funk, A.L.; Fontanet, A.; Talaat, M. The prevalence of hepatitis c virus infection in egypt 2015: Implications for future policy on prevention and treatment. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37, 45–53. [Google Scholar] [CrossRef]
- Lomtadze, N.; Kupreishvili, L.; Salakaia, A.; Vashakidze, S.; Sharvadze, L.; Kempker, R.R.; Magee, M.J.; del Rio, C.; Blumberg, H.M. Hepatitis c virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS ONE 2013, 8, e83892. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; Valiquette, C.; Pelletier, M.; Parisien, I.; Rocher, I.; Menzies, D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Larrubia, J.R.; Moreno-Cubero, E.; Lokhande, M.U.; Garcia-Garzon, S.; Lazaro, A.; Miquel, J.; Perna, C.; Sanz-de-Villalobos, E. Adaptive immune response during hepatitis c virus infection. World J. Gastroenterol. 2014, 20, 3418–3430. [Google Scholar] [CrossRef]
- Cliff, J.M.; Kaufmann, S.H.; McShane, H.; van Helden, P.; O’Garra, A. The human immune response to tuberculosis and its treatment: A view from the blood. Immunol. Rev. 2015, 264, 88–102. [Google Scholar] [CrossRef]
- Esmail, H.; Riou, C.; Bruyn, E.D.; Lai, R.P.; Harley, Y.X.R.; Meintjes, G.; Wilkinson, K.A.; Wilkinson, R.J. The immune response to mycobacterium tuberculosis in hiv-1-coinfected persons. Annu. Rev. Immunol. 2018, 36, 603–638. [Google Scholar] [CrossRef]
- Riou, C.; Bunjun, R.; Muller, T.L.; Kiravu, A.; Ginbot, Z.; Oni, T.; Goliath, R.; Wilkinson, R.J.; Burgers, W.A. Selective reduction of ifn-gamma single positive mycobacteria-specific cd4+ t cells in hiv-1 infected individuals with latent tuberculosis infection. Tuberculosis 2016, 101, 25–30. [Google Scholar] [CrossRef]
- Day, C.L.; Abrahams, D.A.; Harris, L.D.; van Rooyen, M.; Stone, L.; de Kock, M.; Hanekom, W.A. Hiv-1 infection is associated with depletion and functional impairment of mycobacterium tuberculosis-specific cd4 t cells in individuals with latent tuberculosis infection. J. Immunol. 2017, 199, 2069–2080. [Google Scholar] [CrossRef] [Green Version]
- Rueda, C.M.; Marin, N.D.; Garcia, L.F.; Rojas, M. Characterization of cd4 and cd8 t cells producing ifn-gamma in human latent and active tuberculosis. Tuberculosis 2010, 90, 346–353. [Google Scholar] [CrossRef]
- Parsons, L.M.; Somoskovi, A.; Gutierrez, C.; Lee, E.; Paramasivan, C.N.; Abimiku, A.; Spector, S.; Roscigno, G.; Nkengasong, J. Laboratory diagnosis of tuberculosis in resource-poor countries: Challenges and opportunities. Clin. Microbial. Rev. 2011, 24, 314–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-Y.; Liu, C.-H.; Hu, F.-C.; Chang, H.-C.; Liu, J.-L.; Chen, J.-M.; Yu, C.-J.; Lee, L.-N.; Kao, J.-H.; Yang, P.-C.; et al. Risk factors of hepatitis during anti-tuberculous treatment and implications of hepatitis virus load. J. Infect. 2011, 62, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.M.; Shah, S.A.; Jenkins, C.A.; Shepherd, B.E.; Vermund, S.H. Risk factors and prevalence of tuberculosis, human immunodeficiency virus, syphilis, hepatitis b virus, and hepatitis c virus among prisoners in pakistan. Int. J. Infect. Dis. 2010, 14, e60–e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, P.; Zhu, Q.; Teter, C.; Yang, R.; Deng, L.; Yan, Y.; Chen, J.; Zeng, J.; Gui, X.E. Prevalence, drug-induced hepatotoxicity, and mortality among patients multi-infected with hiv, tuberculosis, and hepatitis virus. Int. J. Infect. Dis. Off. Publ. Int. Soc. Infect. Dis. 2014, 28, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nader, L.A.; de Mattos, A.A.; Picon, P.D.; Bassanesi, S.L.; De Mattos, A.Z.; Pineiro Rodriguez, M. Hepatotoxicity due to rifampicin, isoniazid and pyrazinamide in patients with tuberculosis: Is anti-hcv a risk factor? Ann. Hepatol. 2010, 9, 70–74. [Google Scholar] [CrossRef]
- Agha, M.A.; El-Mahalawy, I.I.; Seleem, H.M.; Helwa, M.A. Prevalence of hepatitis c virus in patients with tuberculosis and its impact in the incidence of anti-tuberculosis drugs induced hepatotoxicity. Egypt. J. Chest Dis. Tuberc. 2015, 64, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Richards, D.C.; Mikiashvili, T.; Parris, J.J.; Kourbatova, E.V.; Wilson, J.C.; Shubladze, N.; Tsertvadze, T.; Khechinashvili, G.; del Rio, C.; Blumberg, H.M.; et al. High prevalence of hepatitis c virus but not hiv co-infection among patients with tuberculosis in georgia. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis. 2006, 10, 396–401. [Google Scholar]
- Merza, M.A.; Haji, S.M.; Alsharafani, A.M.; Muhammed, S.U. Low prevalence of hepatitis b and c among tuberculosis patients in duhok province, kurdistan: Are hbsag and anti-hcv prerequisite screening parameters in tuberculosis control program? Int. J. Mycobact. 2016, 5, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Reis, N.; Lopes, C.; Teles, S.A.; Matos, M.; Carneiro, M.; Marinho, T.; JA Filho, A.; Espírito-Santo, M.; Lampe, E.; Martins, R.; et al. Hepatitis c virus infection in patients with tuberculosis in central brazil. Int. J. Tuberc. Lung Dis. 2011, 15, 1397–1402. [Google Scholar] [CrossRef]
- Behzadifar, M.; Heydarvand, S.; Behzadifar, M.; Bragazzi, N.L. Prevalence of hepatitis c virus in tuberculosis patients: A systematic review and meta-analysis. Ethiop. J. Health Sci. 2019, 29, 945–956. [Google Scholar]
- Marsh, S.G.E. Nomenclature for factors of the hla system, update february 2018. Int. J. immunogenet. 2018, 45, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Shubinsky, G.; Schlesinger, M. The cd38 lymphocyte differentiation marker: New insight into its ectoenzymatic activity and its role as a signal transducer. Immunity 1997, 7, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Caruso, A.M.; Serbina, N.; Klein, E.; Triebold, K.; Bloom, B.R.; Flynn, J.L. Mice deficient in cd4 t cells have only transiently diminished levels of ifn-gamma, yet succumb to tuberculosis. J. Immunol. 1999, 162, 5407–5416. [Google Scholar] [PubMed]
- Ottenhoff, T.H. New pathways of protective and pathological host defense to mycobacteria. Trends Microbial. 2012, 20, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, E.; Petrone, L.; Vanini, V.; Sampaolesi, A.; Gualano, G.; Girardi, E.; Palmieri, F.; Goletti, D. Ifngamma/tnfalpha specific-cells and effector memory phenotype associate with active tuberculosis. J. Infect. 2013, 66, 475–486. [Google Scholar] [CrossRef]
- Adekambi, T.; Ibegbu, C.C.; Cagle, S.; Kalokhe, A.S.; Wang, Y.F.; Hu, Y.; Day, C.L.; Ray, S.M.; Rengarajan, J. Biomarkers on patient t cells diagnose active tuberculosis and monitor treatment response. J. Clin. Investing. 2015, 125, 1827–1838. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Bao, D.; Gu, L.; Gu, Y.; Zhou, L.; Gao, Z.; Huang, Y. Co-infection with hepatitis b virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect. Dis. 2018, 18, 295. [Google Scholar] [CrossRef] [Green Version]
- Chukwuanukwu, R.C.; Onyenekwe, C.C.; Martinez-Pomares, L.; Flynn, R.; Singh, S.; Amilo, G.I.; Agbakoba, N.R.; Okoye, J.O. Modulation of the immune response to mycobacterium tuberculosis during malaria/m. Tuberculosis co-infection. Clin. Exp. Immunol. 2017, 187, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.L.; Munkanta, M.; Wilkinson, K.A.; Pathan, A.A.; Ewer, K.; Ayles, H.; Reece, W.H.; Mwinga, A.; Godfrey-Faussett, P.; Lalvani, A.; et al. Rapid detection of active and latent tuberculosis infection in hiv-positive individuals by enumeration of mycobacterium tuberculosis-specific t cells. Aids 2002, 16, 2285–2293. [Google Scholar] [CrossRef]
- Geldmacher, C.; Schuetz, A.; Ngwenyama, N.; Casazza, J.P.; Sanga, E.; Saathoff, E.; Boehme, C.; Geis, S.; Maboko, L.; Singh, M.; et al. Early depletion of mycobacterium tuberculosis-specific t helper 1 cell responses after hiv-1 infection. J. Infect. Dis. 2008, 198, 1590–1598. [Google Scholar] [CrossRef] [Green Version]
- Chetty, S.; Govender, P.; Zupkosky, J.; Pillay, M.; Ghebremichael, M.; Moosa, M.Y.; Ndung’u, T.; Porichis, F.; Kasprowicz, V.O. Co-infection with mycobacterium tuberculosis impairs hiv-specific cd8+ and cd4+ t cell functionality. PLoS ONE 2015, 10, e0118654. [Google Scholar] [CrossRef] [PubMed]
- Stoop, J.N.; van der Molen, R.G.; Baan, C.C.; van der Laan, L.J.; Kuipers, E.J.; Kusters, J.G.; Janssen, H.L. Regulatory t cells contribute to the impaired immune response in patients with chronic hepatitis b virus infection. Hepatology 2005, 41, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Penna, A.; Pilli, M.; Zerbini, A.; Orlandini, A.; Mezzadri, S.; Sacchelli, L.; Missale, G.; Ferrari, C. Dysfunction and functional restoration of hcv-specific cd8 responses in chronic hepatitis c virus infection. Hepatology 2007, 45, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Jacques, M.K.; Zhu, C.; Steblenko, K.M.; Stowell, B.L.; Madi, A.; Anderson, A.C.; Kuchroo, V.K.; Behar, S.M. Tim3 mediates t cell exhaustion during mycobacterium tuberculosis infection. PLoS Pathog. 2016, 12, e1005490. [Google Scholar] [CrossRef]
- Groux, H.; Bigler, M.; de Vries, J.E.; Roncarolo, M.G. Inhibitory and stimulatory effects of il-10 on human cd8+ t cells. J. Immunol. 1998, 160, 3188–3193. [Google Scholar]
- Sugimoto, K.; Ikeda, F.; Stadanlick, J.; Nunes, F.A.; Alter, H.J.; Chang, K.M. Suppression of hcv-specific t cells without differential hierarchy demonstrated ex vivo in persistent hcv infection. Hepatology 2003, 38, 1437–1448. [Google Scholar] [CrossRef]
- Verbon, A.; Juffermans, N.; Van Deventer, S.J.; Speelman, P.; Van Deutekom, H.; Van Der Poll, T. Serum concentrations of cytokines in patients with active tuberculosis (tb) and after treatment. Clin. Exp. Immunol. 1999, 115, 110–113. [Google Scholar] [CrossRef]
- Bertoletti, A.; D’Elios, M.M.; Boni, C.; De Carli, M.; Zignego, A.L.; Durazzo, M.; Missale, G.; Penna, A.; Fiaccadori, F.; Del Prete, G.; et al. Different cytokine profiles of intraphepatic t cells in chronic hepatitis b and hepatitis c virus infections. Gastroenterology 1997, 112, 193–199. [Google Scholar] [CrossRef]

| Parameter | Total TB Patients (n = 64) | TB+HCV− Group (n = 46) | TB+HCV+ Group (n = 18) | TB−HCV− Controls (n = 18) | p-Value a |
|---|---|---|---|---|---|
| Median age (IQR) | 46.8 (21–65) | 40.2 (21–63) | 51.8 (28–65) | 44 (25–51) | 0.076 |
| Sex M/F | 41/23 (64.1%/35.9%) | 28/18 (60.9%/39.61%) | 13/5 (72.2%/27.8%) | 12/6 (66.6%/33.3%) | 0.436 |
| Active TB Pulmonary/Extrapulmonary | 35/29 (54.7%/45.3%) | 25/21 (54.3/45.7%) | 10/8 (55.6%/44.4%) | NA | 0.296 |
| TB toxemia | 41 (64.1%) | 31 (67.4%) | 10 (55.6%) | NA | 0.189 |
| Fever | 32 (50%) | 24 (52.2%) | 8 (44.4%) | NA | 0.171 |
| Weight loss | 28 (43.8%) | 22 (47.8%) | 6 (33.3%) | NA | 0.174 |
| Serum bilirubin (mg/dL) | 0.7 ± 0.3 | 0.8 ± 0.4 | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.420 |
| Serum albumin (g/dL) | 3.9 ± 0.8 | 4.4 ± 0.5 | 3.5 ± 0.8 | 4 ± 0.5 | 0.03 * |
| AST (U/L) | 31 (4–102) | 22 (4–35) | 43.5 (18–102) | 20 (10–30) | 0.004 * |
| ALT (U/L) | 23 (10.4–98.2) | 21 (10.4–87) | 23 (12–98.2) | 25 (15–35) | 0.095 |
| WBC (×109/L) | 8.1 ± 3.8 | 7.5 ± 3.3 | 8.5 ± 3.5 | 5 ± 0.5 | 0.537 |
| Hemoglobin (g/dL) | 12.9 ± 1.4 | 12.5 ± 1.8 | 13.1 ± 1.03 | 13.5 ± 1.53 | 0.288 |
| Platelets (×109/L) | 307.5 ± 56.1 | 281.1 ± 90.7 | 329.5 ± 79.1 | 349 ± 25 | 0.196 |
| Neutrophil count % | 62.2 ± 8.8 | 58.3 ± 6.3 | 64.4 ± 9.6 | 55.3 ± 2.3 | 0.189 |
| Lymphocyte count % | 29.1 ± 9.4 | 34.1 ± 8.7 | 26.1 ± 8.8 | 30 ± 5 | 0.097 |
| Serum creatinine (mg/dL) | 0.9 ± 0.3 | 0.8 ± 0.2 | 1.03 ± 0.3 | 0.8 ± 0.2 | 0.342 |
| ESR (mm/h) | 52.8 ± 19.2 | 51.33 ± 24.56 | 53.71 ± 15.73 | 12 ± 2 | 0.779 |
| HCV RNA, IU/mL | NA | NA | 3.7 × 104 (5 × 103–8.9 × 106) | NA | - |
| Response to anti-TB treatment | 55 (85.9%) | 41 (89.1%) | 14 (77.8%) | NA | 0.176 |
| IL-2 (pg/mL), median (IQR) | NA | 15.1 (13.2–16.6) | 17.3 (17–18.4) | 7.3 (6.3–8.7) | 0.26 |
| IL-4 (pg/mL), median (IQR) | NA | 33 (31.5–34.2) | 36 (34.3–40.3) | 4.9 (4.9–6.3) | 0.436 |
| IFN-γ (pg/mL), median (IQR) | NA | 50 (48–52) | 45.6 (43–49) | 5.3 (4.5–6.6) | 0.296 |
| TNF-α (pg/mL), median (IQR) | NA | 70 (68.8–72) | 64.3 (63–67.3) | 9.5 (9–12) | 0.189 |
| IL-10 (pg/mL), median (IQR) | NA | 45.3 (43.2–47.3) | 29.3 (28.2–31.5) | 4.9 (4–6.1) | 0.012 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mokhtar, M.A.; Elgendy, S.G.; Eldin, A.S.; Hassan, E.A.; Hasan, A.A.A.; Abdel Hameed, M.R.; Sayed, D.; Salama, E.H. Hepatitis C Virus Affects Tuberculosis-Specific T Cells in HIV-Negative Patients. Viruses 2020, 12, 101. https://doi.org/10.3390/v12010101
El-Mokhtar MA, Elgendy SG, Eldin AS, Hassan EA, Hasan AAA, Abdel Hameed MR, Sayed D, Salama EH. Hepatitis C Virus Affects Tuberculosis-Specific T Cells in HIV-Negative Patients. Viruses. 2020; 12(1):101. https://doi.org/10.3390/v12010101
Chicago/Turabian StyleEl-Mokhtar, Mohamed Ahmed, Sherein G. Elgendy, Abeer Sharaf Eldin, Elham Ahmed Hassan, Ali Abdel Azeem Hasan, Muhamad R. Abdel Hameed, Douaa Sayed, and Eman H. Salama. 2020. "Hepatitis C Virus Affects Tuberculosis-Specific T Cells in HIV-Negative Patients" Viruses 12, no. 1: 101. https://doi.org/10.3390/v12010101
APA StyleEl-Mokhtar, M. A., Elgendy, S. G., Eldin, A. S., Hassan, E. A., Hasan, A. A. A., Abdel Hameed, M. R., Sayed, D., & Salama, E. H. (2020). Hepatitis C Virus Affects Tuberculosis-Specific T Cells in HIV-Negative Patients. Viruses, 12(1), 101. https://doi.org/10.3390/v12010101






