Identifying Spanish Areas at More Risk of Monthly BTV Transmission with a Basic Reproduction Number Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monthly Culicoides spp. Maps and Environmental Variables
2.2. Transmission Risk Maps Based on the Basic Reproduction Number (R0)
3. Results
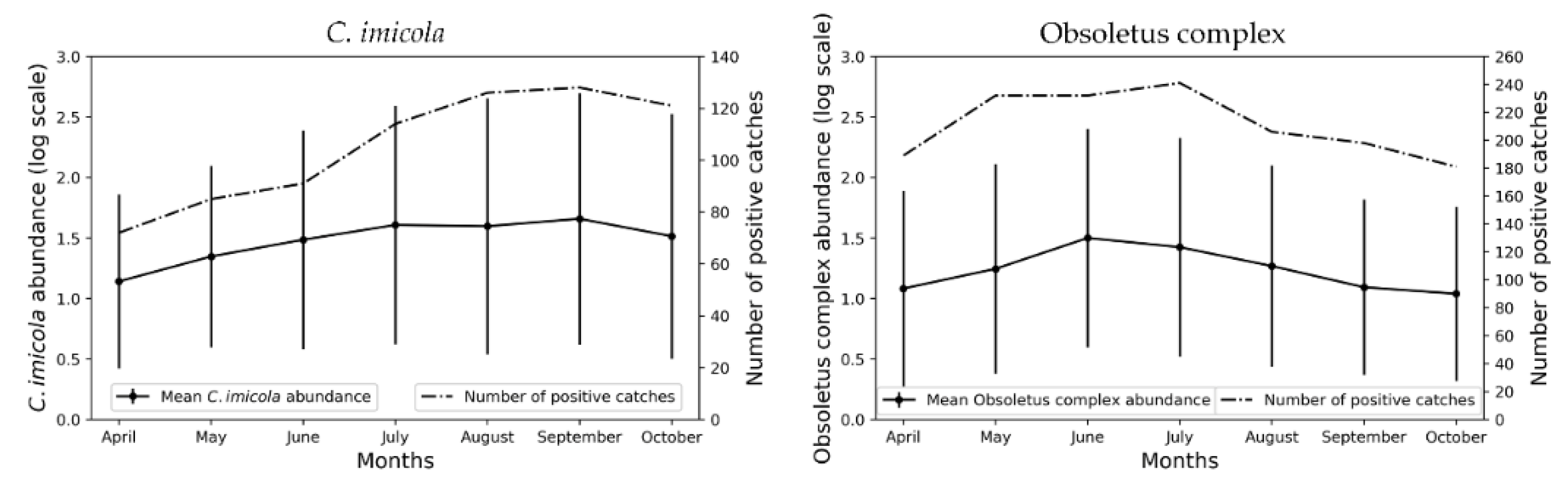
3.1. Monthly Culicoides spp. Maps
3.2. Transmission Risk Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sperlova, A.; Zendulkova, D. Bluetongue: A review. Vet. Med. 2011, 56, 430–452. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcacci, M.; Sant, S.; Mangone, I.; Goria, M.; Dondo, A.; Zoppi, S.; van Gennip, R.G.P.; Radaelli, M.C.; Cammà, C.; van Rijn, P.A.; et al. One after the other: A novel Bluetongue virus strain related to Toggenburg virus detected in the Piedmont region (North-western Italy), extends the panel of novel atypical BTV strains. Transbound Emerg. Dis. 2018, 65, 370–374. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on bluetongue serotype 8. EFSA J. 2011, 9, 2189. [Google Scholar] [CrossRef]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Vectors, epidemiology and climate change. Parasitol. Res. 2008, 103 (Suppl. 1), S69–S77. [Google Scholar] [CrossRef] [PubMed]
- MARM. Lengua Azul: Situación de la Enfermedad en España y Europa; Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2008.
- de Diego, A.C.; Sánchez-Cordón, P.J.; Sánchez-Vizcaíno, J.M. Bluetongue in Spain: From the first outbreak to 2012. Transbound Emerg. Dis. 2014, 61, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- MAGRAMA. Informe sobre la declaración de libre del serotipo 1 del virus de la lengua azul en el norte y este peninsular español); Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015.
- MAGRAMA. Informe de Situación del Virus de la Lengua Azul en España (19/1/2015); Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015.
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, present and future. Philos Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2669–2681. [Google Scholar] [CrossRef]
- Clercq, K.; Vandenbussche, F.; Vandemeulebroucke, E.; Vanbinst, T.; Leeuw, I.; Verheyden, B.; Goris, N.; Mintiens, K.; Méroc, E.; Herr, C.; et al. Transplacental Bluetongue infection in cattle. Vet. Rec. 2008, 162, 564. [Google Scholar] [CrossRef] [PubMed]
- Backx, A.; Heutink, R.; van Rooij, E.; van Rijn, P. Transplacental and oral transmission of wild-type bluetongue virus serotype 8 in cattle after experimental infection. Vet. Microbiol. 2009, 138, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Darpel, K.E.; Barber, J.; Hope, A.; Wilson, A.J.; Gubbins, S.; Henstock, M.; Frost, L.; Batten, C.; Veronesi, E.; Moffat, K.; et al. Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci. Rep. 2016, 6, 20627. [Google Scholar] [CrossRef] [Green Version]
- Bouwknegt, C.; van Rijn, P.A.; Schipper, J.J.M.; Hölzel, D.; Boonstra, J.; Nijhof, A.M.; van Rooij, E.M.A.; Jongejan, F. Potential role of ticks as vectors of bluetongue virus. Exp. Appl. Acarol. 2010, 52, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.E.; Gorman, B.M.; Tesh, R.B.; Knudson, D.L. Isolation of bluetongue and epizootic hemorrhagic disease viruses from mosquitoes collected in Indonesia. Vet. Microbiol. 1992, 32, 241–252. [Google Scholar] [CrossRef]
- Luedke, A.J.; Jochim, M.M.; Bowne, J.G. Preliminary bluetongue Transmission with the sheep ked Melophagus ovinus (L.). Can. J. Comp. Med. Vet. Sci. 1965, 29, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehlhorn, H.; Walldorf, V.; Klimpel, S.; Jahn, B.; Jaeger, F.; Eschweiler, J.; Hoffmann, B.; Beer, M. First occurrence of Culicoides obsoletus-transmitted Bluetongue virus epidemic in Central Europe. Parasitol. Res. 2007, 101, 219–228. [Google Scholar] [CrossRef]
- Meiswinkel, R.; Baldet, T.; de Deken, R.; Takken, W.; Delécolle, J.C.; Mellor, P.S. The 2006 outbreak of bluetongue in northern Europe—The entomological perspective. Prev. Vet. Med. 2008, 87, 55–63. [Google Scholar] [CrossRef]
- Dijkstra, E.; van der Ven, I.J.; Meiswinkel, R.; Holzel, D.R.; Van Rijn, P.A.; Meiswinkel, R. Culicoides chiopterus as a potential vector of bluetongue virus in Europe. Vet. Rec. 2008, 162, 422. [Google Scholar] [CrossRef]
- Foxi, C.; Meloni, G.; Puggioni, G.; Manunta, D.; Rocchigiani, A.; Vento, L.; Cabras, P.; Satta, G. Bluetongue virus detection in new Culicoides species in Sardinia, Italy. Vet. Rec. 2019, 184, 621. [Google Scholar] [CrossRef]
- Lucientes, J.; Alarcón-Elbal, P.M. Culicoides biting midges in Spain: A brief overview. Small Rumin. Res. 2016, 142, 69–71. [Google Scholar] [CrossRef]
- Calvete, C.; Estrada, R.; Miranda, M.A.; Borrás, D.; Calvo, J.H.; Lucientes, J. Modelling the distributions and spatial coincidence of bluetongue vectors Culicoides imicola and the Culicoides obsoletus group throughout the Iberian peninsula. Med. Vet. Entomol. 2008, 22, 124–134. [Google Scholar] [CrossRef]
- Cuéllar, A.C.; Jung Kjær, L.; Baum, A.; Stockmarr, A.; Skovgard, H.; Nielsen, S.A.; Andersson, M.G.; Lindström, A.; Chirico, J.; Lühken, R.; et al. Monthly variation in the probability of presence of adult Culicoides populations in nine European countries and the implications for targeted surveillance. Parasit Vectors 2018, 11, 608. [Google Scholar] [CrossRef]
- Ducheyne, E.; Miranda Chueca, M.A.; Lucientes, J.; Calvete, C.; Estrada, R.; Boender, G.J.; Goossens, E.; De Clercq, E.M.; Hendrickx, G. Abundance modelling of invasive and indigenous Culicoides species in Spain. Geospat Health 2013, 8, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, A.; Goffredo, M.; Ippoliti, C.; Meiswinkel, R. Influence of biotic and abiotic factors on the distribution and abundance of culicoides imicola and the obsoletus complex in Italy. Vet. Parasitol. 2007, 150. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Vega, C.; Fernández-Carrión, E.; Lucientes, J.; Sánchez-Vizcaíno, J.M. A model for the assessment of bluetongue virus serotype 1 persistence in Spain. PLoS ONE 2020, 15, e0232534. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 1992, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Choisy, M.; Guégan, J.F.; Rohani, P. Mathematical Modeling of Infectious Diseases Dynamics. In Encyclopedia of Infectious Diseases; Tibayrenc, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 379–404. [Google Scholar] [CrossRef]
- Li, J.; Blakeley, D.; Smith, R.J. The failure of R0. Comput. Math. Methods Med. 2011, 2011, 527610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, L.F.; Coutinho, F.A.B.; Burattini, M.N.; Massad, E. Threshold conditions for infection persistence in complex host-vectors interactions. C R Biol. 2002, 325, 1073–1084. [Google Scholar] [CrossRef]
- Turner, J.; Bowers, R.G.; Baylis, M. Two-Host, Two-Vector Basic Reproduction Ratio (R0) for Bluetongue. PLoS ONE 2013, 8, e53128. [Google Scholar] [CrossRef] [Green Version]
- Hartemink, N.A.; Purse, B.V.; Meiswinkel, R.; Brown, H.E.; de Koeijer, A.; Elbers, A.R.W.; Boender, G.J.; Rogers, D.J.; Heesterbeek, J.A.P. Mapping the basic reproduction number (R0) for vector-borne diseases: A case study on bluetongue virus. Epidemics 2009, 1, 153–161. [Google Scholar] [CrossRef]
- Calistri, P.; Savini, L.; Candeloro, L.; Di Sabatino, D.; Cito, F.; Bruno, R.; Danzetta, M.L. A Transitional Model for the Evaluation of West Nile Virus Transmission in Italy. Transbound Emerg. Dis. 2016, 63, 485–496. [Google Scholar] [CrossRef]
- Ogden, N.H.; Radojevic, M.; Wu, X.; Duvvuri, V.R.; Leighton, P.A.; Wu, J. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ. Health Perspect 2014, 122, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Rocklov, J.; Quam, M.B.; Sudre, B.; German, M.; Kraemer, M.U.G.; Brady, O.; Bogoch, I.I.; Liu-Helmersson, J.; Wilder-Smith, A.; Semenza, J.C.; et al. Assessing Seasonal Risks for the Introduction and Mosquito-borne Spread of Zika Virus in Europe. EBioMedicine 2016, 9, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guis, H.; Caminade, C.; Calvete, C.; Morse, A.P.; Tran, A.; Baylis, M. Modelling the effects of past and future climate on the risk of bluetongue emergence in Europe. J. R Soc. Interface 2012, 9, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racloz, V.; Venter, G.; Griot, C.; Stärk, K.D.C. Estimating the temporal and spatial risk of bluetongue related to the incursion of infected vectors into Switzerland. BMC Vet. Res. 2008, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Brugger, K.; Rubel, F. Bluetongue Disease Risk Assessment Based on Observed and Projected Culicoides obsoletus spp. Vector Densities. PLoS ONE 2013, 8, e60330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayo, C.; Shelley, C.; MacLachlan, N.J.; Gardner, I.; Hartley, D.; Barker, C. A Deterministic Model to Quantify Risk and Guide Mitigation Strategies to Reduce Bluetongue Virus Transmission in California Dairy Cattle. PLoS ONE 2016, 11, e0165806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MAPA. Red de Alerta Sanitaria Veterinaria (RASVE); Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2020.
- Calvete, C.; Estrada, R.; Miranda, M.A.; Del Río, R.; Borrás, D.; Garrido, L.; Muñoz, B.; Romero, L.J.; Lucientes, J. Evaluación de la eficacia del programa de monitorización de las poblaciones de vectores de lengua azul, Culicoides imicola Kieffer, 1913 y el complejo Culicoides obsoletus Meigen, 1818 (Diptera: Ceratopogonidae), en España. ITEA 2009, 105, 147–160. [Google Scholar]
- Baylis, M.; El Hasnaoui, H.; Bouayoune, H.; Touti, J.; Mellor, P.S. The spatial and seasonal distribution of African horse sickness and its potential Culicoides vectors in Morocco. Med. Vet. Entomol. 1997, 11, 203–212. [Google Scholar] [CrossRef]
- Rigot, T.; Conte, A.; Goffredo, M.; Ducheyne, E.; Hendrickx, G.; Gilbert, M. Predicting the spatio-temporal distribution of Culicoides imicola in Sardinia using a discrete-time population model. Parasit Vectors 2012, 5, 270. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, P.; Ruiz-Fons, F.; Estrada, R.; Márquez, A.L.; Miranda, M.A.; Gortázar, C.; Lucientes, J. A Broad Assessment of Factors Determining Culicoides imicola Abundance: Modelling the Present and Forecasting Its Future in Climate Change Scenarios. PLoS ONE 2010, 5, e14236. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; De Baets, B.; Van Doninck, J.; Calvete, C.; Lucientes, J.; De Clercq, E.M.; Ducheyne, E.; Verhoest, N. Absence reduction in entomological surveillance data to improve niche-based distribution models for Culicoides imicola. Prev. Vet. Med. 2011, 100, 15–28. [Google Scholar] [CrossRef]
- Searle, K.R.; Blackwell, A.; Falconer, D.; Sullivan, M.; Butler, A.; Purse, B.V. Identifying environmental drivers of insect phenology across space and time: Culicoides in Scotland as a case study. Bull. Entomol. Res. 2013, 103, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Falconer, D.; Sullivan, M.J.; Carpenter, S.; Mellor, P.S.; Piertney, S.B.; Mordue, A.J.; Albon, S.; Gunn, G.J.; Blackwell, A. Impacts of climate, host and landscape factors on Culicoides species in Scotland. Med. Vet. Entomol. 2012, 26, 168–177. [Google Scholar] [CrossRef]
- Wan, Z.; Hook, S.; Hulley, G. MOD11A2 MODIS/Terra Land Surface Temperature/Emissivity 8-Day L3 Global 1km SIN Grid V006, V006 [Data set] ed.; NASA: Washington, DC, USA, 2015. [CrossRef]
- Wan, Z. New refinements and validation of the collection-6 MODIS land-surface temperature/emissivity product. Remote Sens. Environ. 2014, 140, 36–45. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017. [Google Scholar] [CrossRef]
- Campbell, J.B.; Wynne, R.H. Introduction to Remote Sensing; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006, V006 [Data set] ed.; NASA: Washington, DC, USA, 2015. [CrossRef]
- Calvete, C.; Estrada, R.; Miranda, M.A.; Borrás, D.; Calvo, J.H.; Lucientes, J. Ecological correlates of bluetongue virus in Spain: Predicted spatial occurrence and its relationship with the observed abundance of the potential Culicoides spp. vector. Vet. J. 2009, 182, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.M.; Carpenter, S.; Prasad, G.; Sedda, L.; Henrys, P.A.; Gajendragad, M.R.; Purse, B.V. Livestock host composition rather than land use or climate explains spatial patterns in bluetongue disease in South India. Sci. Rep. 2019, 9, 4229. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.P.; Wint, G.R.W.; Conchedda, G.; Van Boeckel, T.P.; Ercoli, V.; Palamara, E.; Cinardi, G.; D’Aietti, L.; Hay, S.I.; Gilbert, M. Mapping the Global Distribution of Livestock. PLoS ONE 2014, 9, e96084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wint, W.; Morley, D.; Medlock, J.; Alexander, N. A First Attempt at Modelling Red Deer (Cervus elaphus) Distributions Over Europe. Open Health Data 2014, 2, e1. [Google Scholar] [CrossRef] [Green Version]
- USGS. Global 30 Arc-Second Elevation (GTOPO30); United States Geological Survey: Reston, VA, USA, 1996. [CrossRef]
- Scolamacchia, F.; Van Den Broek, J.; Meiswinkel, R.; Heesterbeek, J.A.P.; Elbers, A.R.W. Principal climatic and edaphic determinants of Culicoides biting midge abundance during the 2007–2008 bluetongue epidemic in the Netherlands, based on OVI light trap data. Med. Vet. Entomol. 2014, 28, 143–156. [Google Scholar] [CrossRef]
- ESA. Land Cover CCI Product User Guide Version 2.0. Available online: http://maps.elie.ucl.ac.be/CCI/viewer/download/ESACCI-LC-Ph2-PUGv2_2.0.pdf (accessed on 12 September 2017).
- Ippoliti, C.; Gilbert, M.; Vanhuysse, S.; Goffredo, M.; Satta, G.; Wolff, E.; Conte, A. Can landscape metrics help determine the Culicoides imicola distribution in Italy? Geospat. Health 2013, 8, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, C.; Panagos, P.; Monatanarella, L. Mapping topsoil physical properties at European scale using the LUCAS database. Geoderma 2016, 261, 110–123. [Google Scholar] [CrossRef]
- Jones, R.J.A.; Hiederer, R.; Rusco, E.; Montanarella, L. Estimating organic carbon in the soils of Europe for policy support. Eur. J. Soil Sci. 2005, 56, 655–671. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Archer, K.J.; Kimes, R.V. Empirical characterization of random forest variable importance measures. Comput. Stat. Data Anal. 2008, 52, 2249–2260. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Int. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Assessing the Fit of the Model. In Applied Logistic Regression; Balding, D.J., Cressie, N.A.C., Fitzmaurice, G.M., Goldstein, H., Johnstone, I.M., Molenberghs, G., Scott, D.W., Smith, A.F.M., Tsay, R.S., Weisberg, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 153–225. [Google Scholar] [CrossRef]
- Aggarwal, C.C. Data Mining: The Textbook; Springer International Publishing: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Saito, T.; Rehmsmeier, M. The Precision-Recall Plot Is More Informative than the ROC Plot When Evaluating Binary Classifiers on Imbalanced Datasets. PLoS ONE 2015, 10, e0118432. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Torgo, L. Data Mining with R, Learning with Case Studies; Chapman and Hall/CRC: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. R package version 6.0-86. Available online: https://CRAN.R-project.org/package=caret (accessed on 13 October 2020).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R package version 3.3-13. Available online: https://CRAN.R-project.org/package=raster (accessed on 13 October 2020).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Gubbins, S.; Carpenter, S.; Baylis, M.; Wood, J.L.N.; Mellor, P.S. Assessing the risk of bluetongue to UK livestock: Uncertainty and sensitivity analyses of a temperature-dependent model for the basic reproduction number. J. R Soc. Interface 2008, 5, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Federici, V.; Goffredo, M.; Mancini, G.; Quaglia, M.; Santilli, A.; Di Nicola, F.; De Ascentis, M.; Cabras, P.; Volpicelli, C.; De Liberato, C.; et al. Vector Competence of Italian Populations of Culicoides for Some Bluetongue Virus Strains Responsible for Recent Northern African and European Outbreaks. Viruses 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- MAPA. Encuestas Ganaderas, Análisis del Número de Animales Por Tipos. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/ganaderia/encuestas-ganaderas/ (accessed on 29 April 2020).
- Baylis, M.; O’Connell, L.; Mellor, P.S. Rates of bluetongue virus transmission between Culicoides sonorensis and sheep. Med. Vet. Entomol. 2008, 22, 228–237. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Panel on Animal Health Welfare. Bluetongue: Control, surveillance and safe movement of animals. EFSA J. 2017, 15, e04698. [Google Scholar] [CrossRef] [Green Version]
- Melville, L.F.; Weir, R.; Harmsen, M.; Walsh, S.; Hunt, N.T.; Daniels, P.W. Characteristics of naturally-occurring bluetongue viral infections of cattle. In Proceedings of Bluetongue disease in Southeast Asia and the Pacific. In Proceedings of the First Southeast Asia and Pacific Bluetongue symposium, Greenlake Hotel, Kunming, China, 22–24 August 1995; pp. 245–250. [Google Scholar]
- Goldsmit, L.; Barzilai, E.; Tadmor, A. The comparative sensitivity of sheep and chicken embryos to bluetongue virus and observations of viraemia in experimentally infected sheep. Aust. Vet. J. 1975, 51, 190–196. [Google Scholar] [CrossRef]
- Veronesi, E.; Hamblin, C.; Mellor, P.S. Live attenuated bluetongue vaccine viruses in Dorset Poll sheep, before and after passage in vector midges (Diptera: Ceratopogonidae). Vaccine 2005, 23, 5509–5516. [Google Scholar] [CrossRef]
- Batten, C.; Darpel, K.; Henstock, M.; Fay, P.; Veronesi, E.; Gubbins, S.; Graves, S.; Frost, L.; Oura, C. Evidence for transmission of bluetongue virus serotype 26 through direct contact. PLoS ONE 2014, 9, e96049. [Google Scholar] [CrossRef] [Green Version]
- Mullens, B.A.; Gerry, A.C.; Lysyk, T.J.; Schmidtmann, E.T. Environmental effects on vector competence and virogenesis of bluetongue virus in Culicoides: Interpreting laboratory data in a field context. Vet. Ital. 2004, 40, 160–166. [Google Scholar]
- Verhoef, F.A.; Venter, G.J.; Weldon, C.W. Thermal limits of two biting midges, Culicoides imicola Kieffer and C. bolitinos Meiswinkel (Diptera: Ceratopogonidae). Parasit Vectors 2014, 7, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittmann, E.J.; Mellor, P.S.; Baylis, M. Effect of temperature on the transmission of orbiviruses by the biting midge, Culicoides sonorensis. Med. Vet. Entomol. 2002, 16, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carrión, E.; Ivorra, B.; Ramos, A.M.; Martínez-López, B.; Aguilar-Vega, C.; Sánchez-Vizcaíno, J.M. An advection-deposition-survival model to assess the risk of introduction of vector-borne diseases through the wind: Application to bluetongue outbreaks in Spain. PLoS ONE 2018, 13, e0194573. [Google Scholar] [CrossRef] [Green Version]
- Braverman, Y.; Frish, K.; Reis, M.; Mumcuoglu, K.Y. Host Preference of Culicoides spp from Israel Based on Sensory Organs and Morphometry (Diptera: Ceratopogonidae). Entomol. Gen. 2012, 34, 97–110. [Google Scholar] [CrossRef]
- Carpenter, S.; Szmaragd, C.; Barber, J.; Labuschagne, K.; Gubbins, S.; Mellor, P. An assessment of Culicoides surveillance techniques in northern Europe: Have we underestimated a potential bluetongue virus vector? J. Appl. Ecol. 2008, 45, 1237–1245. [Google Scholar] [CrossRef]
- Gerry, A.C.; Sarto i Monteys, V.; Moreno Vidal, J.O.; Francino, O.; Mullens, B.A. Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in northeastern Spain in relation to midge capture using UV light and carbon dioxide-baited traps. J. Med. Entomol. 2009, 46, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, E.G.; Venter, G.J.; Labuschagne, K.; Page, P.C.; Mullens, B.A.; MacLachlan, N.J.; Osterrieder, N.; Guthrie, A.J. Comparison of two trapping methods for Culicoides biting midges and determination of African horse sickness virus prevalence in midge populations at Onderstepoort, South Africa. Vet. Parasitol 2012, 185, 265–273. [Google Scholar] [CrossRef]
- Viennet, E.; Garros, C.; Lancelot, R.; Allène, X.; Gardès, L.; Rakotoarivony, I.; Crochet, D.; Delécolle, J.-C.; Moulia, C.; Baldet, T.; et al. Assessment of vector/host contact: Comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of Orbiviruses. Parasit Vectors 2011, 4, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viennet, E.; Garros, C.; Gardès, L.; Rakotoarivony, I.; Allène, X.; Lancelot, R.; Crochet, D.; Moulia, C.; Baldet, T.; Balenghien, T. Host preferences of Palaearctic Culicoides biting midges: Implications for transmission of orbiviruses. Med. Vet. Entomol. 2013, 27, 255–266. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- MATLAB. Version 9.8.0.1323502 (R2020a); The MathWorks Inc.: Natick, MA, USA, 2020. [Google Scholar]
- Jenks, G. The Data Model Concept in Statistical Mapping. Int. J. Cart. 1967, 7, 186–190. [Google Scholar]
- ADNS. Animal Disease Notification System Database. Available online: https://ec.europa.eu/food/animals/animal-diseases/not-system_en (accessed on 11 December 2019).
- Napp, S.; Allepuz, A.; Purse, B.V.; Casal, J.; Garcia-Bocanegra, I.; Burgin, L.E.; Searle, K.R. Understanding Spatio-Temporal Variability in the Reproduction Ratio of the Bluetongue (BTV-1) Epidemic in Southern Spain (Andalusia) in 2007 Using Epidemic Trees. PLoS ONE 2016, 11, e0151151. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Delgado, M.; Cernadas, E.; Barro, S.; Amorim, D. Do we Need Hundreds of Classifiers to Solve Real World Classification Problems? J. Mach. Learn. Res. 2014, 15, 3133–3181. [Google Scholar]
- Gerry, A.C.; Mullens, B.A. Seasonal Abundance and Survivorship of Culicoides sonorensis (Diptera: Ceratopogonidae) at a Southern California Dairy, with Reference to Potential Bluetongue Virus Transmission and Persistence. J. Med. Entomol. 2000, 37, 675–688. [Google Scholar] [CrossRef]
- Caracappa, S.; Torina, A.; Guercio, A.; Vitale, F.; Calabro, A.; Purpari, G.; Ferrantelli, V.; Vitale, M.; Mellor, P.S. Identification of a novel bluetongue virus vector species of Culicoides in Sicily. Vet. Rec. 2003, 153, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, R.; van Rijn, P.; Leijs, P.; Goffredo, M. Potential new Culicoides vector of bluetongue virus in northern Europe. Vet. Rec. 2007, 161, 564–565. [Google Scholar] [CrossRef] [Green Version]
- López-Olvera, J.R.; Falconi, C.; Fernández-Pacheco, P.; Fernández-Pinero, J.; Sánchez, M.A.; Palma, A.; Herruzo, I.; Vicente, J.; Jiménez-Clavero, M.A.; Arias, M.; et al. Experimental infection of European red deer (Cervus elaphus) with bluetongue virus serotypes 1 and 8. Vet. Microbiol. 2010, 145, 148–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.; Balenghien, T.; Viarouge, C.; Faure, E.; Zanella, G.; Sailleau, C.; Mathieu, B.; Delécolle, J.-C.; Ninio, C.; Garros, C.; et al. Red deer (Cervus elaphus) Did Not Play the Role of Maintenance Host for Bluetongue Virus in France: The Burden of Proof by Long-Term Wildlife Monitoring and Culicoides Snapshots. Viruses 2019, 11, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorca-Oró, C.; López-Olvera, J.R.; Ruiz-Fons, F.; Acevedo, P.; García-Bocanegra, I.; Oleaga, Á.; Gortázar, C.; Pujols, J. Long-Term Dynamics of Bluetongue Virus in Wild Ruminants: Relationship with Outbreaks in Livestock in Spain, 2006–2011. PLoS ONE 2014, 9, e100027. [Google Scholar] [CrossRef] [Green Version]
- Martínez-de la Puente, J.; Figuerola, J.; Soriguer, R. Fur or feather? Feeding preferences of species of Culicoides biting midges in Europe. Trends Parasitol. 2015, 31, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Lassen, S.B.; Nielsen, S.A.; Kristensen, M. Identity and diversity of blood meal hosts of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasit Vectors 2012, 5, 143. [Google Scholar] [CrossRef] [Green Version]
- García-Lastra, R.; Leginagoikoa, I.; Plazaola, J.M.; Ocabo, B.; Aduriz, G.; Nunes, T.; Juste, R.A. Bluetongue Virus Serotype 1 Outbreak in the Basque Country (Northern Spain) 2007–2008. Data Support a Primary Vector Windborne Transport. PLoS ONE 2012, 7, e34421. [Google Scholar] [CrossRef] [Green Version]
- Elbers, A.R.W.; Koenraadt, C.J.M.; Meiswinkel, R. Mosquitoes and Culicoides biting midges: Vector range and the influence of climate change. Rev. Sci. Tech. 2015, 34, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Sailleau, C.; Breard, E.; Viarouge, C.; Gorlier, A.; Leroux, A.; Hirchaud, E.; Lucas, P.; Blanchard, Y.; Vitour, D.; Grandcollot-Chabot, M.; et al. Emergence of bluetongue virus serotype 4 in mainland France in November 2017. Transbound Emerg. Dis. 2018, 65, 1158–1162. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control and European Food Safety Authority. The Importance of Vector Abundance and Seasonality–Results from an Expert Consultation; ECDC: Stockholm, Sweden; EFSA: Parma, Italy, 2018; p. 50.





| Non-Temperature Dependent Variables | |||
|---|---|---|---|
| Variable | Variable Description | Selected Value (Range) or Formula | Reference |
| b | Probability of transmission from vector to host | 0.9 (0.8–1.0) | [78] |
| βj | Probability of transmission from host to vector type j | 0.02 | [31,75,79] |
| mij | Ratio of vectors (Nj) to hosts (Hi) | (0–5000) | [31] |
| φCj | Proportion of vectors type j attracted to cattle | [31] | |
| φSj | Proportion of vectors type j attracted to small ruminants | [31] | |
| σ | Vector preference for host | 0.15 (0–1) | [31,75,79] |
| rC | Recovery rate of cattle (1/duration of viremia) | 0.0485 | Duration of viremia (20.6) estimated by fitting a gamma distribution to data presented in [80], by [75] |
| rS | Recovery rate of sheep (1/duration of viremia) | 0.0610 | Duration of viremia (16.4) estimated by fitting a gamma distribution to data presented in [81] and [82], by [75] |
| dC | Mortality rate of cattle | 0 | [83] |
| dS | Mortality rate of sheep | 0.0078 (0.001–0.01) | [79,83] |
| Temperature (θ) dependent variables | |||
| Variable | Variable Description | Formula | Reference |
| a1 | Biting rate for C. imicola | [79] | |
| a2 | Biting rate for the Obsoletus complex species | [84] | |
| μj | Natural mortality rate of vector type j | estimated by the Hermite cubic interpolation of temperature values of [85,86] | [87] |
| vj | Virogenesis rate in the vector type j (1/EIP) | [84] | |
| BTV-1 | |||||||
|---|---|---|---|---|---|---|---|
| Risk Category | April | May | June | July | August | September | October |
| 1 | 1 (0.5) | 0 (0) | 5 (0.03) | 33 (0.03) | 78 (0.05) | 458 (0.12) | 448 (0.10) |
| 2 | 1 (0.5) | 3 (0.33) | 129 (0.72) | 125 (0.13) | 552 (0.34) | 2084 (0.56) | 2455 (0.56) |
| 3 | 0 (0) | 6 (0.67) | 44 (0.25) | 833 (0.84) | 1017 (0.62) | 1147 (0.31) | 1477 (0.34) |
| Total number of outbreaks | 2 | 9 | 178 | 991 | 1647 | 3689 | 4380 |
| BTV-4 | |||||||
| Risk category | April | May | June | July | August | September | October |
| 1 | 0 (0) | 0 (0) | - | - | 0 (0) | 8 (0.05) | 15 (0.06) |
| 2 | 0 (0) | 0 (0) | - | - | 2 (0.50) | 70 (0.44) | 157 (0.62) |
| 3 | 1 (1) | 1 (1) | - | - | 2 (0.50) | 80 (0.51) | 83 (0.33) |
| Total number of outbreaks | 1 | 1 | 0 | 0 | 4 | 158 | 255 |
| BTV-1 | ||||||
|---|---|---|---|---|---|---|
| Month | Risk Category | Expected Probability | Observed Outbreaks | Expected Outbreaks | Residuals | Χ2 * |
| June | 1 | 0.4 | 5 | 71.2 | −7.85 | 112.02 |
| 2 | 0.49 | 129 | 87.22 | 4.47 | ||
| 3 | 0.11 | 44 | 19.58 | 5.52 | ||
| July | 1 | 0.45 | 33 | 445.95 | −19.55 | 2740.5 |
| 2 | 0.36 | 125 | 356.76 | −12.27 | ||
| 3 | 0.19 | 833 | 188.29 | 46.98 | ||
| August | 1 | 0.52 | 78 | 856.44 | −26.6 | 3107.3 |
| 2 | 0.33 | 552 | 543.51 | 0.36 | ||
| 3 | 0.15 | 1017 | 247.05 | 48.99 | ||
| September | 1 | 0.57 | 458 | 2102.73 | −35.87 | 3331.9 |
| 2 | 0.32 | 2084 | 1180.48 | 26.3 | ||
| 3 | 0.11 | 1147 | 405.79 | 36.8 | ||
| October | 1 | 0.65 | 448 | 2847 | −44.96 | 7012.7 |
| 2 | 0.27 | 2455 | 1182.6 | 37 | ||
| 3 | 0.08 | 1477 | 350.4 | 60.18 | ||
| BTV-4 | ||||||
| Month | Risk category | Expected probability | Observed outbreaks | Expected outbreaks | Residuals | Χ2 * |
| September | 1 | 0.57 | 8 | 90.06 | −8.65 | 307.86 |
| 2 | 0.32 | 70 | 50.56 | 2.73 | ||
| 3 | 0.11 | 80 | 17.38 | 15.02 | ||
| October | 1 | 0.65 | 15 | 165.75 | −11.71 | 442.06 |
| 2 | 0.27 | 157 | 68.85 | 10.62 | ||
| 3 | 0.08 | 83 | 20.4 | 13.86 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Vega, C.; Bosch, J.; Fernández-Carrión, E.; Lucientes, J.; Sánchez-Vizcaíno, J.M. Identifying Spanish Areas at More Risk of Monthly BTV Transmission with a Basic Reproduction Number Approach. Viruses 2020, 12, 1158. https://doi.org/10.3390/v12101158
Aguilar-Vega C, Bosch J, Fernández-Carrión E, Lucientes J, Sánchez-Vizcaíno JM. Identifying Spanish Areas at More Risk of Monthly BTV Transmission with a Basic Reproduction Number Approach. Viruses. 2020; 12(10):1158. https://doi.org/10.3390/v12101158
Chicago/Turabian StyleAguilar-Vega, Cecilia, Jaime Bosch, Eduardo Fernández-Carrión, Javier Lucientes, and José Manuel Sánchez-Vizcaíno. 2020. "Identifying Spanish Areas at More Risk of Monthly BTV Transmission with a Basic Reproduction Number Approach" Viruses 12, no. 10: 1158. https://doi.org/10.3390/v12101158




