Wine Phenolic Compounds Differently Affect the Host-Killing Activity of Two Lytic Bacteriophages Infecting the Lactic Acid Bacterium Oenococcus oeni
Abstract
:1. Introduction
2. Materials and Methods
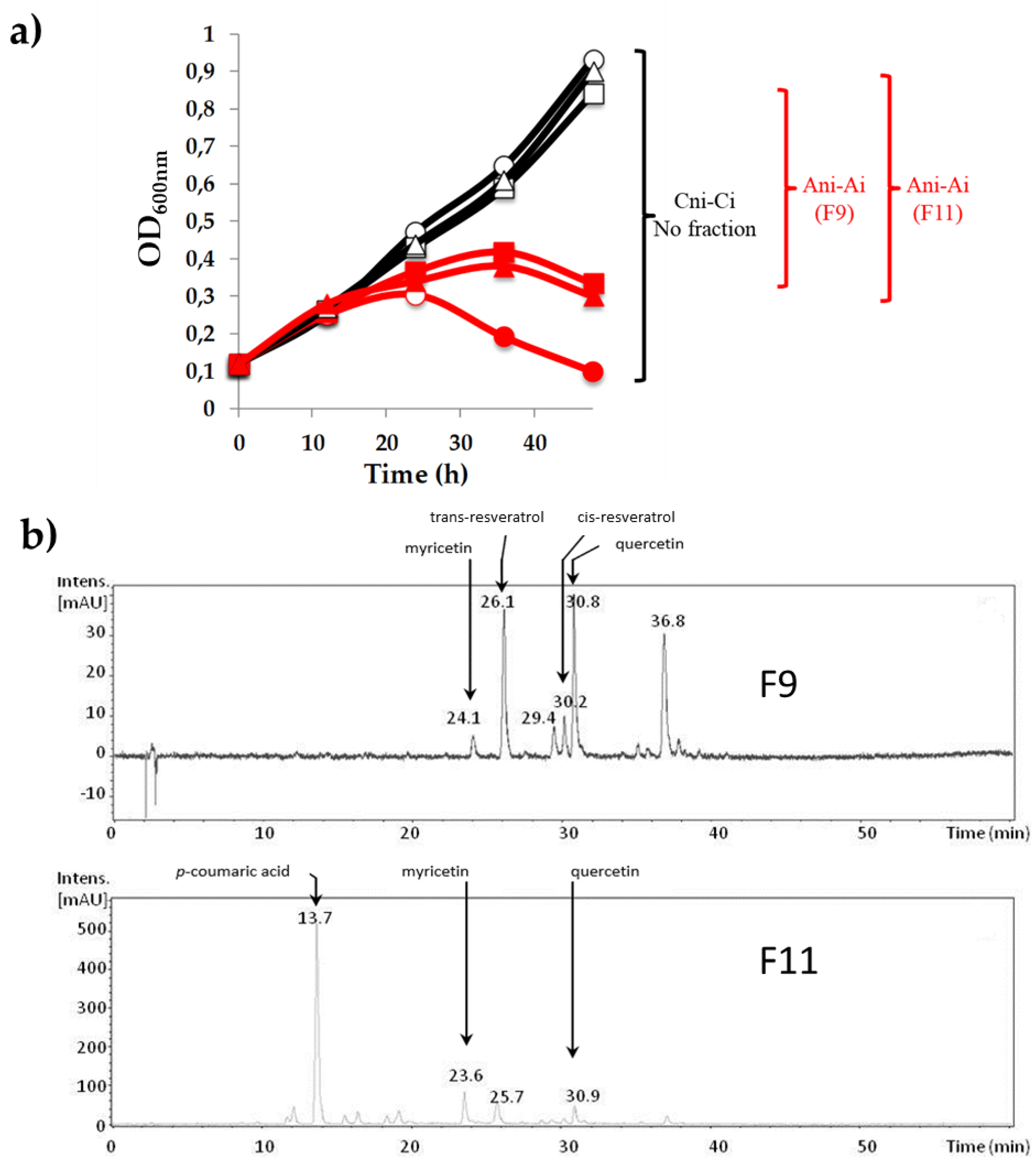
2.1. Preparation of Phenolic-Enriched Extracts from a Red Wine by Centrifugal Partition Chromatography
2.2. Determination of Total Phenols of Fractions
2.3. Identification of Phenolic Compounds in the Biologically Active Fractions
2.4. Phage and Strains
2.5. Killing-Curve Assays with the CPC Fractions
2.6. Killing-Curve Assays with Pure Molecules
2.7. Adsorption Assays
2.8. Isolation of Phage-Insensitive Mutants
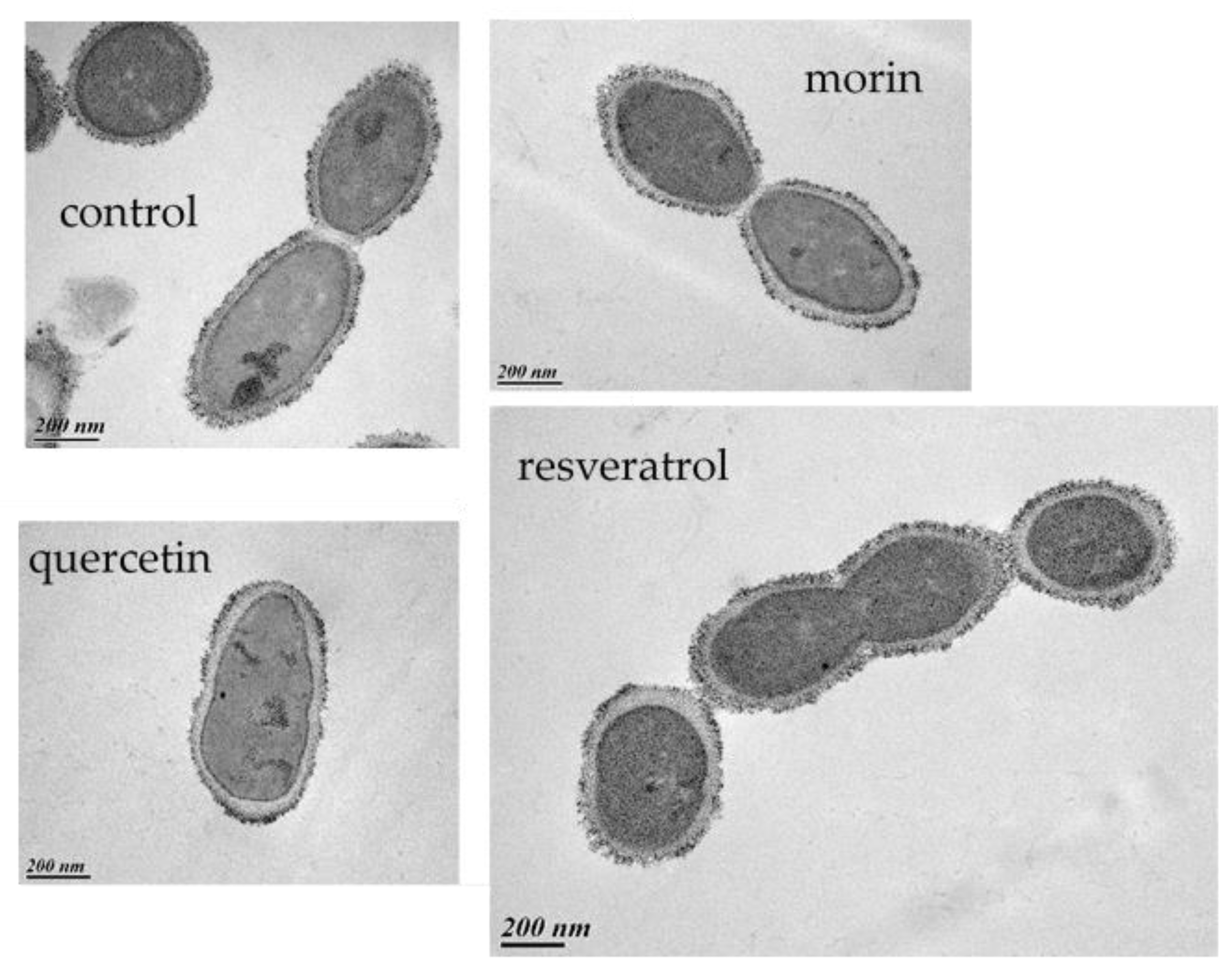
2.9. Transmission Electron Microscopy
2.10. Homology Modeling of OE33PA and Cinnamic Acid and Quercetin Docking
3. Results
3.1. Influence of Phenolic Compounds on the Lytic Development of Phage OE33PA on Its Host
3.2. Other PCs Possess the Ability to Impair Phage Lytic Cycle on O. oeni
3.3. Adsorption of Phage OE33PA Is Impaired by Phenolic Compounds
3.4. PCs Do Not Affect the Host-Killing Activity of Vinitor162 on O. oeni
3.5. Phages’ Adhesion Devices: Homology Detection and Structure Prediction
3.6. How Do PCs Affect the Host-Killing Activity of OE33PA on O. oeni?
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Campos, F.M.; Couto, J.A.; Hogg, T.A. Influence of phenolic acids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2003, 94, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Breniaux, M.; Dutilh, L.; Petrel, M.; Gontier, E.; Campbell-Sills, H.; Deleris-Bou, M.; Krieger, S.; Teissedre, P.L.; Jourdes, M.; Reguant, C.; et al. Adaptation of two groups of Oenococcus oeni strains to red and white wines: The role of acidity and phenolic compounds. J. Appl. Microbiol. 2018, 125, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Anu-Appaiah, K.A. Diverse physiological and metabolic adaptations by Lactobacillus plantarum and Oenococcus oeni in response to the phenolic stress during wine fermentation. Food Chem. 2018, 268, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Balasundrama, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Pastorkowa, E.; Zakova, T.; Landa, P.; Navakova, J.; Vadlejch, J.; Kokoska, L. Growth inhibitory effect of grape phenolics against wine spoilage yeast and acetic acid bacteria. Int. J. Food Microb. 2013, 161, 209–213. [Google Scholar] [CrossRef]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Stivala, M.G.; Villecco, M.B.; Enriz, D.; Aredes Fernández, P. Effect of phenolic compounds on viability of wine spoilage Lactic Acid Bacteria. A structure-activity relationship Study. Am. J. Enol. Vitic. 2017, 68, 228–233. [Google Scholar] [CrossRef]
- Ledesma, S.C.; Stivala, M.G.; Rubio, M.C.; Fanzone, M.L.; Jofré, V.; Aredes-Fernández, P. Influence of wine phenolic compounds on viability and exopolysaccharide production by Pediococcus pentosaceus. J. Wine Res. 2018, 29, 143–150. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; López de Felipe, F.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-Staphylococcus aureus activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food. Prot. 2017, 81, 68–78. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Delsoglio, M.; Brix, S.; Pessione, E.; Svensson, B. Plant polyphenols stimulate adhesion to intestinal mucosa and induce proteome changes in the probiotic Lactobacillus acidophilus NCFM. Mol. Nutr. Food Res. 2018, 62, 1700638. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.S.; de Albuquerque, T.M.R.; de Brito Alves, J.L.; de Souza, E.L. Effects of quercetin and resveratrol on in vitro properties related to the functionality of potentially probiotic Lactobacillus strains. Front. Microbiol. 2019, 10, 2229. [Google Scholar] [CrossRef] [Green Version]
- Veloz, J.J.; Saavedra, N.; Alvear, M.; Zambrano, T.; Barrientos, L.; Salazar, L.A. Polyphenol-rich extract from propolis reduces the expression and activity of Streptococcus mutans glucosyltransferases at subinhibitory concentrations. Biomed. Res. Int. 2016, 2016, 4302706. [Google Scholar] [CrossRef] [Green Version]
- Firrman, J.; Liu, L.; Zhang, L.; Argoty, G.A.; Wang, M.; Tomasula, P.; Kobori, M.; Pontious, S.; Xiao, W. The effect of quercetin on genetic expression of the commensal gut microbes Bifidobacterium catenulatum, Enterococcus caccae and Ruminococcus gauvreauii. Anaerobe 2016, 42, 130–141. [Google Scholar] [CrossRef]
- Lee, A.; Eschenbruch, R.; Waller, J. Effect of phenolic compounds, ethyl alcohol, and sodium metabisulphite on the lytic activity of phage PL-1 on a Lactobacillus casei S strain. Can. J. Microbiol. 1985, 31, 873–875. [Google Scholar] [CrossRef]
- Tayyarcan, E.K.; Soykut, E.A.; Yılmaz, O.M.; Boyaci, I.H.; Khaaladi, M.; Fattouch, S. Investigation of different interactions between Staphylococcus aureus phages and pomegranate peel, grape seed, and black cumin extracts. J. Food Saf. 2019, 39, e12679. [Google Scholar] [CrossRef]
- Jaomanjaka, F.; Claisse, O.; Blanche-Barbat, M.; Petrel, M.; Ballestra, P.; Le Marrec, C. Characterization of a new virulent phage infecting the lactic acid bacterium Oenococcus oeni. Food Microbiol. 2016, 54, 167–177. [Google Scholar] [CrossRef]
- Philipe, C.; Jaomanjaka, F.; Claisse, O.; Laforgue, R.; Maupeu, J.; Petrel, M.; Le Marrec, C. A survey of oenophages during wine making reveals a novel group with unusual genomic characteristics. Int. J. Food Microbiol. 2017, 257, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Philipe, C.; Chaïb, A.; Jaomanjaka, F.; Claisse, O.; Lucas, P.M.; Samot, J.; Cambillau, C.; Goulet, A.; Le Marrec, C. Queen check in the cellars: Characterization of virulent phages infecting Oenococcus oeni, responsible for the malolactic fermentation of wines. Front. Microbiol. 2020, in press. [Google Scholar]
- Pawlus, A.D.; Cantos-Villar, E.; Richard, T.; Bisson, J.; Poupard, P.; Papastamoulis, Y.; Monti, J.P.; Teissedre, P.L.; Waffo-Téguo, P.; Mérillon, J.M. Chemical dereplication of wine stilbenoids using high performance liquid chromatography–nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2013, 1289, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Montoro, P.; Piacente, S.; Oleszek, W.; Pizza, C. Liquid chromatography/tandem mass spectrometry of unusual phenols from Yucca schidigera bark: Comparison with other analytical techniques. J. Mass Spectrom. 2004, 39, 1131–1138. [Google Scholar] [CrossRef]
- Montsko, G.; Pour Nikfardjam, M.S.; Szabo, Z.; Boddi, K.; Lorand, T.; Ohmacht, R.; Mark, L. Determination of products derived from trans-resveratrol UV photoisomerisation by means of HPLC–APCI-MS. J. Photochem. Photobiol. A Chem. 2008, 196, 44–50. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, Q.M.; Van den Heuvel, H.; Claeys, M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef]
- Jaomanjaka, F.; Claisse, O.; Philippe, C.; Le Marrec, C. Complete Genome Sequence of Lytic Oenococcus oeni Bacteriophage OE33PA. Microbiol. Res. Announ. 2018, 7, e00818-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaomanjaka, F.; Ballestra, P.; Dols-lafargue, M.; Le Marrec, C. Expanding the diversity of oenococcal bacteriophages: Insights into a novel group based on the integrase sequence. Int. J. Food Microbial. 2013, 166, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Claisse, O.; Lonvaud-Funel, A. Multiplex variable number of tandem repeats for Oenococcus oeni and applications. Food Microbiol. 2014, 38, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Thiéry, J.P. Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J. Microsc. 1967, 6, 987–1018. [Google Scholar]
- Dimopoulou, M.; Vuillemin, M.; Campbell-Sills, H.; Lucas, P.M.; Ballestra, P.; Miot-Sertier, C.; Favier, M.; Coulon, J.; Moine, V.; Doco, T.; et al. Exopolysaccharide (EPS) synthesis by Oenococcus oeni: From genes to phenotypes. PLoS ONE 2014, 9, e98898. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, L.; Stephens, A.; Nam, S.Z.; Rau, D.; Kubler, J.; Lozajic, M.; Gabler, F.; Soding, J.; Lupas, A.N.; Alva, V. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Cryst. D Biol. Cryst. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cryst. D Biol. Cryst. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Giamogante, F.; Marrocco, I.; Romaniello, D.; Eufemi, M.; Chichiarelli, S.; Altieri, F. Comparative analysis of the interaction between different flavonoids and PDIA3. Oxid. Med. Cell Longev. 2016, 2016, 4518281. [Google Scholar] [CrossRef]
- Chaïb, A. Measurement of the Adsorption of Phage OE33PA on Its Host in the Presence of Polyphenolic Compounds; EA4577-USC1366 INRAE; Unité de Recherche OEnologie, Université de Bordeaux, Institut des Sciences de la Vigne et du Vin (ISVV): Villenave d’Ornon, France, 2019. [Google Scholar]
- Mahony, J.; Cambillau, C.; van Sinderen, D. Host recognition by lactic acid bacterial phages. FEMS Microbiol. Rev. 2017, 41, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Goulet, A.; Spinelli, S.; Mahony, J.; Cambillau, C. Conserved and diverse traits of adhesion devices from Siphoviridae recognizing proteinaceous or saccharidic receptors. Viruses 2020, 12, 512. [Google Scholar] [CrossRef] [PubMed]
- Sciara, G.; Bebeacua, C.; Bron, P.; Tremblay, D.; Ortiz-Lombardia, M.; Lichiere, J.; van Heel, M.; Campanacci, V.; Moineau, S.; Cambillau, C. Structure of lactococcal phage p2 baseplate and its mechanism of activation. Proc. Natl. Acad. Sci. USA 2010, 107, 6852–6857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, S.; Tremblay, D.; Moineau, S.; Cambillau, C.; Goulet, A. Structural Insights into Lactococcal Siphophage p2 Baseplate Activation Mechanism. Viruses 2020, 12, 878. [Google Scholar] [CrossRef]
- Dieterle, M.E.; Fina Martin, J.; Duran, R.; Nemirovsky, S.I.; Sanchez Rivas, C.; Bowman, C.; Russell, D.; Hatfull, G.F.; Cambillau, C.; Piuri, M. Characterization of prophages containing “evolved” Dit/Tal modules in the genome of Lactobacillus casei BL23. Appl. Microbiol. Biotechnol. 2016, 100, 9201–9215. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.E.; Spinelli, S.; Sadovskaya, I.; Piuri, M.; Cambillau, C. Evolved distal tail carbohydrate binding modules of Lactobacillus phage J-1: A novel type of anti-receptor widespread among lactic acid bacteria phages. Mol. Microbiol. 2017, 104, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, S.; Desmyter, A.; Verrips, C.T.; de Haard, H.J.; Moineau, S.; Cambillau, C. Lactococcal bacteriophage p2 receptor-binding protein structure suggests a common ancestor gene with bacterial and mammalian viruses. Nat. Struct. Mol. Biol. 2006, 13, 85–89. [Google Scholar] [CrossRef]
- Tremblay, D.M.; Tegoni, M.; Spinelli, S.; Campanacci, V.; Blangy, S.; Huyghe, C.; Desmyter, A.; Labrie, S.; Moineau, S.; Cambillau, C. Receptor-binding protein of Lactococcus lactis phages: Identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 2006, 188, 2400–2410. [Google Scholar] [CrossRef] [Green Version]
- Hayes, S.; Vincentelli, R.; Mahony, J.; Nauta, A.; Ramond, L.; Lugli, G.A.; Ventura, M.; van Sinderen, D.; Cambillau, C. Functional carbohydrate binding modules identified in evolved dits from siphophages infecting various Gram-positive bacteria. Mol. Microbiol. 2018, 110, 777–795. [Google Scholar] [CrossRef]
- Spinelli, S.; Campanacci, V.; Blangy, S.; Moineau, S.; Tegoni, M.; Cambillau, C. Modular structure of the receptor binding proteins of Lactococcus lactis phages. The RBP structure of the temperate phage TP901-1. J. Biol. Chem. 2006, 281, 14256–14262. [Google Scholar] [CrossRef] [Green Version]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Jaomanjaka, F. Diversité des Bactériophages Infectant la Bactérie Lactique Oenococcus oeni, Responsable de la Fermentation Malolactique des Vins. Ph.D. Thesis, University Bordeaux, Bordeaux, France, 2014. [Google Scholar]
- Davis, C.; Silveira, N.F.A.; Fleet, G.H. Occurrence and properties of bacteriophages of Leuconostoc oenos in australian wines. Appl. Environ. Microbiol. 1985, 50, 872–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henick-Kling, T.; Lee, T.H.; Nicholas, D.J.D. Characterization of the lytic activity of bacteriophages of Leuconostoc oenos isolated from wine. J. Appl. Bacteriol. 1986, 61, 525–534. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalani, S.; Poh, C.L. Flavonoids as antiviral agents for Enterovirus A71 (EV-A71). Viruses 2020, 12, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The beta-lactam-resistance modifier (-)epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [Green Version]
- Theodorou, I.; Courtin, P.; Palussière, S.; Kulakauskas, S.; Bidnenko, E.; Péchoux, C.; Fenaille, F.; Penno, C.; Mahony, J.; van Sinderen, D.; et al. A dual-chain assembly pathway generates the high structural diversity of cell-wall polysaccharides in Lactococcus lactis. J. Biol. Chem. 2019, 294, 17612–17625. [Google Scholar] [CrossRef]
- Mahony, J.; Frantzen, C.; Vinogradov, E.; Sadovskaya, I.; Theodorou, I.; Kelleher, P.; Chapot-Chartier, M.P.; Cambillau, C.; Holo, H.; van Sinderen, D. The CWPS Rubik’s cube: Linking diversity of cell wall polysaccharide structures with the encoded biosynthetic machinery of selected Lactococcus lactis strains. Mol. Microbiol. 2020. [Google Scholar] [CrossRef]
- Prieto-Martínez, F.D.; Medina-Franco, J.L. Flavonoids as putative epi-modulators: Insight into their binding mode with BRD4 bromodomains using molecular docking and dynamics. Biomolecules 2018, 8, 61. [Google Scholar]
- Ivey, K.L.; Chan, A.T.; Izard, J.; Cassidy, A.; Rogers, G.B.; Rimm, E.B. Role of dietary flavonoid compounds in driving patterns of microbial community assembly. mBio 2019, 10, e01205-19. [Google Scholar] [CrossRef] [Green Version]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Chemical Structure | PC Family and Functional Groups | Inhibition of Phage-Killing Activity | |||||||
|---|---|---|---|---|---|---|---|---|---|
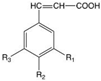 | Hydroxycinnamic acids | R1 | R2 | R3 | OE33PA | Vinitor 162 | |||
| Coumaric acid | H | OH | H | + | - | ||||
| Caffeic acid | H | OH | OH | + | - | ||||
| Cinnamic acid | H | H | H | + | - | ||||
| Flavonols | 2′ | 3′ | 4′ | 5′ | R | 7 | |||
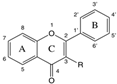 | Quercetin | H | OH | OH | H | OH | OH | + | - |
| Quercetin 3-O-glucuronide | H | OH | OH | H | Gluc. acid | OH | - | - | |
| Myricetin | H | OH | OH | OH | OH | OH | + | - | |
| Kaempferol | H | H | OH | H | OH | OH | - | - | |
| Morin | OH | H | OH | H | OH | OH | + | - | |
| Retusin | H | OCH3 | OCH3 | H | OCH3 | OCH3 | + | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippe, C.; Chaïb, A.; Jaomanjaka, F.; Cluzet, S.; Lagarde, A.; Ballestra, P.; Decendit, A.; Petrel, M.; Claisse, O.; Goulet, A.; et al. Wine Phenolic Compounds Differently Affect the Host-Killing Activity of Two Lytic Bacteriophages Infecting the Lactic Acid Bacterium Oenococcus oeni. Viruses 2020, 12, 1316. https://doi.org/10.3390/v12111316
Philippe C, Chaïb A, Jaomanjaka F, Cluzet S, Lagarde A, Ballestra P, Decendit A, Petrel M, Claisse O, Goulet A, et al. Wine Phenolic Compounds Differently Affect the Host-Killing Activity of Two Lytic Bacteriophages Infecting the Lactic Acid Bacterium Oenococcus oeni. Viruses. 2020; 12(11):1316. https://doi.org/10.3390/v12111316
Chicago/Turabian StylePhilippe, Cécile, Amel Chaïb, Fety Jaomanjaka, Stéphanie Cluzet, Aurélie Lagarde, Patricia Ballestra, Alain Decendit, Mélina Petrel, Olivier Claisse, Adeline Goulet, and et al. 2020. "Wine Phenolic Compounds Differently Affect the Host-Killing Activity of Two Lytic Bacteriophages Infecting the Lactic Acid Bacterium Oenococcus oeni" Viruses 12, no. 11: 1316. https://doi.org/10.3390/v12111316
APA StylePhilippe, C., Chaïb, A., Jaomanjaka, F., Cluzet, S., Lagarde, A., Ballestra, P., Decendit, A., Petrel, M., Claisse, O., Goulet, A., Cambillau, C., & Le Marrec, C. (2020). Wine Phenolic Compounds Differently Affect the Host-Killing Activity of Two Lytic Bacteriophages Infecting the Lactic Acid Bacterium Oenococcus oeni. Viruses, 12(11), 1316. https://doi.org/10.3390/v12111316






