Releasing Intracellular NS1 from Mosquito Cells for the Detection of Dengue Virus-Infected Mosquitoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito, Cell Culture and Virus Maintenance
2.2. Plaque Assays
2.3. Infecting Cells or Mosquitoes with DENV
2.4. Immunoblotting
2.5. Dengue NS1 Rapid Test
2.6. RNA Extraction, Reverse Transcription and qPCR
2.7. Immunofluorescence Staining and Imaging
2.8. Statistical Analysis
3. Results
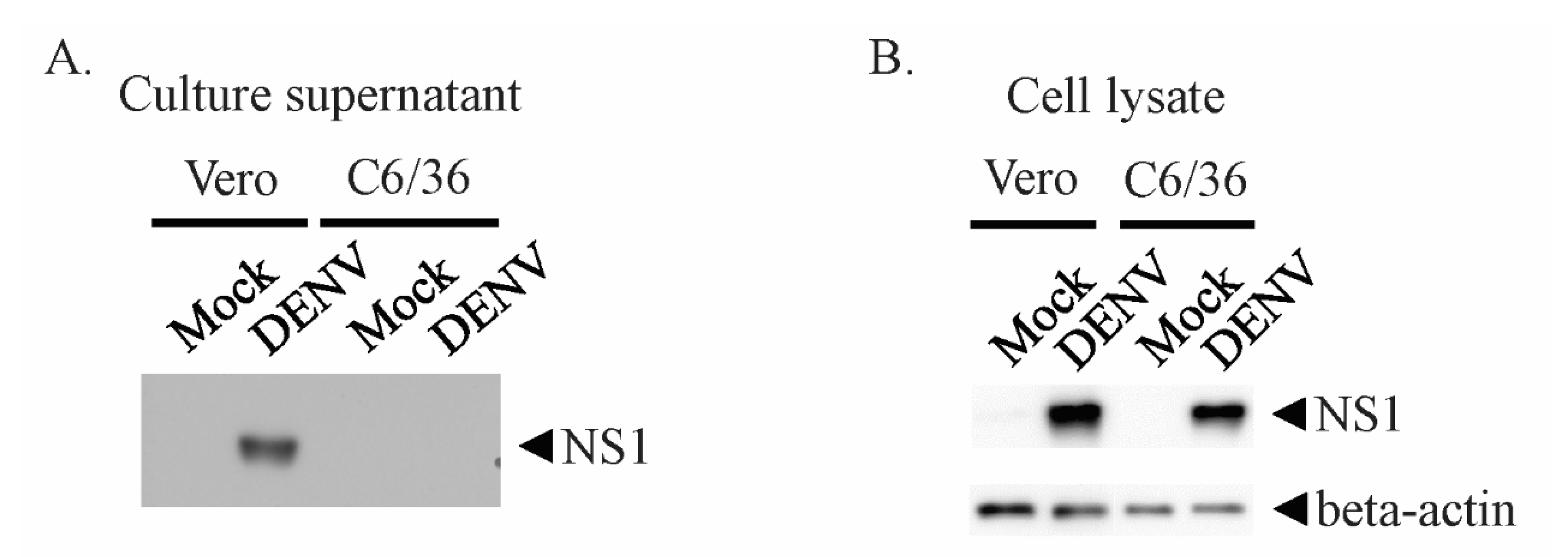
3.1. DENV NS1 Is not Secreted at Detectable Levels by Mosquito Cell Lines
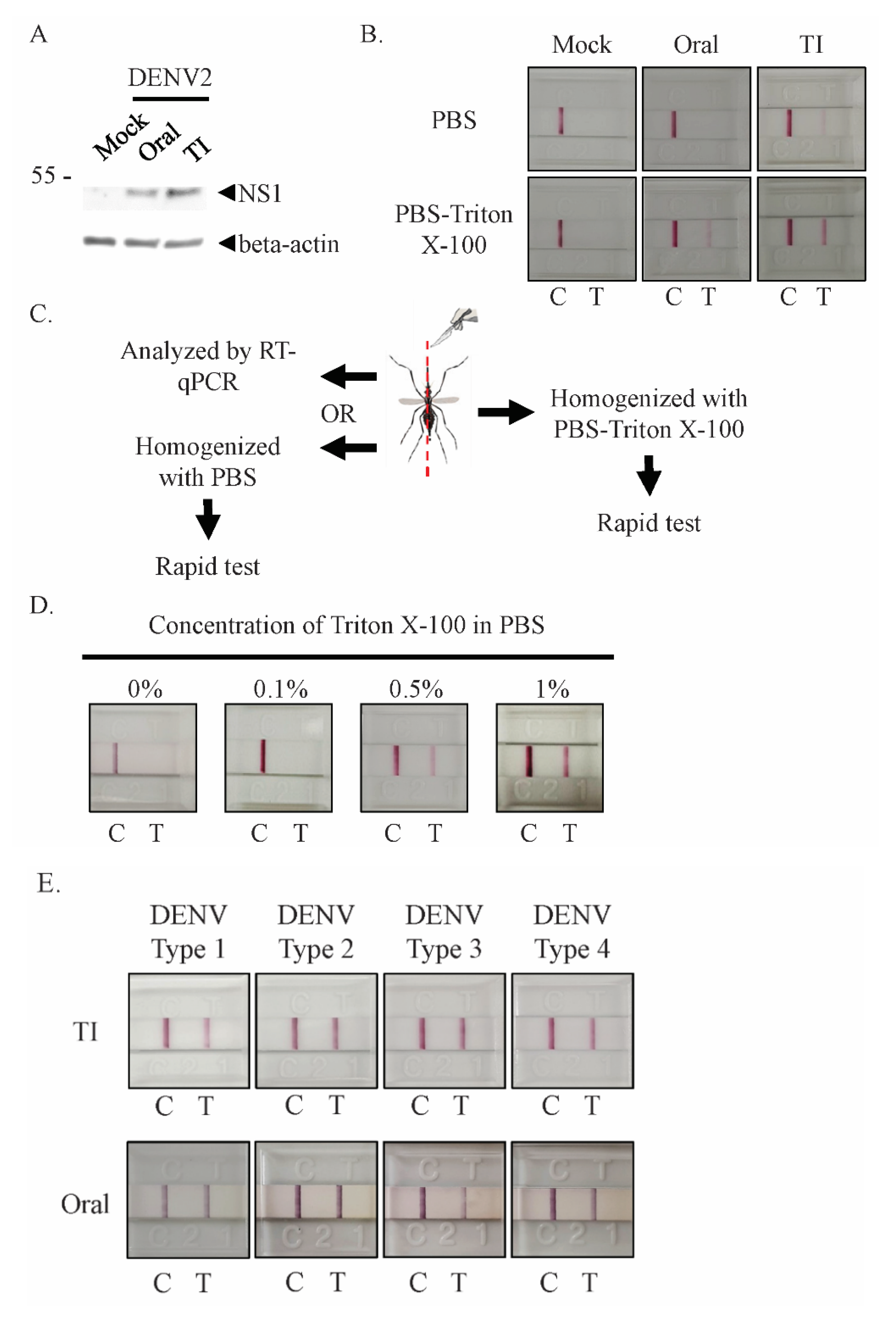
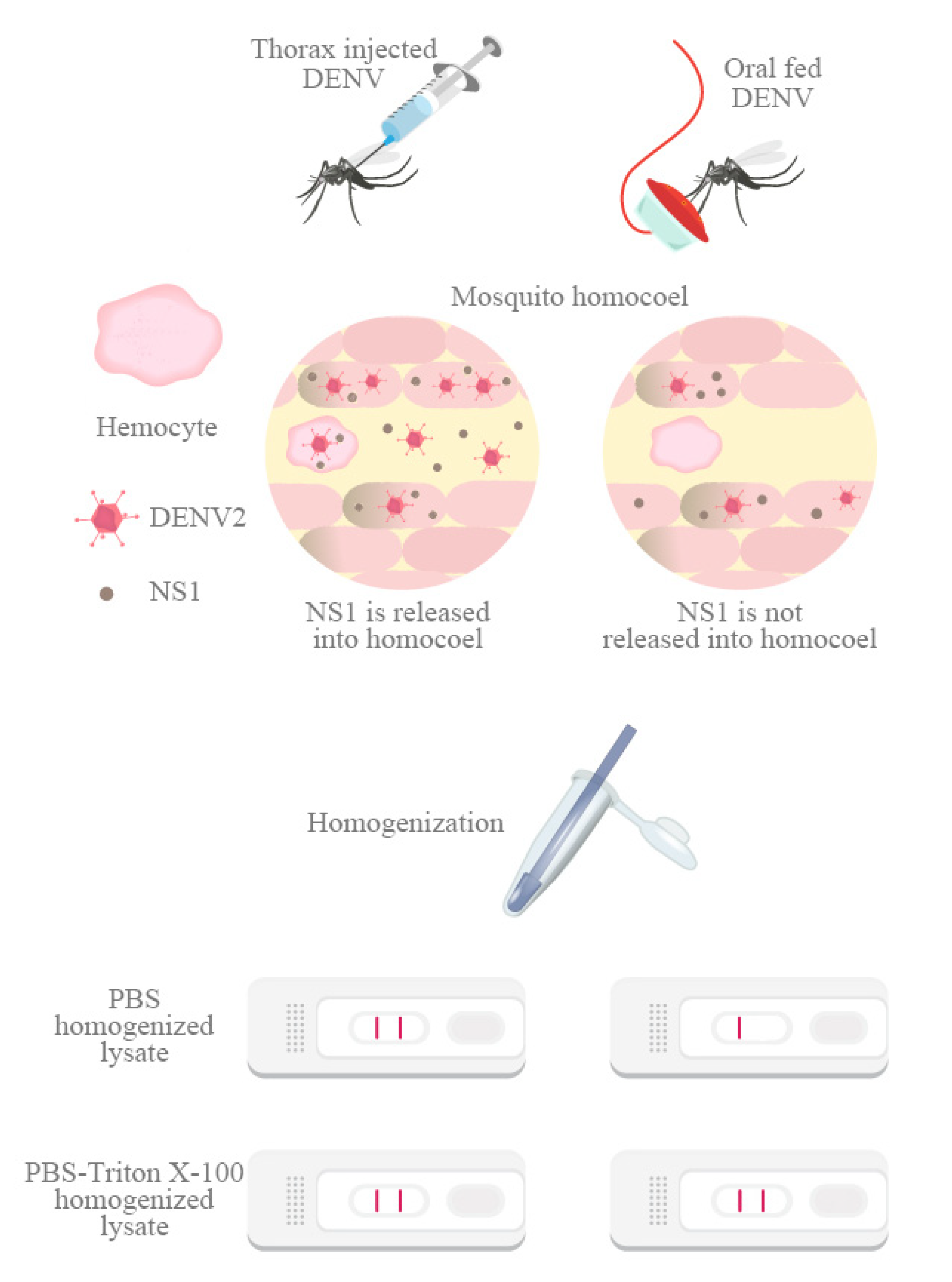
3.2. Detergent Releases Intracellular NS1 from Infected Mosquitoes for Rapid Test Detection
3.3. NS1 Rapid Test Was Able to Correctly Identify Infected Mosquitoes even during Early Stages of DENV Infection
3.4. Sensitivity of the NS1 Rapid Test Increased after DENV2 Had Spread from the Midgut
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| NS1 | Non-structural protein 1 |
| qPCR | Real-time quantitative polymerase chain reaction |
| PBS | Phosphate-buffered saline |
| PI | Post-infection |
References
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- World Health Organization. Special Programme for Research and Training in Tropical Diseases. In Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control; TDR; World Health Organization: Geneva, Switzerland, 2009; p. 147. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Questions and Answers on Dengue Vaccines. Available online: https://www.who.int/immunization/research/development/dengue_q_and_a/en/ (accessed on 15 May 2020).
- Rather, I.A.; Parray, H.A.; Lone, J.B.; Paek, W.K.; Lim, J.; Bajpai, V.K.; Park, Y.H. Prevention and Control Strategies to Counter Dengue Virus Infection. Front. Cell. Infect. Microbiol. 2017, 7, 336. [Google Scholar] [CrossRef]
- Schwab, S.R.; Stone, C.M.; Fonseca, D.M.; Fefferman, N.H. The importance of being urgent: The impact of surveillance target and scale on mosquito-borne disease control. Epidemics 2018, 23, 55–63. [Google Scholar] [CrossRef]
- Eisen, L.; Beaty, B.J.; Morrison, A.C.; Scott, T.W. Proactive Vector control strategies and improved monitoring and evaluation practices for dengue prevention. J. Med. Entomol. 2009, 46, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Unnasch, T.R.; Katholi, C.R.; Lampman, R.; Novak, R.J. Fundamental issues in mosquito surveillance for arboviral transmission. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 817–822. [Google Scholar] [CrossRef]
- Sylvestre, G.; Gandini, M.; de Araujo, J.M.; Kubelka, C.F.; Lourenco-de-Oliveira, R.; Maciel-de-Freitas, R. Preliminary evaluation on the efficiency of the kit Platelia Dengue NS1 Ag-ELISA to detect dengue virus in dried Aedes aegypti: A potential tool to improve dengue surveillance. Parasites Vectors 2014, 7, 155. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, P.S.; Li, M.Z.; Vythilingam, I.; Ng, L.C. Evaluation of the Dengue NS1 Ag Strip(R) for detection of dengue virus antigen in Aedes aegypti (Diptera: Culicidae). Vector Borne Zoonotic Dis. 2011, 11, 789–792. [Google Scholar] [CrossRef]
- Tsai, J.J.; Liu, W.L.; Lin, P.C.; Huang, B.Y.; Tsai, C.Y.; Chou, P.H.; Lee, F.C.; Ping, C.F.; Lee, P.A.; Liu, L.T.; et al. An RT-PCR panel for rapid serotyping of dengue virus serotypes 1 to 4 in human serum and mosquito on a field-deployable PCR system. PLoS ONE 2019, 14, e0214328. [Google Scholar] [CrossRef]
- Tsai, J.J.; Liu, W.L.; Lin, P.C.; Huang, B.Y.; Tsai, C.Y.; Lee, P.A.; Tsai, Y.L.; Chou, P.H.; Chung, S.; Liu, L.T.; et al. A fully automated sample-to-answer PCR system for easy and sensitive detection of dengue virus in human serum and mosquitos. PLoS ONE 2019, 14, e0218139. [Google Scholar] [CrossRef]
- Voge, N.V.; Sanchez-Vargas, I.; Blair, C.D.; Eisen, L.; Beaty, B.J. Detection of dengue virus NS1 antigen in infected Aedes aegypti using a commercially available kit. Am. J. Trop. Med. Hyg. 2013, 88, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Najioullah, F.; Verlaeten, O.; Martial, J.; Brichler, S.; Kaidomar, S.; Moravie, V.; Cabie, A.; Cesaire, R. Short Report: Relationship between Nonstructural Protein 1 Detection and Plasma Virus Load in Dengue Patients. Am. J. Trop. Med. Hyg. 2010, 83, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.N.; da Silva, E.M.; Allonso, D.; Coelho, D.R.; Andrade, I.D.; de Medeiros, L.N.; Menezes, J.L.; Barbosa, A.S.; Mohana-Borges, R. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581. [Google Scholar] [CrossRef] [PubMed]
- Thiemmeca, S.; Tamdet, C.; Punyadee, N.; Prommool, T.; Songjaeng, A.; Noisakran, S.; Puttikhunt, C.; Atkinson, J.P.; Diamond, M.S.; Ponlawat, A.; et al. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization. J. Immunol. 2016, 197, 4053–4065. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Mason, P.W. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology 1989, 169, 354–364. [Google Scholar] [CrossRef]
- Alcala, A.C.; Medina, F.; Gonzalez-Robles, A.; Salazar-Villatoro, L.; Fragoso-Soriano, R.J.; Vasquez, C.; Cervantes-Salazar, M.; Del Angel, R.M.; Ludert, J.E. The dengue virus non-structural protein 1 (NS1) is secreted efficiently from infected mosquito cells. Virology 2016, 488, 278–287. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Danet, L.; Beauclair, G.; Berthet, M.; Moratorio, G.; Gracias, S.; Tangy, F.; Choumet, V.; Jouvenet, N. Midgut barriers prevent the replication and dissemination of the yellow fever vaccine in Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007299. [Google Scholar] [CrossRef]
- Franz, A.W.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Le Coupanec, A.; Tchankouo-Nguetcheu, S.; Roux, P.; Khun, H.; Huerre, M.; Morales-Vargas, R.; Enguehard, M.; Lavillette, D.; Misse, D.; Choumet, V. Co-Infection of Mosquitoes with Chikungunya and Dengue Viruses Reveals Modulation of the Replication of Both Viruses in Midguts and Salivary Glands of Aedes aegypti Mosquitoes. Int. J. Mol. Sci. 2017, 18, 1708. [Google Scholar] [CrossRef]
- Kuno, G.; Oliver, A. Maintaining Mosquito Cell-Lines at High-Temperatures - Effects on the Replication of Flaviviruses. Vitro Cell. Dev. Biol. 1989, 25, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.S.L.; Nguyen-Khuong, T.; Rudd, P.M.; Alonso, S. Dengue Virus Glycosylation: What Do We Know? Front. Microbiol. 2017, 8, 1415. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Cho, H.; Fremont, D.H.; Diamond, M.S. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J. Virol. 2010, 84, 9516–9532. [Google Scholar] [CrossRef] [PubMed]
- Alcala, A.C.; Hernandez-Bravo, R.; Medina, F.; Coll, D.S.; Zambrano, J.L.; del Angel, R.M.; Ludert, J.E. The dengue virus non-structural protein 1 (NS1) is secreted from infected mosquito cells via a non-classical caveolin-1-dependent pathway. J. Gen. Virol. 2017, 98, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Alcala, A.C.; Palomares, L.A.; Ludert, J.E. Secretion of Nonstructural Protein 1 of Dengue Virus from Infected Mosquito Cells: Facts and Speculations. J. Virol. 2018, 92, e00275-18. [Google Scholar] [CrossRef]
- Frank, P.G.; Cheung, M.W.; Pavlides, S.; Llaverias, G.; Park, D.S.; Lisanti, M.P. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am. J. Physiol Heart Circ. Physiol. 2006, 291, H677–H686. [Google Scholar] [CrossRef]
- Ramirez, R.R.; Ludert, J.E. The Dengue Virus Nonstructural Protein 1 (NS1) Is Secreted from Mosquito Cells in Association with the Intracellular Cholesterol Transporter Chaperone Caveolin Complex. J. Virol. 2019, 93, e01985-18. [Google Scholar]
- Ciota, A.T.; Keyel, A.C. The Role of Temperature in Transmission of Zoonotic Arboviruses. Viruses 2019, 11, 1013. [Google Scholar] [CrossRef]
- Kay, B. Dengue vector surveillance and control. Curr. Opin. Infect. Dis. 1999, 12, 425–432. [Google Scholar] [CrossRef]

| Days Post-Infection | Sample No. | Rapid Test | RT-qPCR | Sensitivity | Specificity | Cohen’s Kappa Agreement | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| Day 3 | 41 | 14 | 27 | 24 | 17 | 58.33% | 100% | Moderate |
| Day 5 | 43 | 24 | 19 | 27 | 16 | 88.89% | 100% | Almost perfect |
| Day 7 | 40 | 25 | 15 | 33 | 7 | 84.85% | 100% | Moderate |
| Day 14 | 36 | 24 | 12 | 27 | 9 | 88.89% | 100% | Substantial |
| Day 21 | 36 | 30 | 6 | 34 | 2 | 88.24% | 100% | Moderate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Liu, W.-L.; Li, H.-H.; Su, M.P.; Wu, S.-C.; Chen, H.-W.; Pan, C.-Y.; Tsai, J.-J.; Chen, C.-H. Releasing Intracellular NS1 from Mosquito Cells for the Detection of Dengue Virus-Infected Mosquitoes. Viruses 2020, 12, 1105. https://doi.org/10.3390/v12101105
Cheng L, Liu W-L, Li H-H, Su MP, Wu S-C, Chen H-W, Pan C-Y, Tsai J-J, Chen C-H. Releasing Intracellular NS1 from Mosquito Cells for the Detection of Dengue Virus-Infected Mosquitoes. Viruses. 2020; 12(10):1105. https://doi.org/10.3390/v12101105
Chicago/Turabian StyleCheng, Lie, Wei-Liang Liu, Hsing-Han Li, Matthew P. Su, Shih-Cheng Wu, Hsin-Wei Chen, Chao-Ying Pan, Jih-Jin Tsai, and Chun-Hong Chen. 2020. "Releasing Intracellular NS1 from Mosquito Cells for the Detection of Dengue Virus-Infected Mosquitoes" Viruses 12, no. 10: 1105. https://doi.org/10.3390/v12101105
APA StyleCheng, L., Liu, W.-L., Li, H.-H., Su, M. P., Wu, S.-C., Chen, H.-W., Pan, C.-Y., Tsai, J.-J., & Chen, C.-H. (2020). Releasing Intracellular NS1 from Mosquito Cells for the Detection of Dengue Virus-Infected Mosquitoes. Viruses, 12(10), 1105. https://doi.org/10.3390/v12101105






