Oxygen Levels Affect Macrophage HIV-1 Gene Expression and Delay Resolution of Inflammation in HIV-Tg Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Reagents
2.3. Immunohistochemistry
2.4. Isolation of Intra-Peritoneal Macrophages
2.5. Quantitative RT-PCR
2.6. Trans-Endothelial Migration Assay
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
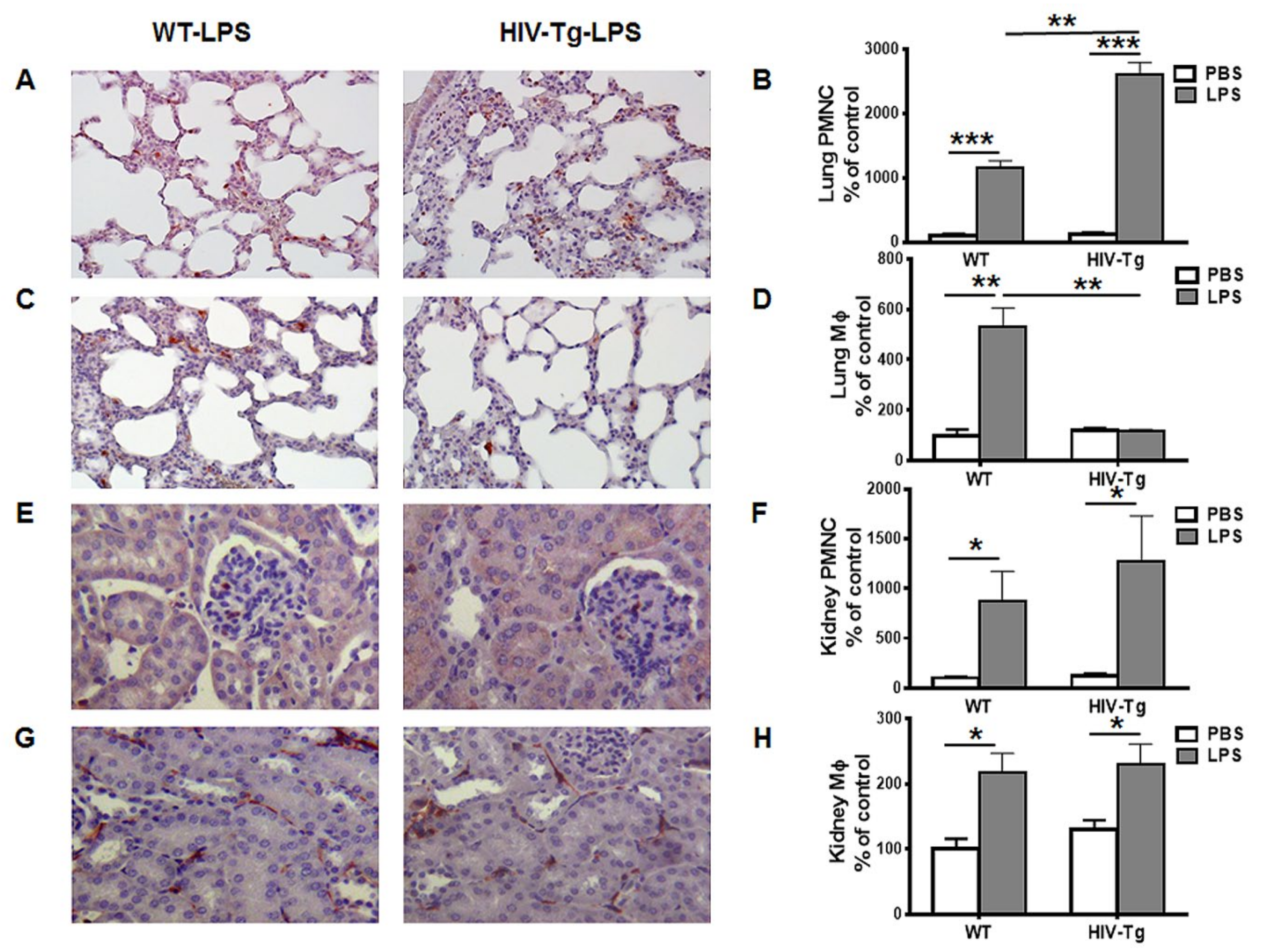
3.1. Renal and Peritoneal Leukocytes Infiltrations are Similar in HIV-Tg and WT Mice
3.2. Expression of HIV-1 Genes is Elevated in Lung Macrophages Compared to Peritoneal Macrophages
3.3. Trans-Endothelial Migration of Macrophages Isolated from HIV-Tg Mice is Reduced at High Oxygen Level in Vitro
3.4. Dynamics of Peritoneal Leukocytes Infiltration and Resolution of Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calligaro, G.L.; Gray, D.M. Lung function abnormalities in HIV-infected adults and children. Respirology 2015, 20, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, P.; Santoro, C.R.; Nofri, M.; Ricci, E.; De Gennaro, N.; Bellacosa, C.; Schiaroli, E.; Orofino, G.; Menzaghi, B.; Di Biagio, A.; et al. Clusterization of co-morbidities and multi-morbidities among persons living with HIV: A cross-sectional study. BMC Infect. Dis. 2019, 19, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsue, P.Y. Mechanisms of Cardiovascular Disease in the Setting of HIV Infection. Can. J. Cardiol. 2019, 35, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Brenchley, J.M.; Price, D.A.; Douek, D.C. HIV disease: Fallout from a mucosal catastrophe? Nat. Immunol. 2006, 7, 235–239. [Google Scholar] [CrossRef]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Somsouk, M.; Estes, J.D.; Deleage, C.; Dunham, R.M.; Albright, R.; Inadomi, J.M.; Martin, J.N.; Deeks, S.G.; McCune, J.M.; Hunt, P.W. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS 2015, 29, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Jerebtsova, M.; Ahmad, A.; Niu, X.; Rutagarama, O.; Nekhai, S. HIV-1 Transcription Inhibitor 1E7-03 Restores LPS-Induced Alteration of Lung Leukocytes’ Infiltration Dynamics and Resolves Inflammation in HIV Transgenic Mice. Viruses 2020, 12, 204. [Google Scholar] [CrossRef] [Green Version]
- Bruggeman, L.A.; Dikman, S.; Meng, C.; Quaggin, S.E.; Coffman, T.M.; Klotman, P.E. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J. Clin. Investig. 1997, 100, 84–92. [Google Scholar] [CrossRef]
- Kopp, J.B.; Klotman, M.E.; Adler, S.H.; Bruggeman, L.A.; Dickie, P.; Marinos, N.J.; Eckhaus, M.; Bryant, J.L.; Notkins, A.L.; Klotman, P.E. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc. Natl. Acad. Sci. USA 1992, 89, 1577–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.E.; Bruggeman, L.A.; Weeks, B.S.; Kopp, J.B.; Bryant, J.L.; Owens, J.W.; Notkins, A.L.; Klotman, P.E. bFGF and its low affinity receptors in the pathogenesis of HIV-associated nephropathy in transgenic mice. Kidney Int. 1994, 46, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barisoni, L.; Bruggeman, L.A.; Mundel, P.; D’Agati, V.D.; Klotman, P.E. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000, 58, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, B.A.; Porter, K.M.; Elms, S.C.; Cheng, P.Y.; Jones, D.P.; Sutliff, R.L. HIV-1-induced pulmonary oxidative and nitrosative stress: Exacerbated response to endotoxin administration in HIV-1 transgenic mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L811–L819. [Google Scholar] [CrossRef] [Green Version]
- Putatunda, R.; Ho, W.Z.; Hu, W. HIV-1 and Compromised Adult Neurogenesis: Emerging Evidence for a New Paradigm of HAND Persistence. AIDS Rev. 2019, 21, 11–22. [Google Scholar] [CrossRef]
- Bruggeman, L.A.; Thomson, M.M.; Nelson, P.J.; Kopp, J.B.; Rappaport, J.; Klotman, P.E.; Klotman, M.E. Patterns of HIV-1 mRNA expression in transgenic mice are tissue-dependent. Virology 1994, 202, 940–948. [Google Scholar] [CrossRef]
- Leonard, J.; Khillan, J.S.; Gendelman, H.E.; Adachi, A.; Lorenzo, S.; Westphal, H.; Martin, M.A.; Meltzer, M.S. The human immunodeficiency virus long terminal repeat is preferentially expressed in Langerhans cells in transgenic mice. AIDS Res. Hum. Retrovir. 1989, 5, 421–430. [Google Scholar] [CrossRef]
- Dickie, P. Nef modulation of HIV type 1 gene expression and cytopathicity in tissues of HIV transgenic mice. AIDS Res. Hum. Retrovir. 2000, 16, 777–790. [Google Scholar] [CrossRef]
- Putatunda, R.; Zhang, Y.; Li, F.; Yang, X.F.; Barbe, M.F.; Hu, W. Adult neurogenic deficits in HIV-1 Tg26 transgenic mice. J. Neuroinflamm. 2018, 15, 287. [Google Scholar] [CrossRef] [Green Version]
- Charles, S.; Ammosova, T.; Cardenas, J.; Foster, A.; Rotimi, J.; Jerebtsova, M.; Ayodeji, A.A.; Niu, X.; Ray, P.E.; Gordeuk, V.R.; et al. Regulation of HIV-1 transcription at 3% versus 21% oxygen concentration. J. Cell. Physiol. 2009, 221, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Ammosova, T.; Platonov, M.; Ivanov, A.; Kont, Y.S.; Kumari, N.; Kehn-Hall, K.; Jerebtsova, M.; Kulkarni, A.A.; Uren, A.; Kovalskyy, D.; et al. 1E7-03, a low MW compound targeting host protein phosphatase-1, inhibits HIV-1 transcription. Br. J. Pharmacol. 2014, 171, 5059–5075. [Google Scholar] [PubMed] [Green Version]
- Jerebtsova, M.; Wong, E.; Przygodzki, R.; Tang, P.; Ray, P.E. A novel role of fibroblast growth factor-2 and pentosan polysulfate in the pathogenesis of intestinal bleeding in mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H743–H750. [Google Scholar] [CrossRef] [PubMed]
- Fasciolo, J.C.; Chiodi, H. Arterial oxygen pressure during pure O2 breathing. Am. J. Physiol. 1946, 147, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Renvall, S.; Niinikoski, J. Kinetics of oxygen in peritoneal cavity. Effects of chemical peritonitis and intraperitoneally administered colloids in rats. J. Surg. Res. 1980, 28, 132–139. [Google Scholar] [CrossRef]
- Pruijm, M.; Hofmann, L.; Vogt, B.; Muller, M.E.; Piskunowicz, M.; Stuber, M.; Burnier, M. Renal tissue oxygenation in essential hypertension and chronic kidney disease. Int. J. Hypertens. 2013, 2013, 696598. [Google Scholar] [CrossRef] [Green Version]
- Jerebtsova, M.; Liu, X.H.; Ye, X.; Ray, P.E. Adenovirus-mediated gene transfer to glomerular cells in newborn mice. Pediatr. Nephrol. 2005, 20, 1395–1400. [Google Scholar] [CrossRef]
- Nunez, D.; Comas, L.; Lanuza, P.M.; Sanchez-Martinez, D.; Perez-Hernandez, M.; Catalan, E.; Domingo, M.P.; Velazquez-Campoy, A.; Pardo, J.; Galvez, E.M. A Functional Analysis on the Interspecies Interaction between Mouse LFA-1 and Human Intercellular Adhesion Molecule-1 at the Cell Level. Front. Immunol. 2017, 8, 1817. [Google Scholar] [CrossRef] [Green Version]
- Torre, D.; Gennero, L.; Baccino, F.M.; Speranza, F.; Biondi, G.; Pugliese, A. Impaired macrophage phagocytosis of apoptotic neutrophils in patients with human immunodeficiency virus type 1 infection. Clin. Diagn. Lab. Immunol. 2002, 9, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Tsachouridou, O.; Skoura, L.; Chatzidimitriou, D.; Margariti, A.; Georgiou, A.; Chatzidimitriou, M.; Bougiouklis, D.; Zebekakis, P.; Metallidis, S. Deficient Phagocytosis Among HIV-1 Infected Adults Over Time Even in HAART Setting. Curr. Hiv Res. 2017, 15, 285–290. [Google Scholar] [CrossRef]
- Verollet, C.; Souriant, S.; Bonnaud, E.; Jolicoeur, P.; Raynaud-Messina, B.; Kinnaer, C.; Fourquaux, I.; Imle, A.; Benichou, S.; Fackler, O.T.; et al. Maridonneau-Parini, I. HIV-1 reprograms the migration of macrophages. Blood 2015, 125, 1611–1622. [Google Scholar] [CrossRef]
- Williams, M.R.; Luscinskas, F.W. Leukocyte rolling and adhesion via ICAM-1 signals to endothelial permeability. Focus on “Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1”. Am. J. Physiol. Cell Physiol. 2011, 301, C777–C779. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M. Endothelial actin-binding proteins and actin dynamics in leukocyte transendothelial migration. J. Immunol. 2015, 194, 3535–3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verani, A.; Scarlatti, G.; Comar, M.; Tresoldi, E.; Polo, S.; Giacca, M.; Lusso, P.; Siccardi, A.G.; Vercelli, D. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J. Exp. Med. 1997, 185, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verani, A.; Sironi, F.; Siccardi, A.G.; Lusso, P.; Vercelli, D. Inhibition of CXCR4-tropic HIV-1 infection by lipopolysaccharide: Evidence of different mechanisms in macrophages and T lymphocytes. J. Immunol. 2002, 168, 6388–6395. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.; Maurais, E.; Gilbert, C.; Tremblay, M.J. LPS reduces HIV-1 replication in primary human macrophages partly through an endogenous production of type I interferons. Clin. Immunol. 2008, 127, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Nordone, S.K.; Ignacio, G.A.; Su, L.; Sempowski, G.D.; Golenbock, D.T.; Li, L.; Dean, G.A. Failure of TLR4-driven NF-kappa B activation to stimulate virus replication in models of HIV type 1 activation. AIDS Res. Hum. Retrovir. 2007, 23, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Bandarra, D.; Biddlestone, J.; Mudie, S.; Muller, H.A.; Rocha, S. HIF-1alpha restricts NF-kappaB-dependent gene expression to control innate immunity signals. Dis. Models Mech. 2015, 8, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Stebbing, J.; Gazzard, B.; Douek, D.C. Where does HIV live? N. Engl. J. Med. 2004, 350, 1872–1880. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerebtsova, M.; Ahmad, A.; Kumari, N.; Rutagarama, O.; Nekhai, S. Oxygen Levels Affect Macrophage HIV-1 Gene Expression and Delay Resolution of Inflammation in HIV-Tg Mice. Viruses 2020, 12, 277. https://doi.org/10.3390/v12030277
Jerebtsova M, Ahmad A, Kumari N, Rutagarama O, Nekhai S. Oxygen Levels Affect Macrophage HIV-1 Gene Expression and Delay Resolution of Inflammation in HIV-Tg Mice. Viruses. 2020; 12(3):277. https://doi.org/10.3390/v12030277
Chicago/Turabian StyleJerebtsova, Marina, Asrar Ahmad, Namita Kumari, Ornela Rutagarama, and Sergei Nekhai. 2020. "Oxygen Levels Affect Macrophage HIV-1 Gene Expression and Delay Resolution of Inflammation in HIV-Tg Mice" Viruses 12, no. 3: 277. https://doi.org/10.3390/v12030277
APA StyleJerebtsova, M., Ahmad, A., Kumari, N., Rutagarama, O., & Nekhai, S. (2020). Oxygen Levels Affect Macrophage HIV-1 Gene Expression and Delay Resolution of Inflammation in HIV-Tg Mice. Viruses, 12(3), 277. https://doi.org/10.3390/v12030277




