Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus
Abstract
1. Introduction
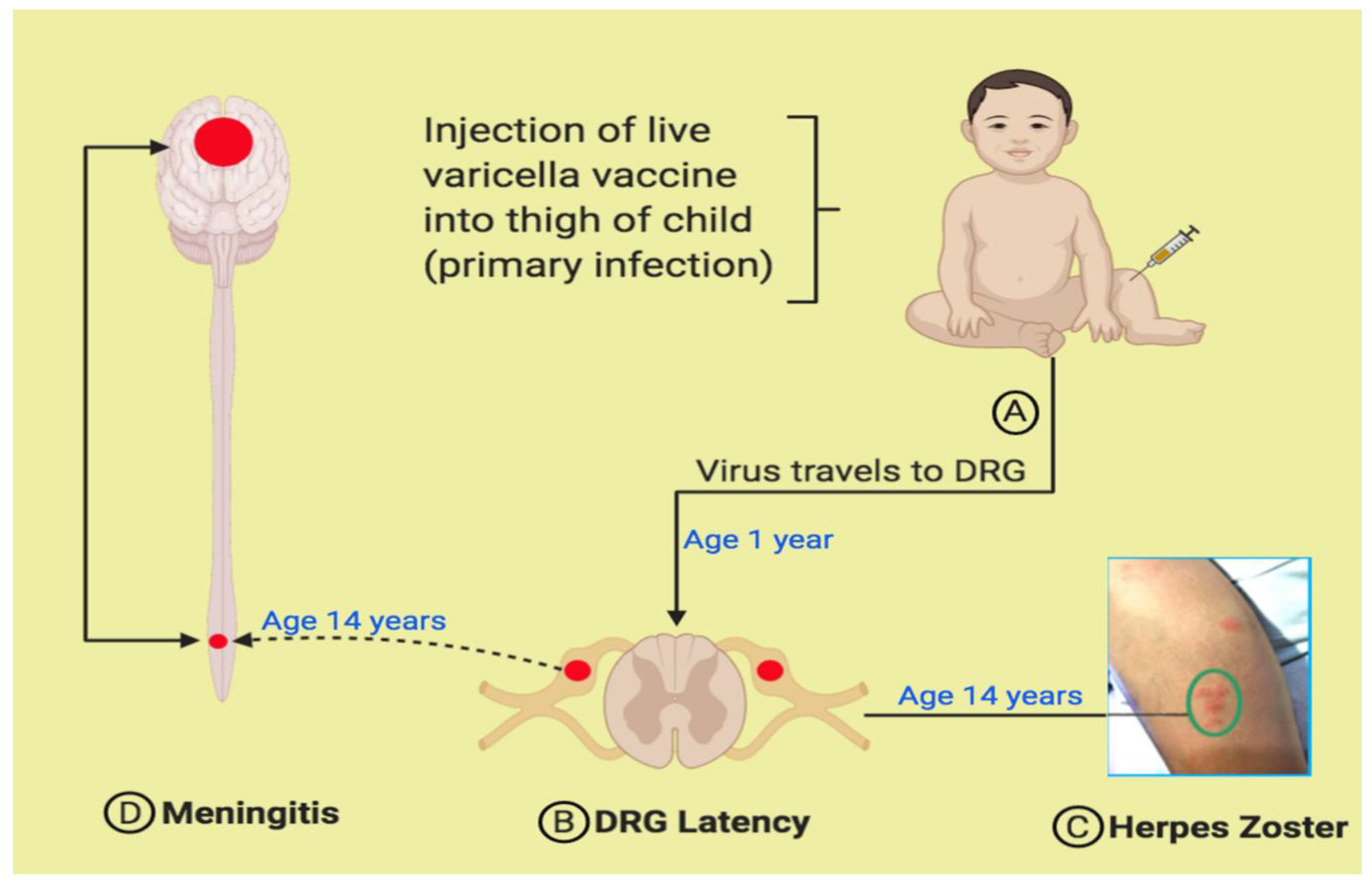
2. Varicella Vaccine Meningitis after Herpes Zoster in Once-Immunized Children
3. Varicella Vaccine Meningitis after Herpes Zoster in Twice-Immunized Children
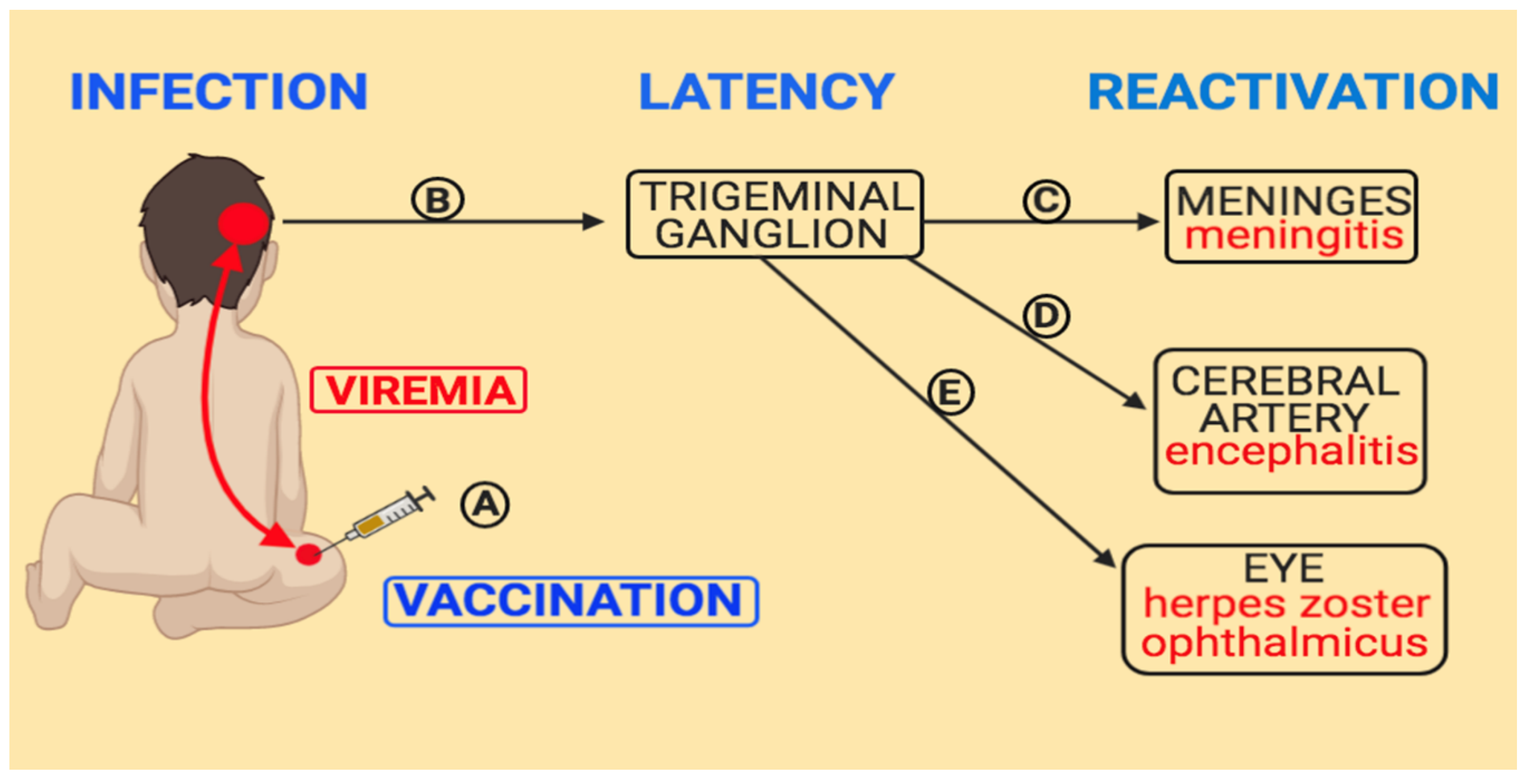
4. Pathogenesis of Varicella Vaccine Meningitis
5. Varicella Vaccine Meningitis after Herpes Zoster Ophthalmicus
6. Varicella Meningitis Caused by Wild Type Varicella
7. Comparison between Wild Type vs. Vaccine-Type Varicella Meningitis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramachandran, V.; Elliott, S.C.; Rogers, K.L.; Cohrs, R.J.; Weinberger, M.; Jackson, W.; Carpenter, J.E.; Grose, C.; Bonthius, D.J. Varicella Vaccine Meningitis as a Complication of Herpes Zoster in Twice-Immunized Immunocompetent Adolescents. J. Child Neurol. 2020, 883073820938597. [Google Scholar] [CrossRef]
- Gershon, A. Varicella vaccine: Its past, present and future. Pediatr. Infect. Dis. J. 1995, 14, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Effectiveness of live varicella vaccine. Expert Opin. Biol. Ther. 2004, 4, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Varela, F.H.; Pinto, L.A.; Scotta, M.C. Global impact of varicella vaccination programs. Hum. Vaccines Immunother. 2018, 15, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Grose, C. Varicella vaccination of children in the United States: Assessment after the first decade 1995–2005. J. Clin. Virol. 2005, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Marin, M. Update on trends in varicella mortality during the varicella vaccine era—United States, 1990–2016. Hum. Vaccines Immunother. 2018, 14, 2460–2463. [Google Scholar] [CrossRef]
- Galea, S.A.; Sweet, A.; Beninger, P.; Steinberg, S.P.; LaRussa, P.S.; Gershon, A.A.; Sharrar, R.G. The Safety Profile of Varicella Vaccine: A 10-Year Review. J. Infect. Dis. 2008, 197 (Suppl. S2), S165–S169. [Google Scholar] [CrossRef]
- Horien, C.; Grose, C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin. Pediatr. Neurol. 2012, 19, 124–129. [Google Scholar] [CrossRef]
- Seward, J.F.; Marin, M.; Vázquez, M. Varicella Vaccine Effectiveness in the US Vaccination Program: A Review. J. Infect. Dis. 2008, 197 (Suppl. S2), S82–S89. [Google Scholar] [CrossRef]
- Chen, Y.-C.; James, A.; Kung, E.; Madhavan, V. A Case of Herpes Zoster and Meningitis in a Twice-Vaccinated Healthy Adolescent. J. Pediatr. Infect. Dis. 2017, 12, 142–144. [Google Scholar] [CrossRef][Green Version]
- E Harrington, W.; Mató, S.; Burroughs, L.; Carpenter, P.A.; Gershon, A.; Schmid, D.S.; Englund, J.A. Vaccine Oka Varicella Meningitis in Two Adolescents. Pediatrics 2019, 144, e20191522. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Hanson, D.C.; Way, S.S. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr. Infect. Dis. J. 2011, 30, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Pahud, B.A.; Glaser, C.A.; Dekker, C.L.; Arvin, A.M.; Schmid, D.S. Varicella Zoster Disease of the Central Nervous System: Epidemiological, Clinical, and Laboratory Features 10 Years after the Introduction of the Varicella Vaccine. J. Infect. Dis. 2011, 203, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Hope-Simpson, R.E. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef]
- Grose, C.; Giller, R.H. Varicella-zoster virus infection and immunization in the healthy and the immunocompromised host. Crit. Rev. Oncol. 1988, 8, 27–64. [Google Scholar] [CrossRef]
- Gershon, A.; Steinberg, S.; Greenberg, S.; Taber, L. Varicella-zoster-associated encephalitis: Detection of specific antibody in cerebrospinal fluid. J. Clin. Microbiol. 1980, 12, 764–767. [Google Scholar] [CrossRef]
- Bieger, R.C.; Van Scoy, R.E.; Smith, T.F. Antibodies to Varicella Zoster in Cerebrospinal Fluid. Arch. Neurol. 1977, 34, 489–491. [Google Scholar] [CrossRef]
- Nagel, M.A.; Forghani, B.; Mahalingam, R.; Wellish, M.C.; Cohrs, R.J.; Russman, A.N.; Katzan, I.; Lin, R.; Gardner, C.J.; Gilden, D.H. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology 2007, 68, 1069–1073. [Google Scholar] [CrossRef]
- Mahalingam, R.; Gershon, A.A.; Gershon, M.; Cohen, J.I.; Arvin, A.M.; Zerboni, L.; Zhu, H.; Gray, W.; Messaoudi, I.; Traina-Dorge, V.L. Current In Vivo Models of Varicella-Zoster Virus Neurotropism. Viruses 2019, 11, 502. [Google Scholar] [CrossRef]
- Gold, E. Serologic and Virus-Isolation Studies of Patients with Varicella or Herpes-Zoster Infection. N. Engl. J. Med. 1966, 274, 181–185. [Google Scholar] [CrossRef]
- Haanpaa, M.; Dastidar, P.; Weinberg, A.; Levin, M.; Miettinen, A.; Lapinlampi, A.; Laippala, P.; Nurmikko, T. CSF and MRI findings in patients with acute herpes zoster. Neurology 1998, 51, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, R.; Leung, J.W. The Epidemiology of Herpes Zoster in the United States During the Era of Varicella and Herpes Zoster Vaccines: Changing Patterns Among Children. Clin. Infect. Dis. 2018, 69, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Moodley, A.; Swanson, J.; Grose, C.; Bonthius, D.J. Severe Herpes Zoster Following Varicella Vaccination in Immunocompetent Young Children. J. Child Neurol. 2019, 34, 184–188. [Google Scholar] [CrossRef]
- Detty, S.Q.; Peebles, J.K.; Guerrieri, J.M.; Seroogy, C.M.; Struck, M.C.; Arkin, L.M.; Henderson, S.L. Vaccine-Strain Herpes Zoster Ophthalmicus in a 14-month-old Boy Prompting an Immunodeficiency Workup. Pediatr. Infect. Dis. J. 2020, 39, e25–e27. [Google Scholar] [CrossRef]
- Grose, C.; Enquist, L.W. The round trip model for severe herpes zoster caused by live attenuated varicella vaccine virus. J. Med. Virol. 2020, 92, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Brittle, E.E.; Reynolds, A.E.; Enquist, L.W. Two Modes of Pseudorabies Virus Neuroinvasion and Lethality in Mice. J. Virol. 2004, 78, 12951–12963. [Google Scholar] [CrossRef]
- Grose, C.; Adams, H.P. Reassessing the link between herpes zoster ophthalmicus and stroke. Expert Rev. Anti-infective Ther. 2014, 12, 527–530. [Google Scholar] [CrossRef]
- Chouliaras, G.; Spoulou, V.; Quinlivan, M.; Breuer, J.; Theodoridou, M. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child. Pediatrics 2010, 125, e969–e972. [Google Scholar] [CrossRef]
- Ozaki, T.; Masuda, S.; Asano, Y.; Kondo, K.; Namazue, J.; Yamanishi, K. Investigation of varicella-zoster virus DNA by the polymerase chain reaction in healthy children with varicella vaccination. J. Med. Virol. 1994, 42, 47–51. [Google Scholar] [CrossRef]
- Qureshi, A.I. Artery of Trigeminal Nerve Ganglion. J. Vasc. Interv. Neurol. 2017, 9, 57–58. [Google Scholar]
- Esiri, M.M.; Tomlinson, A.H. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J. Neurol. Sci. 1972, 15, 35–48. [Google Scholar] [CrossRef]
- Weinmann, S.; Chun, C.S.; Schmid, D.S.; Roberts, M.; VanderMeer, M.; Riedlinger, K.; Bialek, S.R.; Marin, M. Incidence and Clinical Characteristics of Herpes Zoster Among Children in the Varicella Vaccine Era, 2005–2009. J. Infect. Dis. 2013, 208, 1859–1868. [Google Scholar] [CrossRef]
- Weller, T.H. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N. Engl. J. Med. 1983, 309, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Wellish, M.; Wolf, W.; Dueland, A.N.; Cohrs, R.J.; Vafai, A.; Gilden, D. Latent Varicella–Zoster Viral DNA in Human Trigeminal and Thoracic Ganglia. N. Engl. J. Med. 1990, 323, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Guess, H.A.; Broughton, D.D.; Melton, L.J.; Kurland, L.T. Epidemiology of herpes zoster in children and adolescents: A population-based study. Pediatricts 1985, 76, 512–517. [Google Scholar]
- Itoh, N.; Motokura, K.; Kumakura, A.; Hata, D.; Hata, A. Herpes zoster meningitis in immunocompetent children: Two case reports and a literature review. Pediatr. Int. 2017, 59, 1116–1118. [Google Scholar] [CrossRef]
- Yasuda, R.; Minami, K.; Ogawa, A.; Okada, H.; Terakawa, R.; Koike, Y.; Ogura, S.; Takeuchi, K.; Higuchi, T. Herpes zoster and meningitis in an immunocompetent child: A case report. J. Med. Case Rep. 2019, 13, 182. [Google Scholar] [CrossRef]
- Barry, R.; Prentice, M.; Costello, D.; O’Mahony, O.; Degascun, C.; Felsenstein, S. Varicella zoster reactivation causing aseptic meningitis in healthy adolescents. Pediatr. Infect. Dis. J. 2020, 39, e278–e282. [Google Scholar] [CrossRef]
- Brunell, P.A.; Kotchmar, G.S., Jr. Zoster in infancy: Failure to maintain virus latency following intrauterine infection. J. Pediatr. 1981, 98, 71–73. [Google Scholar] [CrossRef]
- Takayama, N.; Yamada, H.; Kaku, H.; Minamitani, M. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr. Int. 2000, 42, 275–279. [Google Scholar] [CrossRef]
- Echevarría, J.M.; Casas, I.; Tenorio, A.; De Ory, F.; Martinez-Martin, P. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J. Med. Virol. 1994, 43, 331–335. [Google Scholar] [CrossRef]
- Sadaoka, T.; Depledge, D.P.; Rajbhandari, L.; Venkatesan, A.; Breuer, J.; Cohen, J.I. In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc. Natl. Acad. Sci. USA 2016, 113, E2403–E2412. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, L.; Reichelt, M.; Arvin, A.M. Varicella-Zoster Virus Neurotropism in SCID Mouse–Human Dorsal Root Ganglia Xenografts. Curr. Top Microbiol. Immunol. 2010, 342, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Halling, G.; Giannini, C.; Britton, J.W.; Lee, R.W.; Watson, R.E.; Terrell, C.L.; Parney, I.F.; Buckingham, E.M.; Carpenter, J.E.; Grose, C. Focal encephalitis following varicella-zoster virus reactivation without rash in a healthy immunized young adult. J. Infect. Dis. 2014, 210, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.; Marko, A.; Galea, S.; Eagel, B.; Straus, W. Varicella Virus Vaccine Live: A 22-Year Review of Postmarketing Safety Data. Open Forum Infect. Dis. 2019, 6, ofz295. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.R.; Witkop, C.T.; Webber, B.J.; Costello, A.A. Varicella seroepidemiology in United States air force recruits: A retrospective cohort study comparing immunogenicity of varicella vaccination and natural infection. Vaccine 2017, 35, 2351–2357. [Google Scholar] [CrossRef]
- Jenke, A.C.W.; Klein, S.; Baiker, A.; Wirth, S.; Sander, M.; Noelting, C.; Boecher, O.; Vizoso-Pinto, M.G. Serologic Analysis of the IgG Antibody Response in Children With Varicella Zoster Virus Wild-type Infection and Vaccination. Pediatr. Infect. Dis. J. 2012, 31, 1148–1152. [Google Scholar] [CrossRef]
- Grose, C.; Juhn, Y. COMMENTARY: Significantly less anti-gC antibody detectable in sera collected after varicella vaccination than after the disease varicella. Pediatr. Infect. Dis. J. 2012, 31, 1153–1154. [Google Scholar] [CrossRef]
- Storlie, J.; Carpenter, J.E.; Jackson, W.; Grose, C. Discordant varicella-zoster virus glycoprotein C expression and localization between cultured cells and human skin vesicles. Virology 2008, 382, 171–181. [Google Scholar] [CrossRef]
- Moffat, J.F.; Zerboni, L.; Kinchington, P.R.; Grose, C.; Kaneshima, H.; Arvin, A.M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998, 72, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Kinchington, P.R.; Ruyechan, W.T.; Hay, J. A detailed analysis of transcripts mapping to varicella zoster virus gene 14 (glycoprotein V). Virology 1991, 184, 625–635. [Google Scholar] [CrossRef]
- Peters, G.A.; Tyler, S.D.; Carpenter, J.E.; Jackson, W.; Mori, Y.; Arvin, A.M.; Grose, C. The Attenuated Genotype of Varicella-Zoster Virus Includes an ORF0 Transitional Stop Codon Mutation. J. Virol. 2012, 86, 10695–10703. [Google Scholar] [CrossRef] [PubMed]
- Grose, C. Pangaea and the Out-of-Africa Model of Varicella-Zoster Virus Evolution and Phylogeography. J. Virol. 2012, 86, 9558–9565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Jouanguy, E.; Ugolini, S.; Smahi, A.; Elain, G.; Romero, P.; Segal, D.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A.; et al. TLR3 Deficiency in Patients with Herpes Simplex Encephalitis. Science 2007, 317, 1522–1527. [Google Scholar] [CrossRef]
- Lim, H.K.; Seppänen, M.; Hautala, T.; Ciancanelli, M.J.; Itan, Y.; Lafaille, F.G.; Dell, W.; Lorenzo, L.; Byun, M.; Pauwels, E.; et al. TLR3 deficiency in herpes simplex encephalitis: High allelic heterogeneity and recurrence risk. Neurology 2014, 83, 1888–1897. [Google Scholar] [CrossRef]
- Ogunjimi, B.; Zhang, S.-Y.; Sørensen, K.B.; Skipper, K.A.; Carter-Timofte, M.; Kerner, G.; Luecke, S.; Prabakaran, T.; Cai, Y.; Meester, J.; et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J. Clin. Investig. 2017, 127, 3543–3556. [Google Scholar] [CrossRef]
- Carter-Timofte, M.E.; Paludan, S.R.; Mogensen, T.H. RNA Polymerase III as a Gatekeeper to Prevent Severe VZV Infections. Trends Mol. Med. 2018, 24, 904–915. [Google Scholar] [CrossRef]
- Jouanguy, E.; Béziat, V.; Mogensen, T.H.; Casanova, J.-L.; Tangye, S.G.; Zhang, S.-Y. Human inborn errors of immunity to herpes viruses. Curr. Opin. Immunol. 2020, 62, 106–122. [Google Scholar] [CrossRef]
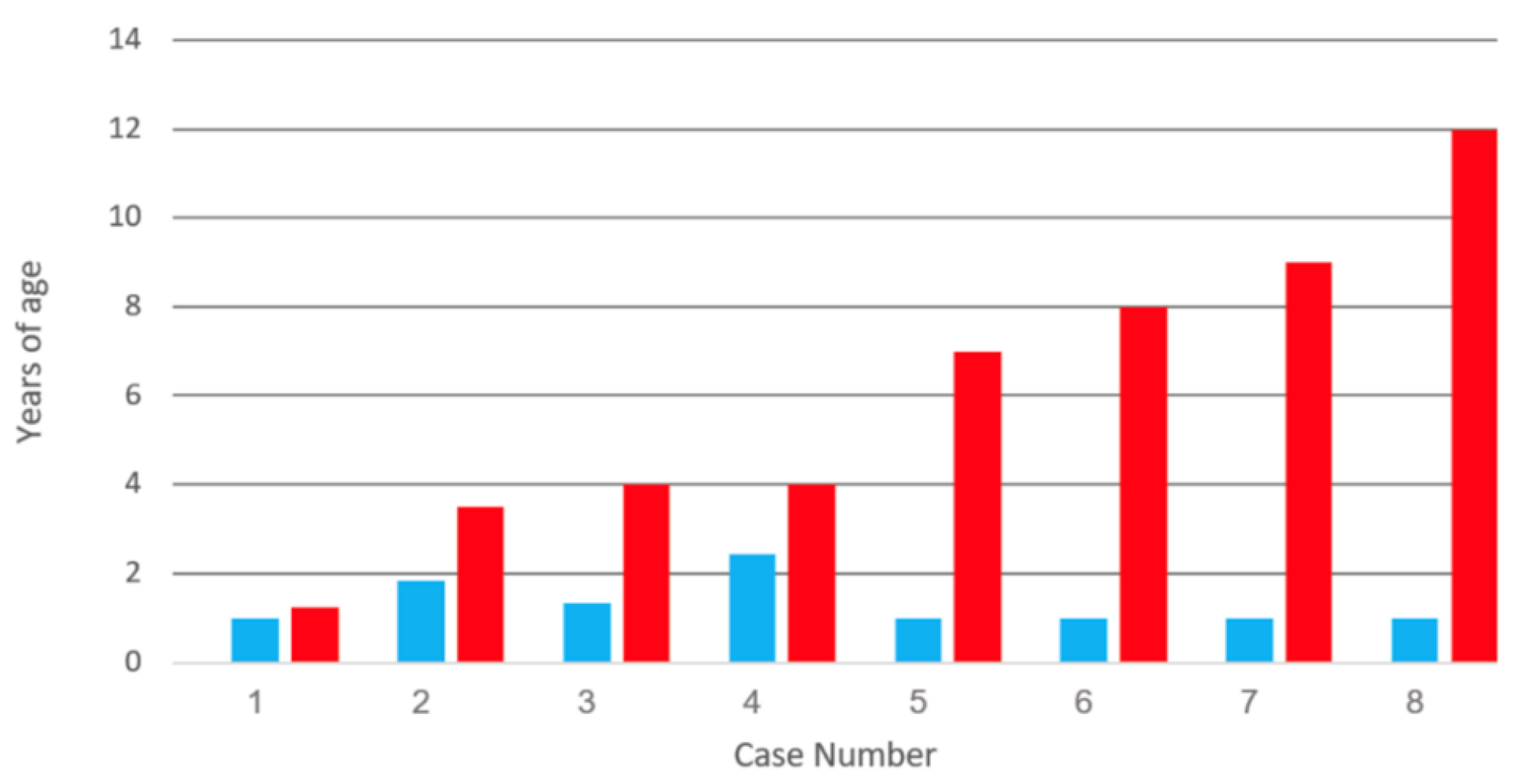






| Category | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Vaccination (y) | 1 | 1.8 | 1.3 | 2.4 | 1 | 1 | 1 | 1 |
| Meningitis (y) | 1.3 | 3 | 4 | 4 | 7 | 8 | 9 | 12 |
| Zoster | Lum | Tri | Cer | Cer | Cer | Cer | Cer | Cer |
| Cancer | Yes | No | No | Yes | No | No | No | No |
| IV Acyclovir | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Year | 2003 | 2009 | 2008 | 2008 | 2011 | 2008 | 2008 | 2011 |
| VZV Lab | CDC | UK | CDC | CDC | CDC | CDC | CDC | CDC |
| Gender | Boy | Girl | NG | NG | Boy | NG | NG | Girl |
| Category | Case 1 [10] | Case 2 [11] | Case 3 [11] | Case 4 [1] |
|---|---|---|---|---|
| Geography | Boston | Seattle | Seattle | Des Moines |
| Gender | Girl | Boy | Boy | Girl |
| Age 1st vaccine (y) | 1.5 | ~1 | ~1 | ~1 |
| Age 2nd vaccine (y) | 12 | 4 | 10 | 5 |
| Age of meningitis (y) | 14 | 14 | 14 | 14 |
| Location of zoster | T5 | L1/L2 | T8 | L4 |
| Years after 1st vaccine | 13 | 13 | 13 | 13 |
| Years after 2nd vaccine | 2 | 10 | 4 | 9 |
| CSF Screening PCR | Yes | Yes | Yes | Yes |
| CSF cell count | 568 | 140 | 81 | 775 |
| CSF VZV antibody | Not done | No | Not done | Yes |
| Acyclovir IV (days) | 7 | 7 | 27 | 7 |
| Valacyclovir (days) | 14 | 0 | Indefinite | 14 |
| Immunocompromised | No | No | Yes | No |
| Year | 2017 | 2019 | 2019 | 2020 |
| VZV Lab | CDC | CDC | CDC | Iowa |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heusel, E.H.; Grose, C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses 2020, 12, 1078. https://doi.org/10.3390/v12101078
Heusel EH, Grose C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses. 2020; 12(10):1078. https://doi.org/10.3390/v12101078
Chicago/Turabian StyleHeusel, Ethan H., and Charles Grose. 2020. "Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus" Viruses 12, no. 10: 1078. https://doi.org/10.3390/v12101078
APA StyleHeusel, E. H., & Grose, C. (2020). Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses, 12(10), 1078. https://doi.org/10.3390/v12101078






