Influenza Vaccines toward Universality through Nanoplatforms and Given by Microneedle Patches
Abstract
:1. Introduction
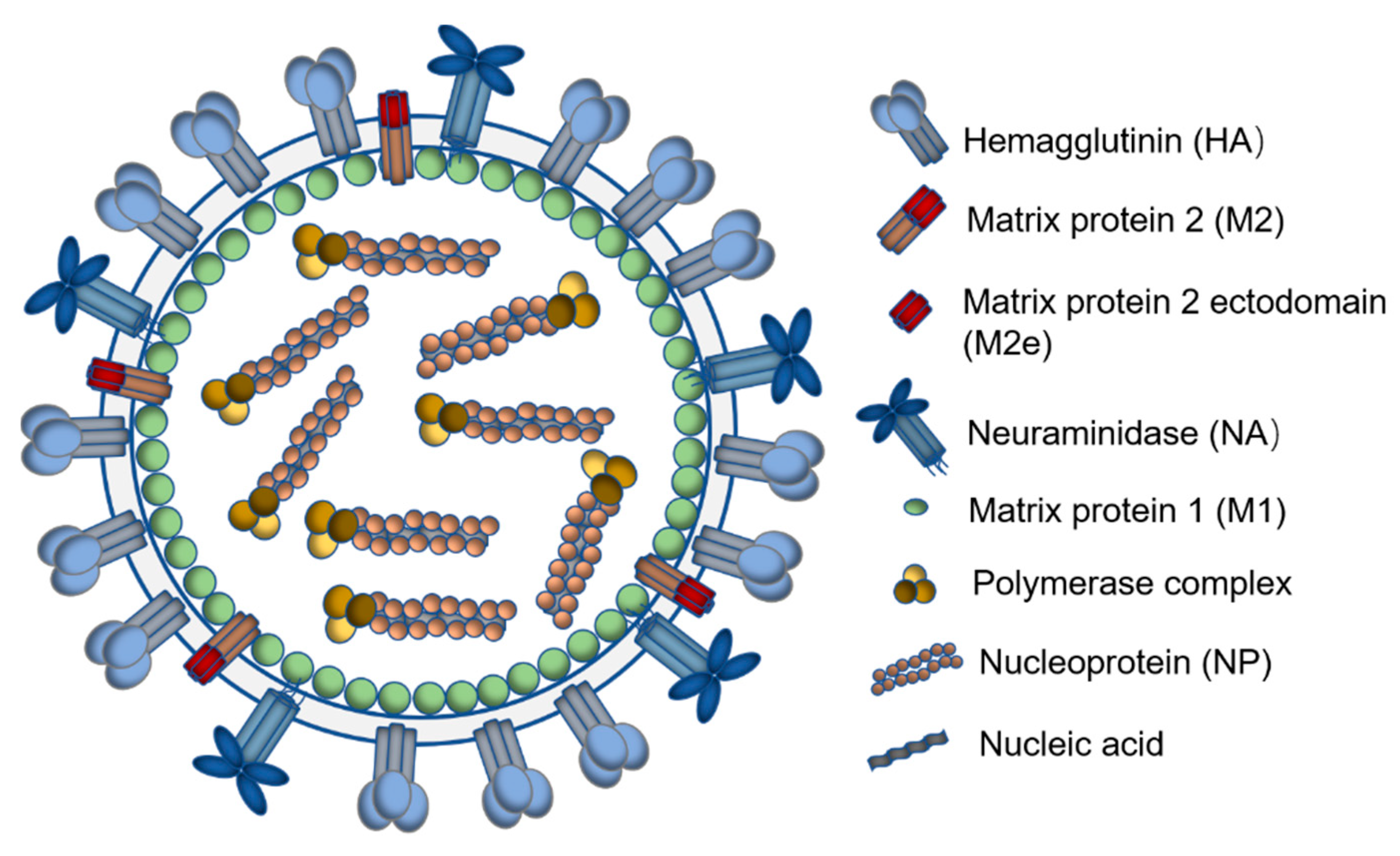
2. Antigenic Structures Conserved over Different Influenza Types Are Ideal Immunogens for a Universal Influenza Vaccine
2.1. Hemagglutinin Stalk Domain
2.2. Neuraminidase
2.3. The Ectodomain of Matrix Protein 2
2.4. Influenza Nucleoprotein
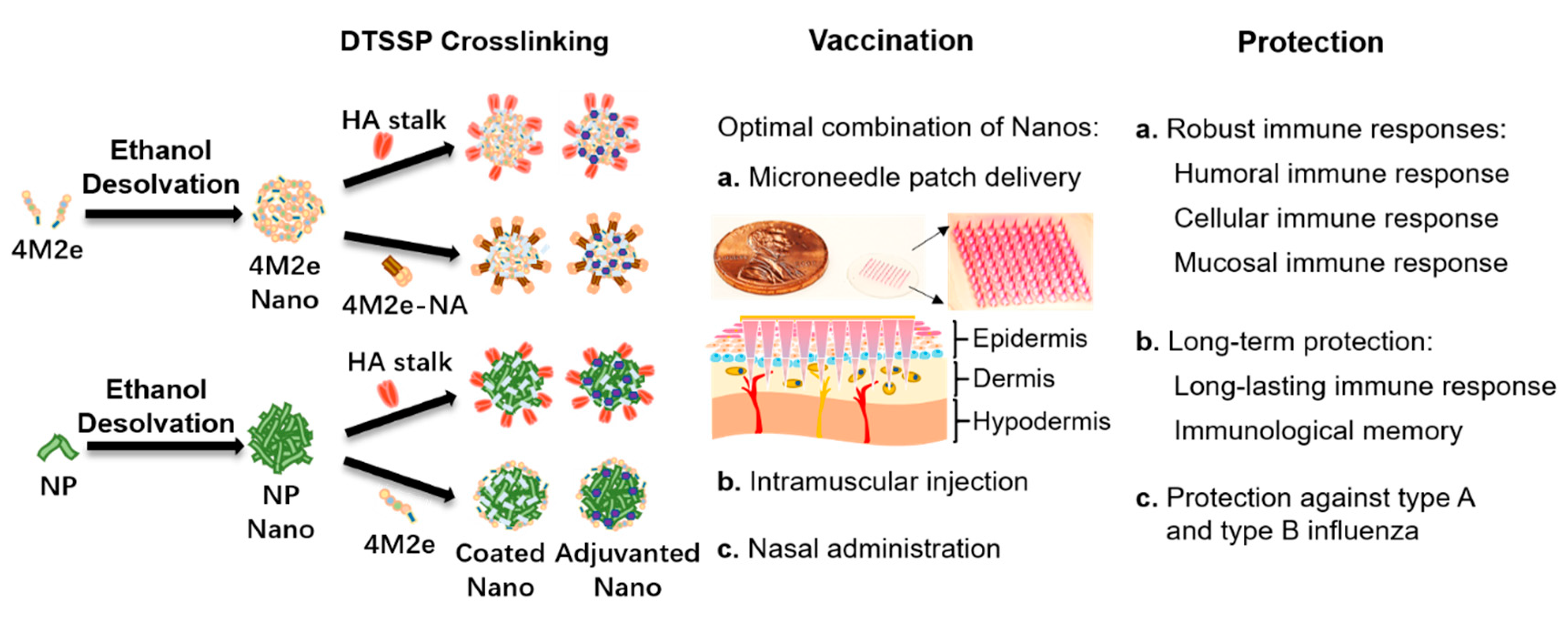
3. Nanoplatforms for Conserved Influenza Structures
4. Different Route for Vaccine Delivery
5. Dry Formulation of Influenza Vaccines on Microneedle Patches
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hause, B.M.; Collin, E.A.; Liu, R.; Huang, B.; Sheng, Z.; Lu, W.; Wang, D.; Nelson, E.A.; Li, F. Characterization of a novel influenza virus in cattle and Swine: Proposal for a new genus in the Orthomyxoviridae family. mBio 2014, 5, e00031-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferhadian, D.; Contrant, M.; Printz-Schweigert, A.; Smyth, R.P.; Paillart, J.C.; Marquet, R. Structural and functional motifs in influenza virus RNAs. Front. Microbiol. 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, E.; Liang, X.; Ingallinella, P.; Finotto, M.; Chastain, M.A.; Fan, J.; Fu, T.M.; Song, H.C.; Horton, M.S.; Freed, D.C.; et al. Universal influenza B vaccine based on the maturational cleavage site of the hemagglutinin precursor. J. Virol. 2005, 79, 7380–7388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, T.; Mamahit, A.; Cox, N.J. 65 years of influenza surveillance by a World Health Organization-coordinated global network. Influenza Other Respir. Viruses 2018, 12, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef] [Green Version]
- Jazayeri, S.D.; Poh, C.L. Development of universal influenza vaccines targeting conserved viral proteins. Vaccines 2019, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Meldgaard, T.S.; Bertholet, S. Novel platforms for the development of a universal influenza vaccine. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Sautto, G.A.; Kirchenbaum, G.A.; Ross, T.M. Towards a universal influenza vaccine: Different approaches for one goal. Virol. J. 2018, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [Green Version]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Newman, E. Stalking flu: Development and characterization of a broadly neutralizing monoclonal antibody targeting the influenza hemagglutinin stem. NYMC Stud. Theses Diss. 2019, 10. Available online: https://touroscholar.touro.edu/nymc_students_theses/10 (accessed on 23 October 2020).
- Margine, I.; Krammer, F.; Hai, R.; Heaton, N.S.; Tan, G.S.; Andrews, S.A.; Runstadler, J.A.; Wilson, P.C.; Albrecht, R.A.; Garcia-Sastre, A.; et al. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza a viruses. J. Virol. 2013, 87, 10435–10446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.-W.; Chien, C.-Y.; Li, S.-W.; King, C.-C.; Chang, C.-H. Highly conserved influenza A virus epitope sequences as candidates of H3N2 flu vaccine targets. Genomics 2012, 100, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Mohan, T.; Chang, T.Z.; Gonzalez, G.X.; Wang, Y.; Kwon, Y.M.; Kang, S.M.; Compans, R.W.; Champion, J.A.; Wang, B.Z. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 2018, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Palese, P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr. Opin. Virol. 2013, 3, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zheng, A.; Miller, R.; Krammer, F.; Palese, P. An inactivated influenza virus vaccine approach to targeting the conserved hemagglutinin stalk and m2e domains. Vaccines 2019, 7, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Karikó, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Welsh, J.P.; Swartz, J.R. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc. Natl. Acad. Sci. USA 2014, 111, 125. [Google Scholar] [CrossRef] [Green Version]
- Westgeest, K.B.; Russell, C.A.; Lin, X.; Spronken, M.I.; Bestebroer, T.M.; Bahl, J.; van Beek, R.; Skepner, E.; Halpin, R.A.; de Jong, J.C.; et al. Genomewide analysis of reassortment and evolution of human influenza A(H3N2) viruses circulating between 1968 and 2011. J. Virol. 2014, 88, 2844–2857. [Google Scholar] [CrossRef] [Green Version]
- Johansson, B.E.; Moran, T.M.; Kilbourne, E.D. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc. Natl. Acad. Sci. USA 1987, 84, 6869–6873. [Google Scholar] [CrossRef] [Green Version]
- Johansson, B.E.; Moran, T.M.; Bona, C.A.; Popple, S.W.; Kilbourne, E.D. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. II. Sequential infection of mice simulates human experience. J. Immunol. 1987, 139, 2010–2014. [Google Scholar] [PubMed]
- Wan, H.; Yang, H.; Shore, D.A.; Garten, R.J.; Couzens, L.; Gao, J.; Jiang, L.; Carney, P.J.; Villanueva, J.; Stevens, J.; et al. Structural characterization of a protective epitope spanning A(H1N1)pdm09 influenza virus neuraminidase monomers. Nat. Commun. 2015, 6, 6114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Wohlbold, T.J.; Zheng, N.Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 2018, 173, 417–429.e410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, T.M.; Hashem, A.M.; Li, C.; van Domselaar, G.; Larocque, L.; Wang, J.; Smith, D.; Cyr, T.; Farnsworth, A.; He, R.; et al. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antivir. Res. 2013, 100, 567–574. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Gonzalez, G.X.; Luthra, L.; Dong, C.; Ma, Y.; Zou, J.; Kang, S.M.; Wang, B.Z. Double-layered m2e-na protein nanoparticle immunization induces broad cross-protection against different influenza viruses in mice. Adv. Healthc. Mater. 2020, 9, e1901176. [Google Scholar] [CrossRef] [Green Version]
- Holsinger, L.J.; Lamb, R.A. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology 1991, 183, 32–43. [Google Scholar] [CrossRef]
- Holsinger, L.J.; Nichani, D.; Pinto, L.H.; Lamb, R.A. Influenza A virus M2 ion channel protein: A structure-function analysis. J. Virol. 1994, 68, 1551–1563. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Lamb, R.A. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. J. Virol. 1994, 68, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebedee, S.L.; Richardson, C.D.; Lamb, R.A. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J. Virol. 1985, 56, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozdzanowska, K.; Feng, J.; Eid, M.; Kragol, G.; Cudic, M.; Otvos, L., Jr.; Gerhard, W. Induction of influenza type A virus-specific resistance by immunization of mice with a synthetic multiple antigenic peptide vaccine that contains ectodomains of matrix protein 2. Vaccine 2003, 21, 2616–2626. [Google Scholar] [CrossRef]
- Turley, C.B.; Rupp, R.E.; Johnson, C.; Taylor, D.N.; Wolfson, J.; Tussey, L.; Kavita, U.; Stanberry, L.; Shaw, A. Safety and immunogenicity of a recombinant M2e–flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 2011, 29, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Song, J.M.; Eunju, O.; Kwon, Y.M.; Lee, Y.J.; Compans, R.W.; Kang, S.M. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hess, A.; Chang, T.Z.; Wang, Y.C.; Champion, J.A.; Compans, R.W.; Wang, B.Z. Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezhenskaya, D.; Isakova-Sivak, I.; Rudenko, L. M2e-based universal influenza vaccines: A historical overview and new approaches to development. J. Biomed. Sci. 2019, 26. [Google Scholar] [CrossRef]
- Lee, Y.T.; Kim, K.H.; Ko, E.J.; Kim, M.C.; Lee, Y.N.; Hwang, H.S.; Lee, Y.; Jung, Y.J.; Kim, Y.J.; Santos, J.; et al. Enhancing the cross protective efficacy of live attenuated influenza virus vaccine by supplemented vaccination with M2 ectodomain virus-like particles. Virology 2019, 529, 111–121. [Google Scholar] [CrossRef]
- Chenavas, S.; Estrozi, L.F.; Slama-Schwok, A.; Delmas, B.; di Primo, C.; Baudin, F.; Li, X.; Crépin, T.; Ruigrok, R.W. Monomeric nucleoprotein of influenza A virus. PLoS Pathog. 2013, 9, e1003275. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Fu, T.M.; Deck, R.R.; Friedman, A.; Guan, L.; DeWitt, C.; Liu, X.; Wang, S.; Liu, M.A.; Donnelly, J.J.; et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 1998, 72, 5648–5653. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Moreno, J.; Carragher, D.M.; Misra, R.S.; Kusser, K.; Hartson, L.; Moquin, A.; Lund, F.E.; Randall, T.D. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J. Immunol. 2008, 180, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.-Y.; Wang, S.-C.; Ko, Y.-A.; Lin, K.-I.; Ma, C.; Cheng, T.-J.R.; Wong, C.-H. Chimeric hemagglutinin vaccine elicits broadly protective CD4 and CD8 T cell responses against multiple influenza strains and subtypes. Proc. Natl. Acad. Sci. USA 2020, 117, 17757–17763. [Google Scholar] [CrossRef]
- Uddbäck, I.; Kohlmeier, J.E.; Thomsen, A.R.; Christensen, J.P. Harnessing cross-reactive CD8(+) T(RM) cells for long-standing protection against influenza a virus. Viral. Immunol. 2020, 33, 201–207. [Google Scholar] [CrossRef]
- la Gruta, N.L.; Turner, S.J. T cell mediated immunity to influenza: Mechanisms of viral control. Trends Immunol. 2014, 35, 396–402. [Google Scholar] [CrossRef]
- Wahl, A.; Schafer, F.; Bardet, W.; Buchli, R.; Air, G.M.; Hildebrand, W.H. HLA class I molecules consistently present internal influenza epitopes. Proc. Natl. Acad. Sci. USA 2009, 106, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babon, J.A.B.; Cruz, J.; Ennis, F.A.; Yin, L.; Terajima, M. A Human CD4+ T cell epitope in the influenza hemagglutinin is cross-reactive to influenza a virus subtypes and to influenza B virus. J. Virol. 2012, 86, 9233. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.T.; Khan, A.M.; August, J.T. Highly conserved influenza A sequences as T cell epitopes-based vaccine targets to address the viral variability. Hum. Vaccines 2011, 7, 402–409. [Google Scholar] [CrossRef]
- Deng, L.; Chang, T.Z.; Wang, Y.; Li, S.; Wang, S.; Matsuyama, S.; Yu, G.; Compans, R.W.; Li, J.-D.; Prausnitz, M.R.; et al. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E7758. [Google Scholar] [CrossRef] [Green Version]
- Patterson, D.P.; Rynda-Apple, A.; Harmsen, A.L.; Harmsen, A.G.; Douglas, T. Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano 2013, 7, 3036–3044. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, W.; Li, Y.; Zhang, S.; Duan, Y.; Xing, L.; Zhao, Z.; Zhang, P.; Li, Z.; Li, R.; et al. Enhanced Influenza VLP vaccines comprising matrix-2 ectodomain and nucleoprotein epitopes protects mice from lethal challenge. Antivir. Res. 2013, 98, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.A.; Chen, L.; Baker, J.L.; Putnam, D.; DeLisa, M.P. Pathogen-like particles: Biomimetic vaccine carriers engineered at the nanoscale. Curr. Opin. Biotechnol. 2014, 28, 51–58. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Kang, S.M.; Wang, B.Z. Universal influenza vaccines: From viruses to nanoparticles. Expert Rev. Vaccines 2018, 17, 967–976. [Google Scholar] [CrossRef]
- Gallagher, J.R.; McCraw, D.M.; Torian, U.; Gulati, N.M.; Myers, M.L.; Conlon, M.T.; Harris, A.K. Characterization of hemagglutinin antigens on influenza virus and within vaccines using electron microscopy. Vaccines 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Kim, J.R.; Chang, T.Z.; Zhang, H.; Mohan, T.; Champion, J.A.; Wang, B.Z. Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology 2017, 509, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, W.; Wang, B.Z. Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int. J. Nanomed. 2017, 12, 4747–4762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCraw, D.M.; Myers, M.L.; Gulati, N.M.; Gallagher, J.R.; Kim, A.J.; Torian, U.; Harris, A.K. Designed nanoparticles elicit cross-reactive antibody responses to conserved influenza virus hemagglutinin stem epitopes. bioRxiv 2019, 707984. [Google Scholar] [CrossRef]
- Sexton, A.; Whitney, P.G.; Chong, S.F.; Zelikin, A.N.; Johnston, A.P.; de Rose, R.; Brooks, A.G.; Caruso, F.; Kent, S.J. A protective vaccine delivery system for in vivo T cell stimulation using nanoengineered polymer hydrogel capsules. ACS Nano 2009, 3, 3391–3400. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1349–1360. [Google Scholar] [CrossRef]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.D.; Ozören, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Papania, M.J.; Zehrung, D.; Jarrahian, C. Technologies to improve immunization. Plotkin’s Vaccines 2018, 1320–1353.e1317. [Google Scholar] [CrossRef]
- Ols, S.; Yang, L.; Thompson, E.A.; Pushparaj, P.; Tran, K.; Liang, F.; Lin, A.; Eriksson, B.; Hedestam, G.B.K.; Wyatt, R.T.; et al. Route of vaccine administration alters antigen trafficking but not innate or adaptive immunity. Cell Rep. 2020, 30, 3964–3971.e3967. [Google Scholar] [CrossRef]
- Foy, J.E.; Hendriksz, T.; Malouf, P.; Tobin, A. Acceptability of fluzone intradermal vaccine to patients and vaccine administrators. J. Am. Osteopath. Assoc. 2013, 113, 134–143. [Google Scholar]
- Zhu, Q.; Talton, J.; Zhang, G.; Cunningham, T.; Wang, Z.; Waters, R.C.; Kirk, J.; Eppler, B.; Klinman, D.M.; Sui, Y.; et al. Large intestine–targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 2012, 18, 1291–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sable, S.B.; Cheruvu, M.; Nandakumar, S.; Sharma, S.; Bandyopadhyay, K.; Kellar, K.L.; Posey, J.E.; Plikaytis, B.B.; Amara, R.R.; Shinnick, T.M. Cellular immune responses to nine Mycobacterium tuberculosis vaccine candidates following intranasal vaccination. PLoS ONE 2011, 6, e22718. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajrovic, I.; Schafer, S.C.; Romanovicz, D.K.; Croyle, M.A. Novel technology for storage and distribution of live vaccines and other biological medicines at ambient temperature. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuft, L.; Yagle, E.M.; Rogers, S. Comparative study of the antibody response after various methods of administration of mixed typhoid vaccine: With particular reference to the intradermal and oral methods. J. Infect. Dis. 1932, 50, 98–110. [Google Scholar] [CrossRef]
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef]
- Alarcon, J.B.; Hartley, A.W.; Harvey, N.G.; Mikszta, J.A. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin. Vaccine Immunol. CVI 2007, 14, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Ansaldi, F.; Canepa, P.; Ceravolo, A.; Valle, L.; de Florentiis, D.; Oomen, R.; Vogel, F.R.; Denis, M.; Samson, S.I.; Icardi, G. Intanza((R)) 15 mcg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine 2012, 30, 2908–2913. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.d.; Weldon, W.C.; Zarnitsyn, V.G.; Koutsonanos, D.G.; Akbari, H.; Skountzou, I.; Jacob, J.; Prausnitz, M.R.; Compans, R.W. Local response to microneedle-based influenza immunization in the skin. mBio 2012, 3, e00012-12. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Z.; Gill, H.S.; He, C.; Ou, C.; Wang, L.; Wang, Y.C.; Feng, H.; Zhang, H.; Prausnitz, M.R.; Compans, R.W. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J. Control. Release Off. J. Control. Release Soc. 2014, 178, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Pewin, W.; Wang, C.; Luo, Y.; Gonzalez, G.X.; Mohan, T.; Prausnitz, M.R.; Wang, B.Z. A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J. Control. Release Off. J. Control. Release Soc. 2017, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Li, N.; Zhen, Y.; Wang, T. Multifunctional particle-constituted microneedle arrays as cutaneous or mucosal vaccine adjuvant-delivery systems. Hum. Vaccin. Immunother. 2016, 12, 2075–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.D.; Li, S.; Wang, C.; Yu, G.Y.; Prausnitz, M.R.; Wang, B.Z. Enhanced immune responses conferring cross-protection by skin vaccination with a tri-component influenza vaccine using a microneedle patch. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, S. Skin vaccination with dissolvable microneedle patches incorporating M2e-NA-FliC nanoparticles induces immune protection against various influenza viruses. In manuscript.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Zhu, W.; Wang, B.-Z. Influenza Vaccines toward Universality through Nanoplatforms and Given by Microneedle Patches. Viruses 2020, 12, 1212. https://doi.org/10.3390/v12111212
Tang S, Zhu W, Wang B-Z. Influenza Vaccines toward Universality through Nanoplatforms and Given by Microneedle Patches. Viruses. 2020; 12(11):1212. https://doi.org/10.3390/v12111212
Chicago/Turabian StyleTang, Sijia, Wandi Zhu, and Bao-Zhong Wang. 2020. "Influenza Vaccines toward Universality through Nanoplatforms and Given by Microneedle Patches" Viruses 12, no. 11: 1212. https://doi.org/10.3390/v12111212




