Abstract
In diseases where epigenetic mechanisms are changed, such as cancer, many genes show altered gene expression and inhibited genes become activated. Human endogenous retrovirus type K (HERV-K) expression is usually inhibited in normal cells from healthy adults. In tumor cells, however, HERV-K mRNA expression has been frequently documented to increase. Importantly, HERV-K-derived proteins can act as tumor-specific antigens, a class of neoantigens, and induce immune responses in different types of cancer. In this review, we describe the function of the HERV-K HML-2 subtype in carcinogenesis as biomarkers, and their potential as targets for cancer immunotherapy.
1. Introduction
Endogenous retroviruses make up about 8% of the human genome [1]. They originated millions of years ago by retroviral infections in germline cells and currently remain in the human genome as “fossil” sequences [1,2,3].
There are several human endogenous retrovirus (HERV) families, as shown in Table 1, some of them composed of a full-length or almost complete retroviral genome, with gag, pol and env genes flanked by LTR regions. The env genes are commonly mutated and are therefore unable to produce infectious viral particles. However, HERV proteins synthesized by env transcripts play an important role in cellular regulation [2,3,4]. For instance, the env transcript from HERV-W is fundamental to the formation of the placenta during embryonic development [5].

Table 1.
Classifications of human endogenous retroviruses (HERVs) and phylogenetic relationship to exogenous retrovirus families.
The transcription of HERVs is mainly controlled by epigenetic mechanisms, such as the methylation of CpG regions [6,7,8,9]. HERV expression is inhibited in normal healthy adult cells [10,11]. However, in diseases where epigenetic mechanisms are altered, such as in cancer, HERV expression is upregulated and HERV proteins play an important role in carcinogenesis [3,7,12,13]. For example, env transcripts encoded by the HERV-K HML-2 subtype—hereafter abbreviated to HERV-K—the most studied HERV element, produce two oncogenic proteins (Rec and Np9) which are able to modulate cellular gene expression and induce cancer development [3,12]. Furthermore, its derived proteins could behave as tumor-associated neoantigens and induce immune responses in different types of cancer [3,4,12,14,15,16]. In this review, we describe the function of HERV-K in carcinogenesis, as well as its use as biomarkers and as targets for cancer immunotherapy.
2. HERVs: Classification and Genome
2.1. Nomenclature and Classification
Since HERVs were discovered, at least 31 distinct HERV groups have been described, with copy numbers ranging from one to many thousands in the human genome [17,18]. HERV group classification is based on the tRNA type used as primers during reverse transcription. However, the nomenclature system is not unified and there are multiple names for each unique HERV. HERV-K nomenclature is a good example of this inconsistency, as shown in Table 1 [17,19,20]. HERV-K has been described with multiple names, such as HLM-2, HML-2, HERV-K10, HTDV/HERV-K, HERV-K (HML-2), HERV-K, HERVK or ERKV. The letter “K” in the group name is based on the lysine-tRNA used during reverse transcription. In addition, the HERV-K group (HML1-HML10) can also be classified into type I and II proviruses, however phylogenetic type I and II classification from the HERV-K group is not the same for all HML subtypes. For instance, HERV-K HML-2, HML-6 and HLM-10 subtypes are classified into type I or II, based on differences from their genomes; however type I and II from each of them are unrelated [21,22,23,24]. Moreover, phylogenetic analysis using LTR sequences can be used to classify HERV-K, HML-2 subtype, into three subgroups, known as LTR5Hs, LTR5A and LTR5B [2,19,24,25]. The LTR5Hs is the most recently integrated subgroup. Interestingly, the HERV-K HML-2 types I and II are not equally distributed within LTR subgroups. While types I and II are found in equal frequency within the LTR5Hs subgroup, LTR5B and LTR5A subgroups show only the type II variant [24]. Due to this complex classification system, some authors have proposed a unified system of endogenous retrovirus nomenclature [20,26,27].
HERVs are phylogenetically similar to exogenous retroviruses due to their origin and can therefore be categorized into three retrovirus classes [28,29,30]. HERVs of Class I are similar to exogenous Gammaretroviruses; Class II to Betaretroviruses and Class III are distantly related to Lentiviruses and Spumaviruses, as shown in Table 1. Unlike exogenous retroviruses, HERV genomes show many mutations and deletions that prevent the production of infectious viral particles.
2.2. Genome Structure
HERV elements originated in the human genome through many insertion events of exogenous retroviruses in germline cells throughout evolution [3,31]. Over evolutionary time, HERV genomes suffered many mutations and this modification rate can be used to define the approximate time in the past of a particular HERV introduction into the human genome [2]. HERVs with recent introductions show full-length or almost complete sequence genomes, which are composed of gag, pol and env genes flanked by LTR regions [2].
The majority of HERV-related sequences in the genome are solitary LTR (solo LTR) sequences [32]. They were generated in the genome by homologous recombination between two LTRs flanking gag, pol and env genes, resulting in the deletion of these regions [33,34]. These HERV sequences do not produce viral proteins but are able to regulate cellular gene expression through their promoter regions [35]. In contrast, in full-length or almost complete genome HERV sequences, such as in HERV-K, the gag, pol and env genes produce their respective viral proteins, and non-infectious viral-like particles in diseases such as cancer [36,37,38,39]. The main env gene product is the Env protein, displaying a surface (SU) and a transmembrane (TM) domain. However, the HERV-K subtype encodes two alternative proteins, called Rec and Np9 by alternative splicing events.
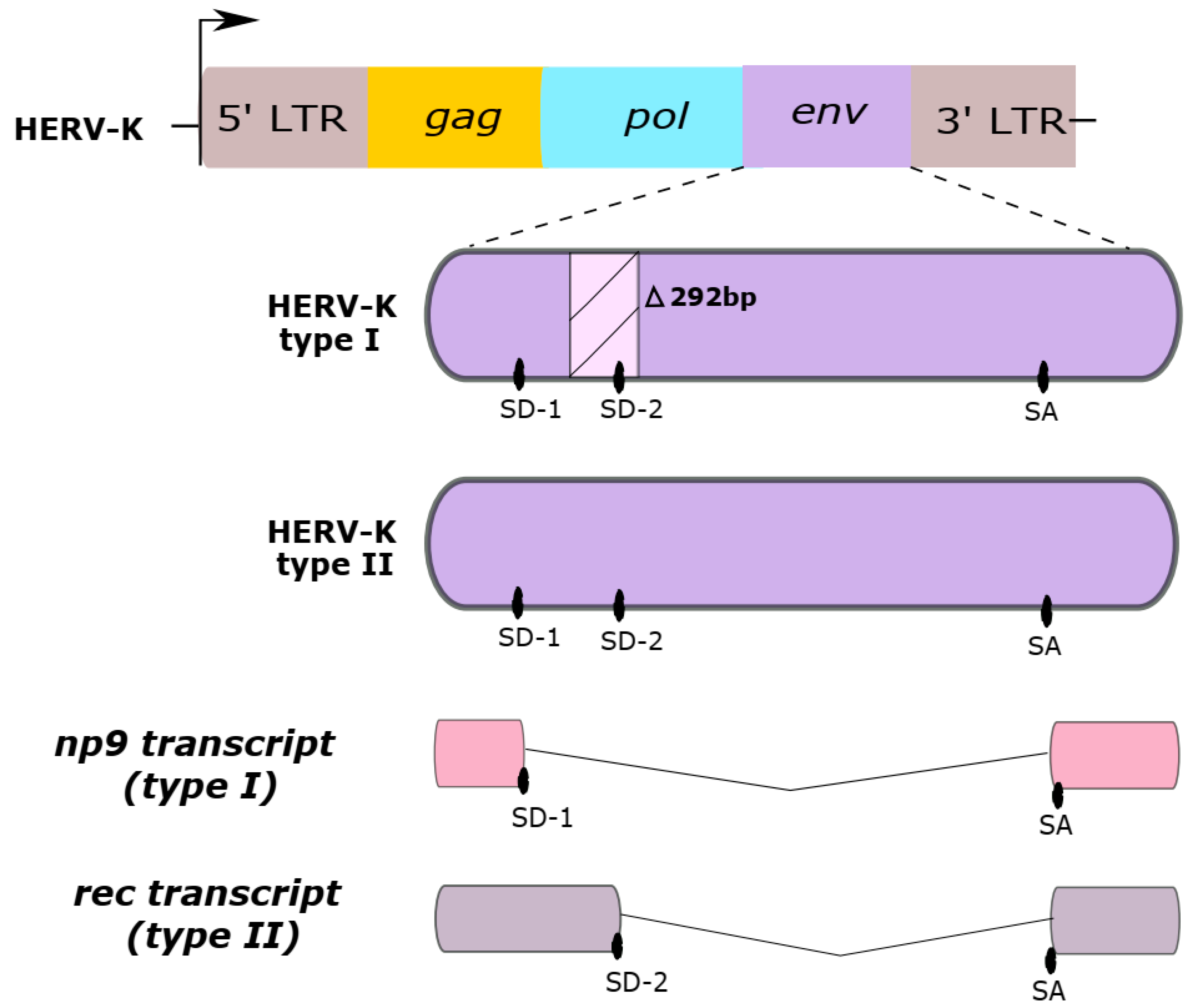
HERV-K HML-2 subtype sequences are classified as type I or type II, according to either the absence or presence of a 292-base pair portion in the env coding region, as shown in Figure 1. While type II sequences are able to produce the viral accessory Rec protein, type I sequences cannot produce the Rec protein but are instead able to synthesize an alternative protein, called Np9. Both Rec and Np9 proteins have been postulated to be involved in carcinogenesis [3,19,36,40,41,42].
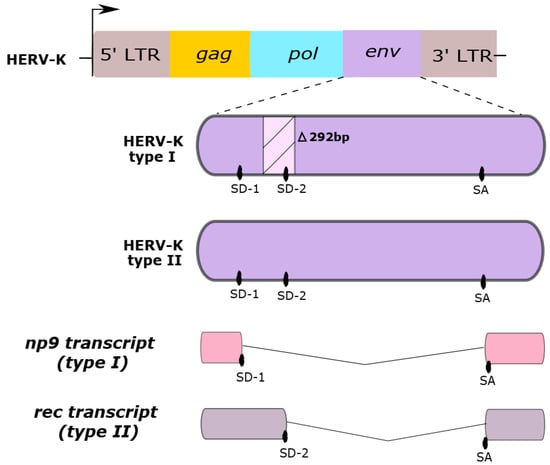
Figure 1.
Two HERV-K HML-2 genome types. Type I sequences have a deletion in the env gene of a 292-base pair region (∆292pb). This region has a splice donor (SD) site 2, which is absent in the HERV-K type I. Type II sequences contain two splice donor sites (SD-1 and SD-2). Differences in the presence of the SD sites are responsible for generating the distinct np9 or rec transcripts from Type I and II sequences, respectively. SA, splice acceptor site.
In healthy adult cells, HERV gene expression is inhibited by epigenetic regulation. In some diseases, such as cancer, the epigenetic mechanisms become dysregulated and many previously repressed genes become expressed, including HERV-K genes [43,44]. DNA methylation, the addition of methyl groups onto the cytidines of CpG regions in the DNA, and histone modification, such as the removal of acetyl groups of histones, cause chromatin condensation and make promoter regions inaccessible to transcription factors and to transcription machinery. In addition, the methylation of histones can also control HERV expression. SETDB1 (SET domain bifurcated histone lysine methyltransferase 1) is a histone methyltransferase, which methylates Lys-9 of histone H3, leading to transcriptional repression. SETDB1 is upregulated in various tumor cells and plays an important role on the silencing of endogenous retroelements [45,46]. These mechanisms contribute to the regulation of HERV expression [11,46,47,48,49]. HERV DNA methylation occurs in CpG regions of the LTR promoters and inhibits or downregulates HERV gene expression in normal cells [11,50,51,52]. The hypomethylation of HERV-K has been associated with poor ovarian cancer prognosis [53]. Interestingly, HERV-K also shows differential gene expression between individuals due to LTR polymorphisms at transcription factor binding sites [54].
The first investigations into the association of HERV expression with carcinogenesis were reported in research from the early 1970s exploring reverse transcriptase (RT) protein activity and viral particles in cancer cells [55,56]. Currently, HERV-K transcription is related to many kinds of cancer, such as breast cancer, melanoma and prostate cancer [57,58,59]. However, its gene expression in breast cancer and melanoma is the most studied as biomarkers and immunologic therapeutic targets [59].
In breast cancer, HERV-K RT expression is found in about 28% of samples and in 18% of adjacent normal breast tissues. There is also a significant correlation between HERV-K RT expression and poor prognosis for disease-free patients that go on to develop disease, suggesting HERV-K could be an early prognostic biomarker for breast cancer [60]. Furthermore, HERV-K env, gag and np9 mRNA expression levels are also elevated in breast cancer cells and their use as biomarkers for early breast cancer diagnosis has been proposed [61]. The HERV-K env gene is expressed in 70% of breast cancers and its expression is associated with breast cancer progression [62]. HERV-K env gene expression was associated with tumor size, tumor stage, and lymph node metastasis. Furthermore, breast cancer patients with high HERV-K env expression show decreased overall survival compared to patients who had tumors with moderate or low HERV-K expression [62]. HERV-K env expression was not detected in normal breast tissues, suggesting that its expression is absent in the normal tissues. In addition, HERV-K was significantly overexpressed in basal breast cancer subtypes—the breast cancer subtype with the worst prognosis [63]. Finally, HERV-K gag mRNA overexpression has been reported in breast cancer patients who developed metastatic tumors when compared with those with tumors that did not metastasize [64]. Similarly, melanoma also shows high HERV-K gene expression, along with the production of retrovirus-like particles in tumor cells [65,66].
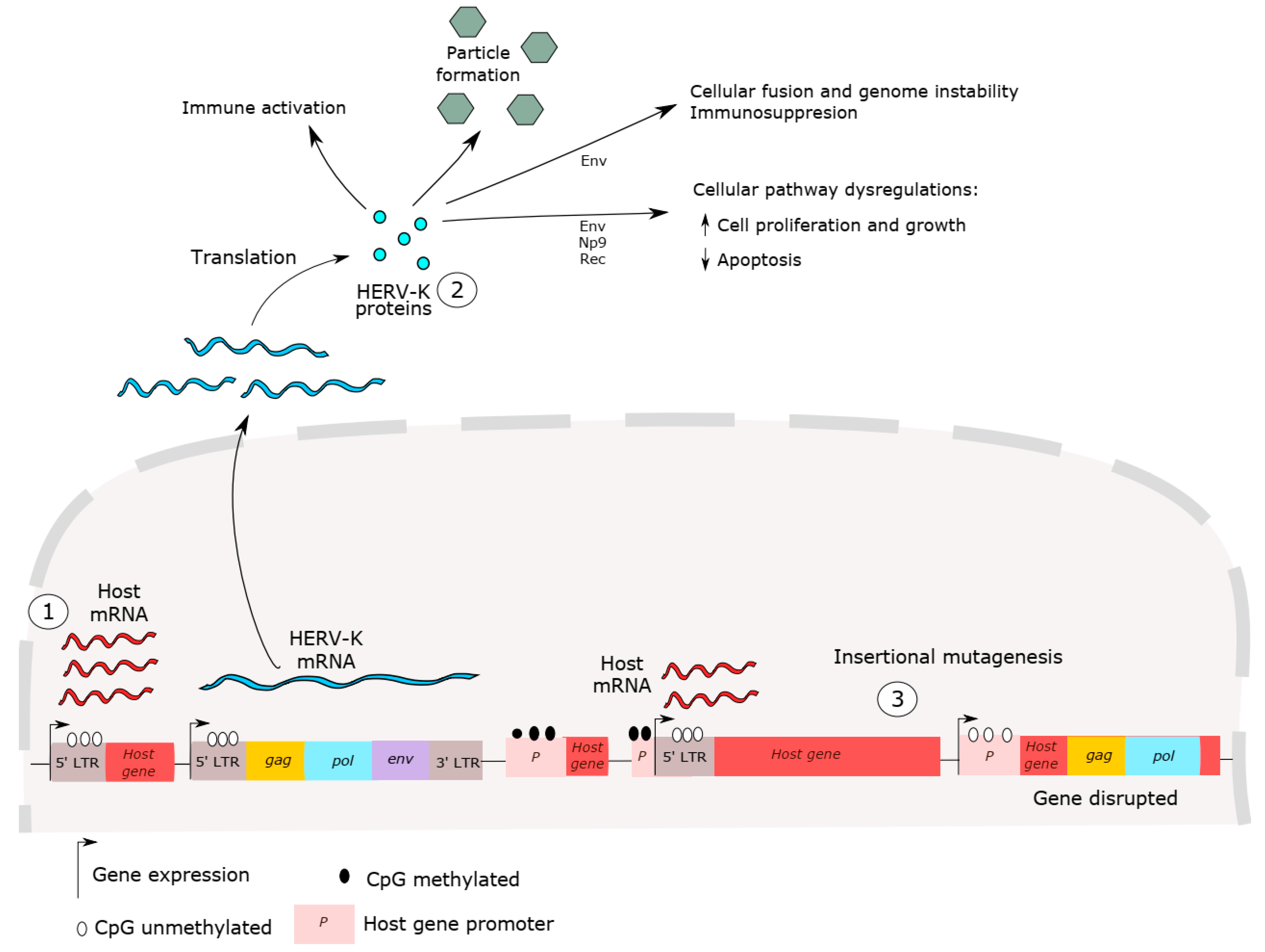
Many studies have discussed the mechanisms of carcinogenesis mediated by HERV-K expression, as shown in Figure 2 [2,12,67]. However, whether HERV has a role in cancer initiation or cancer progression is still controversial. HERV-K insertional polymorphisms, responsible for HERV haplotype diversity in the human population, can influence disease susceptibility, including cancer [40,68,69,70,71]. HERV-K LTR sequences are able to up- and downregulate host genes [72]. Host gene expression dysregulation, such as that of oncogenes, proto-oncogenes and growth factors has been reported in cancer and has been associated with HERV LTR promoter activity [35]. These sequences may influence the expression of neighboring host genes and act as alternative promoters or enhancers of host genes [35,73,74]. For example, hypomethylation in LTR promoters is able to induce carcinogenesis in B cell-derived Hodgkin’s lymphoma by deregulating the expression of the colony-stimulating factor 1 receptor (CSF1R), a proto-oncogene [75]. In addition, despite the high HERV-K expression in cancer, de novo insertion by re-infection events has not been detected, which is not surprising since no HERV-K copies are known to be retrotransposition competent.
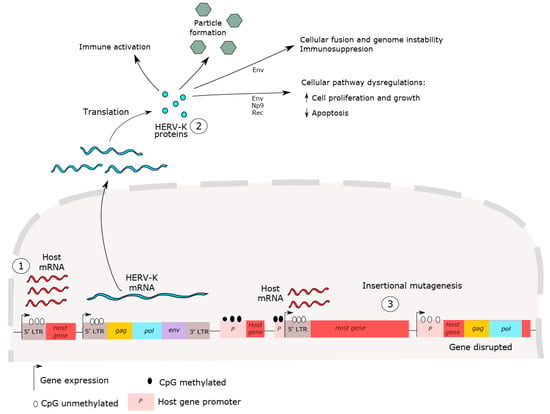
Figure 2.
HERV-K carcinogenesis mechanisms. (1) Dysregulation of host gene expression by LTR promoter sequences. HERV LTR may influence neighboring host gene expressions, such as those of oncogenes, proto-oncogenes and growth factors. (2) HERV proteins can induce immune activation and suppression, lead to cell fusion, genome instability and cellular dysregulation. (3) HERV-K insertion mutagenesis, induced by recent retrotransposition events, is also able to cause host gene alterations, such as disruption in host genes, inducing host gene expression and causing genome instability. No HERV-K copies are competent for genomic reinsertion, but HERV-K insertional polymorphisms exist in the human population, suggesting these elements might provide a platform for genomic rearrangement. In short, all these events are able to disrupt cellular processes and lead to cancer initiation and progression.
HERV-K proteins have also been shown to interact with host proteins and lead to cancer progression [76]. Intriguingly, the HERV-K Env protein exhibits many functions, including cancer cell fusion and host immunosuppression, as shown in Figure 2. Cancer cells are able to fuse with other cells, leading to chromosomal instability. This process may be associated with cancer progression and metastasis and chemoresistance [77,78]. Additionally, the immunosuppressive activity of the Env protein may lead to a tumor’s ability to evade immune responses, through the inhibition of the CD8-T cell cytotoxic activity against cancer cells and the prevention of apoptotic responses [79,80]. This property is co-opted in the fetal–maternal tolerance promoted by the expression of Syncitin 2, a HERV-FRD-derived Env protein. This property of Syncitin 2 led to the identification of an immunosuppressive domain (ISD) in the transmembrane region of the Env protein. Further studies exposing human PBMCs to ISD-derived proteins from HERV-K and other retroviruses, such as HIV, showed increases in the expression of numerous immunomodulatory factors, such as IL-10, IL-6 and IL-8, with decreases in the expression of the immune stimulatory factors IL-2 and CXCL9. However, the overall effect of this modulation remains to be clarified [81]. Furthermore, Env proteins produced by HERV-K mimic the oxygen response element binding protein (OREBP), affecting glutathione peroxidase expression and resulting in increased levels of free radicals in melanoma cells [82]. In vivo, HERV-K env RNA knockdown led to reduced metastasis [83]. Finally, the HERV-K Env protein is able to affect cellular networks and tumor-associated gene expression that play key roles in carcinogenesis (EGFR, c-Myc, TGFB1, NF-κB, p53, p-ERK, p-RSK, p-AKT and Ras) [76,83,84]. In particular, the HERV-K env gene can also produce Np9 or Rec proteins through alternative splicing from the env transcript.
2.3. HERV-K Oncoproteins
2.3.1. Rec
Rec is a 14.5 kDa protein with functional homology to HIV-1 Rev and HTLV Rex proteins, which are responsible for translocating both partially spliced and unspliced retroviral transcripts from the cellular nucleus to the cytoplasm [23,85]. HERV-K RNA transport is mediated by Rec protein binding to the Rec-responsive element (RcRE) that is located within the LTR sequence on the 3′ end of unspliced viral RNAs [19,86].
The description of Rec expression in cancer was first reported in human germ cell tumors [87]. Following this, it was shown that nude mice that received a cell line expressing Rec eventually developed cancer, but not mice treated with cells expressing the full-length env or gag genes [88]. Supporting these findings, a study found that transgenic mice expressing Rec were able to develop in situ testicular carcinomas and predecessor lesions [89]. Moreover, in breast cancer, anti-Rec antibodies were detected in early-stage patients, suggesting a predictive biomarker for breast cancer progression [64].
The Rec protein has been shown to interact with zinc-finger proteins, such as the tumor suppressor, promyelocytic leukemia zinc-finger protein (PLZF) [88], associated with leukemia development. The Rec binding for PLZF leads to the higher expression of the c-myc proto-oncogene, consequently stimulating cell growth and proliferation [90]. Rec is also able to interact with the androgen receptor (AR), PLZF-related testicular zinc-finger protein co-repressor (TZFP) and with the human small glutamine-rich tetratricopeptide repeat-containing protein co-chaperone (hSGT), forming the complex Rec/AR/TZFP/hSGT [91,92]. This Rec-containing complex might lead to carcinogenesis by inducing cellular proliferation and reducing apoptosis [91,93].
2.3.2. Np9
Np9 is a 9kDa protein that shares a region of 14 amino acids (MNPSEMQRKGPPRR) with the Rec protein in its N-terminal portion [90,94]. Np9 is also able to bind to PLZF in the nucleus, interfering with c-Myc repression in a similar fashion to Rec [90]. However, Np9 has also been shown to interact with E3 ubiquitin ligases, such as the ligand of numb protein X (LNX) and the murine double minute 2 (MDM2) protein, which are involved in the proteasome-dependent degradation pathways [95,96,97]. MDM2 has an essential function in the negative regulation of P53 through its degradation by ubiquitination. Therefore, dysregulation in this pathway leads to cell cycle dysfunction and is able to promote cellular proliferation and cancer initiation [97]. Finally, the expression of Np9 in leukemia cells is able to activate leukemia-associated signaling pathways and induce alteration in pERK, c-Myc and β-catenin expression, each of which has been shown to be altered in cancer cells [98].
3. HERV-K in Cancer Immunotherapy
HERV proteins are characterized within the group of alternative tumor-specific antigens, a neoantigen class, due to their expression in many kinds of cancer [4,99]. Tumor-specific antigens are defined as peptide antigens expressed in cancer cells and with minimal to no expression in normal healthy adult cells, as is the case of the expression of HERV-K antigens [99]. They are able to impact both innate and adaptive immune responses through distinct mechanisms. HERVs can induce the innate immune response by RIG-I-like and Toll-like receptor pathways through HERV nucleic acids [4,100,101,102]. The RIG-I-like and Toll-like are pattern recognition receptors able to recognize conserved pathogen-associated molecular patterns, such as ssRNA and dsRNA from viruses. RIG-I receptors mediate antiviral signaling via CARD–CARD interactions with the mitochondrial outer-membrane-localized adaptor molecule through mitochondrial antiviral signaling (MAVS). Such signal transduction leads to type I IFN induction and pro-inflammatory cytokine production, via the activation of IFN regulatory factors 3 (IRF3), and induce nuclear factor κB (NF-kB) expression [4]. Both pattern recognition receptors can induce inflammation that causes immune activation and the expression of class I MHC on tumor cells. In short, the innate immune response activation leads to B- and T-cell stimulation, inducing antibodies and cytotoxic T cell responses [64,103,104,105]. Thus, proteins encoded by the HERV-K env gene are immunogenic and humoral and cellular responses against these HERV-K have been described [67].
Antibodies to HERV-K were shown to inhibit cancer growth in vitro and in animal models [106]. In conjunction with a dendritic vaccine, HERV-K Env antigens demonstrated in vitro activity in ovarian and breast cancer [16,107]. Additionally, new modalities, such as CAR-T cells, have shown novel potential for HERV-related cancer immunotherapy. By using the Sleeping Beauty system, HERV-K Env-specific chimeric antigen mouse monoclonal antibodies were inserted into CAR-T cells and showed anti-tumor activity in vitro [108]. Finally, a recombinant vaccine using modified Ankara virus, expressing HERV-K Env glycoprotein (MVA HERV-K Env), demonstrated activity in vitro and in animal models [109]. However, concerns remain about the possible safety issues of vaccinating patients against endogenous HERV antigens, given the possible roles of these gene products in normal physiological function [67].
Of note, the homology between the HERV-K-MEL protein, Bacillus Calmette–Guerin (BCG) and yellow fever virus vaccine has been described [110]. Interestingly, a case-control study showed that the BCG vaccine was associated with lower melanoma risk in patients compared to the unvaccinated population [82]. Similarly, immunoreactivity to melanoma has been described in vitro with sera from rhesus macaques, vaccinated with the yellow fever virus vaccine, and this vaccine has been proposed as a prophylactic vaccine against melanoma. Given HERV over-expression, noted following BCG, yellow fever virus vaccine and the post-febrile process in melanoma patients, there is strong evidence to suggest that HERV-K gene expression may play a role in anti-melanoma immunoreactivity [111]. Additionally, the dependency of Env expression from a single provirus in a subset of individuals and a pattern of tissue-specific expression among proviruses in Mantle Cell lymphoma cell lines implies that HERV-K-targeted immunotherapy could be a precision medicine technique to specifically target the cell-specific aberrant transcription of this tumor-associated antigen in blood cancers. This could lead to a more targeted proteome-based screening protocol for HERV-K polymorphisms in blood cancers [15]. In short, all these studies show HERV-K expression as a target for cancer immunotherapy.
Several studies have reported humoral and cell-mediated immunity against HERV-K in cancer, as shown in Table 2. Breast cancer, melanoma and prostate cancer are the most studied types of cancer with HERV-K expression as new target in cancer immunotherapy, yet other types have also been studied in that regard.

Table 2.
Studies that have shown HERV-K expression as a biomarker for cancer screening and as an immunotherapeutic target.
3.1. Melanoma
Cytotoxic CD8 T-cell responses against HERV-K and their ability to lyse melanoma cells in vitro were first reported in 2002 [112]. Melanoma patients from stages I to IV showed significant differences in the seroprevalence of anti-HERV-K antibodies when compared to healthy subjects [114]. The serological HERV-K reactivity was inversely correlated with both disease specific (stage I–IV) and overall survival (stage I–III), providing new prognostic information on the disease [115].
In addition, chimeric antigen receptor (CAR) T-cells have been developed against HERV-K Env protein (HERV-K Env-specific CAR+ T-cells), which were found to be overexpressed in melanoma samples. HERV-K Env-specific CAR+ T-cells were able to lyse tumor cells expressing HERV-K Env on their surfaces in vitro. Furthermore, these CAR+ T-cells decreased tumor burden and the number of metastatic lesions to the liver in a mouse xenograft model of metastatic melanoma [116].
3.2. Breast Cancer
Anti-HERV-K antibodies have also been detected in breast cancer patients [16,64], and peripheral blood mononuclear cells (PBMC) from breast cancer patients stimulated in vitro with HERV-K are able to induce T-cell responses, such as T-cell proliferation, IFN-γ production and proinflammatory cytokine secretion [16]. Cytotoxic T-cells respond to breast cancer cells that express HERV-K, suggesting that HERV expression can be used as tumor-associated antigens for activating both T-cell and B-cell responses [16].
Anti-HERV-K antibodies and HERV-K gag mRNA detection showed diagnostic value for early breast cancer detection in women and can be used as sensitive and specific biomarkers for screening tests [64]. The HERV-K antibody’s diagnostic performance was comparable to mammography screening and can be performed as an additional option for early detection in women with increased breast cancer risk [64]. Furthermore, higher levels of HERV-K gag mRNA were detected in serum from breast cancer patients who developed metastasis in comparison with patients that did not [64].
Monoclonal anti-HERV-K Env antibodies showed antitumor effects as therapeutics against breast cancer in vitro and in vivo [105]. They blocked the growth and proliferation of tumor cells through the activation of apoptotic signaling pathways and, consequently cellular death in vitro. Likewise, mice receiving xenografts treated with the antibodies showed a reduction in breast tumor growth compared to mice with no antibody treatment [105].
HERV-K Env-specific CAR+ T-cells generated through monoclonal anti-HERV-K Env antibodies inhibited tumor growth and showed cytotoxic activity against breast cancer cell lines in vitro [108]. A significant reduction in tumor growth and tumor weight was also observed in mice xenograft models for breast cancer. In addition, HERV-K Env-specific CAR+ T-cells also prevented breast cancer metastasis in those mice.
3.3. Prostate Cancer
Both anti-HERV antibodies and HERV mRNA have been reported as biomarkers for prostate cancer and antibody production has been discussed as potential cancer immunotherapy [118,119,123,124,125,126]. HERV-K gag mRNA expression in prostate cells is regulated by both HERV promoter demethylation and androgen stimulation [117]. Results have suggested that the combination of HERV-K gag expression with prostate-specific antigen (PSA) testing using blood samples may be efficient to detect early prostate cancer, specifically in older men and smokers who at higher risk of developing more aggressive prostate cancer [118].
A panel for the detection of autoantibodies, including those against HERV and three host proteins, has been tested for analyzing the potential of using these autoantibodies in the diagnosis of prostate cancer [119]. The results showed that the detection of the anti HERV-K Gag antibody along with other host antibodies was successful in differentiating cancer patients from healthy subjects [119]. Furthermore, the anti-HERV-K Gag antibody is more frequent in serum from patients with advanced prostate cancer (stage III–IV) when compared to patients with early prostate cancer (stages I–II) [117]. The presence of the anti-HERV Gag antibody in patients’ sera has also been correlated with worse disease survival [117].
4. Other Cancers
Several studies have reported high HERV-K expression, adaptive immune responses and HERV-K antibodies in germline cell cancers [53,89,107,120,127,128,129,130,131]. These tumors occur in the testes and ovaries. HERV-K env and RT expression was higher in ovarian cancer in comparison to normal adjacent tissues and blood from ovarian cancer patients showed HERV-K antibody reactivity [107]. Autologous in vitro stimulation of T-lymphocytes from ovarian cancer patients with HERV-K Env protein exhibited cytotoxic activity against ovarian cancer cells [107]. In addition, we previously showed the HERV-K Gag protein and T-cell reactivity to HERV-K in seminoma patients [127].
HERV-K env is expressed in about 20% to 80% of pancreatic cancer tissues but not in normal counterparts [83,121]. High levels of HERV-K antibodies and HERV-K viral RNA have been reported in plasma from pancreatic cancer patients, suggesting HERV-K expression as a biomarker and a tumor-associated antigen that may be used for diagnosis and cancer immunotherapy [83,121].
HERV-K expression has also been associated with hepatocellular carcinoma (HCC) progression and poor outcome. HERV-K expression is upregulated in HCC, which was significantly associated with cancer staging, cirrhosis and tumor differentiation [122]. Furthermore, HCC patients with high HERV-K expression levels showed a poorer overall survival compared to patients with lower expression. In addition, HERV-K expression in HCC showed a diagnostic accuracy value, with 74.7% sensitivity and 67.8% specificity, which may be used as HCC diagnostics and as prognostic biomarkers for the disease [122].
5. Conclusions
HERVs are retroviral fossil sequences in the human genome that originated millions of years ago through retrovirus infections in germline cells and they now compose about 8% of the human genome. There are several HERV families, some of them are composed of full-length or almost complete genome retroviruses, showing gag, pol and env genes flanked by LTR regions. The env genes are mutated and, therefore, unable to produce infectious viral particles. However, HERV proteins synthesized by env transcripts play an important role in cellular regulation attributed to many kinds of HERVs.
The HERV-K family, the most studied, is expressed in many types of cancer. The env gene can give rise to two oncoproteins derived by alternative env mRNA splicing, called Np9 and Rec. Both oncoproteins are able to induce carcinogenesis by the dysregulation of essential cellular pathways, leading to the inhibition of apoptosis and to cellular growth and proliferation. Additionally, HERV-K proteins are classified within the neoantigen class of alternative tumor-specific antigens. They are able to impact both innate and adaptive immune responses, inducing B- and T-cell stimulation and activation. This can then lead to specific antibody and cytotoxic T-cell immune responses in many kinds of cancer, including breast cancer, prostate cancer, melanoma and renal cell carcinoma, and could be used as an immunotherapeutic target in these cancers.
Author Contributions
Conceptualization, G.C., J.L.M., D.F.N. and M.A.S.; writing—original draft preparation, G.C. and J.L.M.; writing—review and editing, M.d.M.R., F.E.L., D.F.N. and M.A.S.; supervision, M.d.M.R., F.E.L., D.F.N. and M.A.S.; project administration, F.E.L., D.F.N. and M.A.S.; funding acquisition, D.F.N. and M.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported in part by the US National Institutes of Health, grant: CA206488 (D.F.N.) and by intramural grants of the Brazilian Ministry of Health (M.A.S.). M.M.R. is funded in part by the Department of Medicine, “Fund for the Future” program at Weill Cornell Medicine. G.C. is the recipient of a Ph.D. fellowship by the Brazilian National Cancer Institute (INCA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bannert, N.; Kurth, R. Retroelements and the human genome: New perspectives on an old relation. Proc. Natl. Acad. Sci. USA 2004, 101, 14572–14579. [Google Scholar] [CrossRef] [PubMed]
- Hohn, O.; Hanke, K.; Bannert, N. HERV-K(HML-2), the Best Preserved Family of HERVs: Endogenization, Expression, and Implications in Health and Disease. Front. Oncol. 2013, 3, 246. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef] [PubMed]
- Magiorkinis, G.; Hurst, T.P. Activation of the innate immune response by endogenous retroviruses. J. Gen. Virol. 2015, 96, 1207–1218. [Google Scholar] [CrossRef]
- Blond, J.-L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.-L. An Envelope Glycoprotein of the Human Endogenous Retrovirus HERV-W Is Expressed in the Human Placenta and Fuses Cells Expressing the Type D Mammalian Retrovirus Receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Groh, S.; Schotta, G. Silencing of endogenous retroviruses by heterochromatin. Cell. Mol. Life Sci. 2017, 74, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Ishak, C.A.; Classon, M.; De Carvalho, D.D. Deregulation of Retroelements as an Emerging Therapeutic Opportunity in Cancer. Trends Cancer 2018, 4, 583–597. [Google Scholar] [CrossRef]
- Goering, W.; Ribarska, T.; Schulz, W.A. Selective changes of retroelement expression in human prostate cancer. Carcinogenesis 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Schulz, W.A.; Steinhoff, C.; Florl, A.R. Methylation of Endogenous Human Retroelements in Health and Disease. In DNA Methylation: Development, Genetic Disease and Cancer; Springer: Berlin/Heidelberg, Germany, 2006; Volume 310, pp. 211–250. ISBN 3540311807. [Google Scholar]
- Misiak, B.; Ricceri, L.; Sąsiadek, M.M. Transposable Elements and Their Epigenetic Regulation in Mental Disorders: Current Evidence in the Field. Front. Genet. 2019, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Hurst, T.; Magiorkinis, G. Epigenetic Control of Human Endogenous Retrovirus Expression: Focus on Regulation of Long-Terminal Repeats (LTRs). Viruses 2017, 9, 130. [Google Scholar] [CrossRef]
- Bannert, N.; Hofmann, H.; Block, A.; Hohn, O. HERVs New Role in Cancer: From Accused Perpetrators to Cheerful Protectors. Front. Microbiol. 2018, 9, 178. [Google Scholar] [CrossRef]
- Loo Yau, H.; Ettayebi, I.; De Carvalho, D.D. The Cancer Epigenome: Exploiting Its Vulnerabilities for Immunotherapy. Trends Cell Biol. 2019, 29, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Attermann, A.S.; Bjerregaard, A.M.; Saini, S.K.; Grønbæk, K.; Hadrup, S.R. Human endogenous retroviruses and their implication for immunotherapeutics of cancer. Ann. Oncol. 2018, 29, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Tatkiewicz, W.; Dickie, J.; Bedford, F.; Jones, A.; Atkin, M.; Kiernan, M.; Maze, E.A.; Agit, B.; Farnham, G.; Kanapin, A.; et al. Characterising a human endogenous retrovirus(HERV)-derived tumour-associated antigen: Enriched RNA-Seq analysis of HERV-K(HML-2) in mantle cell lymphoma cell lines. Mob. DNA 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Radvanyi, L.; Rycaj, K.; Plummer, J.B.; Yan, P.; Sastry, K.J.; Piyathilake, C.J.; Hunt, K.K.; Johanning, G.L. Human Endogenous Retrovirus K Triggers an Antigen-Specific Immune Response in Breast Cancer Patients. Cancer Res. 2008, 68, 5869–5877. [Google Scholar] [CrossRef]
- Stoye, J.P. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat. Rev. Microbiol. 2012, 10, 395–406. [Google Scholar] [CrossRef]
- Martin, M.A.; Bryan, T.; Rasheed, S.; Khan, A.S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc. Natl. Acad. Sci. USA 1981, 78, 4892–4896. [Google Scholar] [CrossRef]
- Garcia-Montojo, M.; Doucet-O’Hare, T.; Henderson, L.; Nath, A. Human endogenous retrovirus-K (HML-2): A comprehensive review. Crit. Rev. Microbiol. 2018, 44, 715–738. [Google Scholar] [CrossRef]
- Gifford, R.J.; Blomberg, J.; Coffin, J.M.; Fan, H.; Heidmann, T.; Mayer, J.; Stoye, J.; Tristem, M.; Johnson, W.E. Nomenclature for endogenous retrovirus (ERV) loci. Retrovirology 2018, 15, 59. [Google Scholar] [CrossRef]
- Grandi, N.; Cadeddu, M.; Pisano, M.P.; Esposito, F.; Blomberg, J.; Tramontano, E. Identification of a novel HERV-K(HML10): Comprehensive characterization and comparative analysis in non-human primates provide insights about HML10 proviruses structure and diffusion. Mob. DNA 2017, 8, 15. [Google Scholar] [CrossRef]
- Pisano, M.P.; Grandi, N.; Cadeddu, M.; Blomberg, J.; Tramontano, E. Comprehensive Characterization of the Human Endogenous Retrovirus HERV-K(HML-6) Group: Overview of Structure, Phylogeny, and Contribution to the Human Genome. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Magin, C.; Löwer, R.; Löwer, J. cORF and RcRE, the Rev/Rex and RRE/RxRE Homologues of the Human Endogenous Retrovirus Family HTDV/HERV-K. J. Virol. 1999, 73, 9496–9507. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.P.; Wildschutte, J.H.; Russo, C.; Coffin, J.M. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology 2011, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, C.; Simmonds, P. Allelic Variation of HERV-K(HML-2) Endogenous Retroviral Elements in Human Populations. J. Mol. Evol. 2004, 59, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Benachenhou, F.; Blikstad, V.; Sperber, G.; Mayer, J. Classification and nomenclature of endogenous retroviral sequences (ERVs). Gene 2009, 448, 115–123. [Google Scholar] [CrossRef]
- Mayer, J.; Blomberg, J.; Seal, R.L. A revised nomenclature for transcribed human endogenous retroviral loci. Mob. DNA 2011, 2, 7. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Zhu, F. Human Endogenous Retroviral Envelope Protein Syncytin-1 and Inflammatory Abnormalities in Neuropsychological Diseases. Front. Psychiatry 2018, 9, 422. [Google Scholar] [CrossRef]
- van der Kuyl, A.C. HIV infection and HERV expression: A review. Retrovirology 2012. [Google Scholar] [CrossRef]
- Jern, P.; Sperber, G.O.; Blomberg, J. Use of endogenous retroviral sequences (ERVs) and structural markers for retroviral phylogenetic inference and taxonomy. Retrovirology 2005, 2, 50. [Google Scholar] [CrossRef]
- Parrish, N.F.; Tomonaga, K. Endogenized viral sequences in mammals. Curr. Opin. Microbiol. 2016, 31, 176–183. [Google Scholar] [CrossRef]
- Gemmell, P.; Hein, J.; Katzourakis, A. Phylogenetic Analysis Reveals That ERVs “Die Young” but HERV-H Is Unusually Conserved. PLOS Comput. Biol. 2016, 12, e1004964. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.F.; Coffin, J.M. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: Implications for human and viral evolution. Proc. Natl. Acad. Sci. USA 2004, 101, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Perron, H.; Feschotte, C. Variation in proviral content among human genomes mediated by LTR recombination. Mob. DNA 2018, 9, 36. [Google Scholar] [CrossRef]
- Cohen, C.J.; Lock, W.M.; Mager, D.L. Endogenous retroviral LTRs as promoters for human genes: A critical assessment. Gene 2009, 448, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; Kaplan, M.H.; Leissner, P.; Verjat, T.; Ferlenghi, I.; Bagnoli, F.; Giusti, F.; Dosik, M.H.; Hayes, D.F.; Gitlin, S.D.; et al. Human Endogenous Retrovirus K (HML-2) Elements in the Plasma of People with Lymphoma and Breast Cancer. J. Virol. 2008, 82, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Lower, R.; Lower, J.; Frank, H.; Harzmann, R.; Kurth, R. Human Teratocarcinomas Cultured in vitro Produce Unique Retrovirus-like Viruses. J. Gen. Virol. 1984, 65, 887–898. [Google Scholar] [CrossRef]
- Büscher, K.; Trefzer, U.; Hofmann, M.; Sterry, W.; Kurth, R.; Denner, J. Expression of Human Endogenous Retrovirus K in Melanomas and Melanoma Cell Lines. Cancer Res. 2005, 65, 4172–4180. [Google Scholar] [CrossRef]
- Schmitt, K.; Reichrath, J.; Roesch, A.; Meese, E.; Mayer, J. Transcriptional Profiling of Human Endogenous Retrovirus Group HERV-K(HML-2) Loci in Melanoma. Genome Biol. Evol. 2013, 5, 307–328. [Google Scholar] [CrossRef]
- Moyes, D.; Griffiths, D.J.; Venables, P.J. Insertional polymorphisms: A new lease of life for endogenous retroviruses in human disease. Trends Genet. 2007, 23, 326–333. [Google Scholar] [CrossRef]
- Löwer, R.; Löwer, J.; Tondera-Koch, C.; Kurth, R. A General Method for the Identification of Transcribed Retrovirus Sequences (R-U5 PCR) Reveals the Expression of the Human Endogenous Retrovirus Loci HERV-H and HERV-K in Teratocarcinoma Cells. Virology 1993, 192, 501–511. [Google Scholar] [CrossRef]
- Chan, S.M.; Sapir, T.; Park, S.-S.; Rual, J.-F.; Contreras-Galindo, R.; Reiner, O.; Markovitz, D.M. The HERV-K accessory protein Np9 controls viability and migration of teratocarcinoma cells. PLoS ONE 2019, 14, e0212970. [Google Scholar] [CrossRef] [PubMed]
- Kassiotis, G. Endogenous Retroviruses and the Development of Cancer. J. Immunol. 2014, 192, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Kitova, M.; Maldener, E.; Meese, E.; Mayer, J. CpG Methylation Directly Regulates Transcriptional Activity of the Human Endogenous Retrovirus Family HERV-K(HML-2). J. Virol. 2005, 79, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Shinkai, Y. SETDB1-Mediated Silencing of Retroelements. Viruses 2020, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, T.L.; Herzner, A.-M.; Zhang, X.; Goyal, Y.; Watanabe, C.; Friedman, B.A.; Janakiraman, V.; Durinck, S.; Stinson, J.; Arnott, D.; et al. Silencing of retrotransposons by SETDB1 inhibits the interferon response in acute myeloid leukemia. J. Cell Biol. 2017, 216, 3535–3549. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, J.Q. Expressional activation and functional roles of human endogenous retroviruses in cancers. Rev. Med. Virol. 2019, 29, e2025. [Google Scholar] [CrossRef]
- Laska, M.J.; Nissen, K.K.; Nexø, B.A. (Some) Cellular Mechanisms Influencing the Transcription of Human Endogenous Retrovirus, HERV-Fc1. PLoS ONE 2013, 8, e53895. [Google Scholar] [CrossRef]
- Goodier, J.L. Restricting retrotransposons: A review. Mob. DNA 2016, 7, 16. [Google Scholar] [CrossRef]
- Stengel, S.; Fiebig, U.; Kurth, R.; Denner, J. Regulation of human endogenous retrovirus-K expression in melanomas by CpG methylation. Genes Chromosomes Cancer 2010, 49, 401–411. [Google Scholar] [CrossRef]
- Kreimer, U.; Schulz, W.A.; Koch, A.; Niegisch, G.; Goering, W. HERV-K and LINE-1 DNA Methylation and Reexpression in Urothelial Carcinoma. Front. Oncol. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Hurst, T.; Pace, M.; Katzourakis, A.; Phillips, R.; Klenerman, P.; Frater, J.; Magiorkinis, G. Human endogenous retrovirus (HERV) expression is not induced by treatment with the histone deacetylase (HDAC) inhibitors in cellular models of HIV-1 latency. Retrovirology 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Iramaneerat, K.; Rattanatunyong, P.; Khemapech, N.; Triratanachat, S.; Mutirangura, A. HERV-K Hypomethylation in Ovarian Clear Cell Carcinoma Is Associated With a Poor Prognosis and Platinum Resistance. Int. J. Gynecol. Cancer 2011, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Montesion, M.; Williams, Z.H.; Subramanian, R.P.; Kuperwasser, C.; Coffin, J.M. Promoter expression of HERV-K (HML-2) provirus-derived sequences is related to LTR sequence variation and polymorphic transcription factor binding sites. Retrovirology 2018, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.M.; Soloviev, V.D.; Bektemirov, T.A.; Ilyin, K.V.; Bykovsky, A.F.; Mazurenko, N.P.; Irlin, I.S.; Yershov, F.I. Isolation of Oncornaviruses from Continuous Human Cell Cultures. Intervirology 1973, 1, 19–26. [Google Scholar] [CrossRef]
- Sarngadharan, M.G.; Sarin, P.S.; Reitz, M.S.; Gallo, R.C. Reverse Transcriptase Activity of Human Acute Leukaemic Cells: Purification of the Enzyme, Response to AMV 70S RNA, and Characterization of the DNA Product. Nat. New Biol. 1972, 240, 67–72. [Google Scholar] [CrossRef]
- Matteucci, C.; Balestrieri, E.; Argaw-Denboba, A.; Sinibaldi-Vallebona, P. Human endogenous retroviruses role in cancer cell stemness. Semin. Cancer Biol. 2018, 53, 17–30. [Google Scholar] [CrossRef]
- Barth, M.; Gröger, V.; Cynis, H.; Staege, M.S. Identification of human endogenous retrovirus transcripts in Hodgkin Lymphoma cells. Mol. Biol. Rep. 2019, 46, 1885–1893. [Google Scholar] [CrossRef]
- Grabski, D.F.; Hu, Y.; Sharma, M.; Rasmussen, S.K. Close to the Bedside: A Systematic Review of Endogenous Retroviruses and Their Impact in Oncology. J. Surg. Res. 2019, 240, 145–155. [Google Scholar] [CrossRef]
- Golan, M.; Hizi, A.; Resau, J.H.; Yaal-Hahoshen, N.; Reichman, H.; Keydar, I.; Tsarfaty, I. Human Endogenous Retrovirus (HERV-K) Reverse Transcriptase as a Breast Cancer Prognostic Marker. Neoplasia 2008, 10, 521-IN2. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. Evaluating the expression level of HERV-K env, np9, rec and gag in breast tissue. Infect. Agent. Cancer 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Zhao, J.; Rycaj, K.; Geng, S.; Li, M.; Plummer, J.B.; Yin, B.; Liu, H.; Xu, X.; Zhang, Y.; Yan, Y.; et al. Expression of Human Endogenous Retrovirus Type K Envelope Protein is a Novel Candidate Prognostic Marker for Human Breast Cancer. Genes Cancer 2011, 2, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Johanning, G.L.; Malouf, G.G.; Zheng, X.; Esteva, F.J.; Weinstein, J.N.; Wang-Johanning, F.; Su, X. Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Sci. Rep. 2017, 7, 41960. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Li, M.; Esteva, F.J.; Hess, K.R.; Yin, B.; Rycaj, K.; Plummer, J.B.; Garza, J.G.; Ambs, S.; Johanning, G.L. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int. J. Cancer 2014, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Muster, T.; Waltenberger, A.; Grassauer, A.; Hirschl, S.; Caucig, P.; Romirer, I.; Födinger, D.; Seppele, H.; Schanab, O.; Magin-Lachmann, C.; et al. An Endogenous Retrovirus Derived from Human Melanoma Cells. Cancer Res. 2003, 63, 8735–8741. [Google Scholar]
- Singh, S.; Kaye, S.; Francis, N.; Peston, D.; Gore, M.; McClure, M.; Bunker, C. Human endogenous retrovirus K (HERV-K) rec mRNA is expressed in primary melanoma but not in benign naevi or normal skin. Pigment Cell Melanoma Res. 2013, 26, 426–428. [Google Scholar] [CrossRef]
- María, G.-C.; Paola, I.; Niki, K.; Mariacarmela, S.; Julià, B.; Rafael, R. Human endogenous retroviruses and cancer. Cancer Biol. Med. 2016, 13, 483. [Google Scholar] [CrossRef]
- Wallace, A.D.; Wendt, G.A.; Barcellos, L.F.; de Smith, A.J.; Walsh, K.M.; Metayer, C.; Costello, J.F.; Wiemels, J.L.; Francis, S.S. To ERV Is Human: A Phenotype-Wide Scan Linking Polymorphic Human Endogenous Retrovirus-K Insertions to Complex Phenotypes. Front. Genet. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Kahyo, T.; Tao, H.; Shinmura, K.; Yamada, H.; Mori, H.; Funai, K.; Kurabe, N.; Suzuki, M.; Tanahashi, M.; Niwa, H.; et al. Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis 2013, 34, 2531–2538. [Google Scholar] [CrossRef]
- Burmeister, T.; Ebert, A.D.; Pritze, W.; Loddenkemper, C.; Schwartz, S.; Thiel, E. Insertional Polymorphisms of Endogenous HERV-K113 and HERV-K115 Retroviruses in Breast Cancer Patients and Age-Matched Controls. AIDS Res. Hum. Retrovir. 2004, 20, 1223–1229. [Google Scholar] [CrossRef]
- Wildschutte, J.H.; Ram, D.; Subramanian, R.; Stevens, V.L.; Coffin, J.M. The distribution of insertionally polymorphic endogenous retroviruses in breast cancer patients and cancer-free controls. Retrovirology 2014, 11, 62. [Google Scholar] [CrossRef]
- Fuentes, D.R.; Swigut, T.; Wysocka, J. Systematic perturbation of retroviral LTRs reveals widespread long-range effects on human gene regulation. Elife 2018, 7, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Shah, N.M.; Du, A.Y.; Dailey, Z.Z.; Pehrsson, E.C.; Godoy, P.M.; Zhang, D.; Li, D.; Xing, X.; Kim, S.; et al. Transposable elements drive widespread expression of oncogenes in human cancers. Nat. Genet. 2019, 51, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Babaian, A.; Mager, D.L. Endogenous retroviral promoter exaptation in human cancer. Mob. DNA 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; Walter, K.; Kreher, S.; Kumar, R.; Hummel, M.; Lenze, D.; Köchert, K.; Bouhlel, M.A.; Richter, J.; Soler, E.; et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat. Med. 2010, 16, 571–579. [Google Scholar] [CrossRef]
- Grandi, N.; Tramontano, E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Berndt, B.; Haverkampf, S.; Reith, G.; Keil, S.; Niggemann, B.; Zänker, K.S.; Dittmar, T. Fusion of CCL21 Non-Migratory Active Breast Epithelial and Breast Cancer Cells Give Rise to CCL21 Migratory Active Tumor Hybrid Cell Lines. PLoS ONE 2013, 8, e63711. [Google Scholar] [CrossRef]
- Bastida-Ruiz, D.; Van Hoesen, K.; Cohen, M. The Dark Side of Cell Fusion. Int. J. Mol. Sci. 2016, 17, 638. [Google Scholar] [CrossRef]
- Kassiotis, G.; Stoye, J.P. Making a virtue of necessity: The pleiotropic role of human endogenous retroviruses in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160277. [Google Scholar] [CrossRef]
- Ruprecht, K.; Mayer, J.; Sauter, M.; Roemer, K.; Mueller-Lantzsch, N. Endogenous retroviruses. Cell. Mol. Life Sci. 2008, 65, 3366–3382. [Google Scholar] [CrossRef] [PubMed]
- Alcazer, V.; Bonaventura, P.; Depil, S. Human Endogenous Retroviruses (HERVs): Shaping the Innate Immune Response in Cancers. Cancers 2020, 12, 610. [Google Scholar] [CrossRef]
- Krone, B.; Kölmel, K.F.; Henz, B.M.; Grange, J.M. Protection against melanoma by vaccination with Bacille Calmette-Guérin (BCG) and/or vaccinia: An epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur. J. Cancer 2005, 41, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Radvanyi, L.; Yin, B.; Rycaj, K.; Li, J.; Chivukula, R.; Lin, K.; Lu, Y.; Shen, J.; Chang, D.Z.; et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin. Cancer Res. 2017, 23, 5892–5911. [Google Scholar] [CrossRef] [PubMed]
- Ibba, G.; Piu, C.; Uleri, E.; Serra, C.; Dolei, A. Disruption by SaCas9 Endonuclease of HERV-Kenv, a Retroviral Gene with Oncogenic and Neuropathogenic Potential, Inhibits Molecules Involved in Cancer and Amyotrophic Lateral Sclerosis. Viruses 2018, 10, 412. [Google Scholar] [CrossRef]
- Magin-Lachmann, C.; Hahn, S.; Strobel, H.; Held, U.; Löwer, J.; Löwer, R. Rec (Formerly Corf) Function Requires Interaction with a Complex, Folded RNA Structure within Its Responsive Element rather than Binding to a Discrete Specific Binding Site. J. Virol. 2001, 75, 10359–10371. [Google Scholar] [CrossRef]
- Johnson, W.E. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019, 17, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Lower, R.; Boller, K.; Hasenmaier, B.; Korbmacher, C.; Muller-Lantzsch, N.; Lower, J.; Kurth, R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc. Natl. Acad. Sci. USA 1993, 90, 4480–4484. [Google Scholar] [CrossRef]
- Boese, A.; Sauter, M.; Galli, U.; Best, B.; Herbst, H.; Mayer, J.; Kremmer, E.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus protein cORF supports cell transformation and associates with the promyelocytic leukemia zinc finger protein. Oncogene 2000, 19, 4328–4336. [Google Scholar] [CrossRef]
- Galli, U.M.; Sauter, M.; Lecher, B.; Maurer, S.; Herbst, H.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus rec interferes with germ cell development in mice and may cause carcinoma in situ, the predecessor lesion of germ cell tumors. Oncogene 2005, 24, 3223–3228. [Google Scholar] [CrossRef]
- Denne, M.; Sauter, M.; Armbruester, V.; Licht, J.D.; Roemer, K.; Mueller-Lantzsch, N. Physical and Functional Interactions of Human Endogenous Retrovirus Proteins Np9 and Rec with the Promyelocytic Leukemia Zinc Finger Protein. J. Virol. 2007, 81, 5607–5616. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Chudak, C.; Kurth, R.; Bannert, N. The Rec protein of HERV-K(HML-2) upregulates androgen receptor activity by binding to the human small glutamine-rich tetratricopeptide repeat protein (hSGT). Int. J. Cancer 2013, 132, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Sauter, M.; Schmitt, M.; Baumert, B.; Best, B.; Boese, A.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus protein Rec interacts with the testicular zinc-finger protein and androgen receptor. J. Gen. Virol. 2010, 91, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Hohn, O.; Bannert, N. HERV-K(HML-2), a seemingly silent subtenant—But still waters run deep. APMIS 2016, 124, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Armbruester, V.; Sauter, M.; Krautkraemer, E.; Meese, E.; Kleiman, A.; Best, B.; Roemer, K.; Mueller-Lantzsch, N. A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin. Cancer Res. 2002, 8, 1800–1807. [Google Scholar]
- Armbruester, V.; Sauter, M.; Roemer, K.; Best, B.; Hahn, S.; Nty, A.; Schmid, A.; Philipp, S.; Mueller, A.; Mueller-Lantzsch, N. Np9 Protein of Human Endogenous Retrovirus K Interacts with Ligand of Numb Protein X. J. Virol. 2004, 78, 10310–10319. [Google Scholar] [CrossRef][Green Version]
- Flores, A.N.; McDermott, N.; Meunier, A.; Marignol, L. NUMB inhibition of NOTCH signalling as a therapeutic target in prostate cancer. Nat. Rev. Urol. 2014, 11, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Heyne, K.; Kölsch, K.; Bruand, M.; Kremmer, E.; Grässer, F.A.; Mayer, J.; Roemer, K. Np9, a cellular protein of retroviral ancestry restricted to human, chimpanzee and gorilla, binds and regulates ubiquitin ligase MDM2. Cell Cycle 2015, 14, 2619–2633. [Google Scholar] [CrossRef]
- Chen, T.; Meng, Z.; Gan, Y.; Wang, X.; Xu, F.; Gu, Y.; Xu, X.; Tang, J.; Zhou, H.; Zhang, X.; et al. The viral oncogene Np9 acts as a critical molecular switch for co-activating β-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia 2013, 27, 1469–1478. [Google Scholar] [CrossRef]
- Smith, C.C.; Selitsky, S.R.; Chai, S.; Armistead, P.M.; Vincent, B.G.; Serody, J.S. Alternative tumour-specific antigens. Nat. Rev. Cancer 2019, 19, 465–478. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Smith, C.C.; Beckermann, K.E.; Bortone, D.S.; De Cubas, A.A.; Bixby, L.M.; Lee, S.J.; Panda, A.; Ganesan, S.; Bhanot, G.; Wallen, E.M.; et al. Endogenous retroviral signatures predict immunotherapy response in clear cell renal cell carcinoma. J. Clin. Investig. 2018, 128, 4804–4820. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Harashima, N.; Kajigaya, S.; Yokoyama, H.; Cherkasova, E.; McCoy, J.P.; Hanada, K.; Mena, O.; Kurlander, R.; Abdul, T.; et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J. Clin. Investig. 2008. [Google Scholar] [CrossRef]
- Cherkasova, E.; Scrivani, C.; Doh, S.; Weisman, Q.; Takahashi, Y.; Harashima, N.; Yokoyama, H.; Srinivasan, R.; Linehan, W.M.; Lerman, M.I.; et al. Detection of an Immunogenic HERV-E Envelope with Selective Expression in Clear Cell Kidney Cancer. Cancer Res. 2016, 76, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Rycaj, K.; Plummer, J.B.; Li, M.; Yin, B.; Frerich, K.; Garza, J.G.; Shen, J.; Lin, K.; Yan, P.; et al. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J. Natl. Cancer Inst. 2012, 104, 189–210. [Google Scholar] [CrossRef]
- Kraus, B.; Fischer, K.; Büchner, S.M.; Wels, W.S.; Löwer, R.; Sliva, K.; Schnierle, B.S. Vaccination Directed against the Human Endogenous Retrovirus-K Envelope Protein Inhibits Tumor Growth in a Murine Model System. PLoS ONE 2013, 8, e72756. [Google Scholar] [CrossRef]
- Rycaj, K.; Plummer, J.B.; Yin, B.; Li, M.; Garza, J.; Radvanyi, L.; Ramondetta, L.M.; Lin, K.; Johanning, G.L.; Tang, D.G.; et al. Cytotoxicity of Human Endogenous Retrovirus K-Specific T Cells toward Autologous Ovarian Cancer Cells. Clin. Cancer Res. 2015, 21, 471–483. [Google Scholar] [CrossRef]
- Zhou, F.; Krishnamurthy, J.; Wei, Y.; Li, M.; Hunt, K.; Johanning, G.L.; Cooper, L.J.N.; Wang-Johanning, F. Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of Ras. Oncoimmunology 2015, 4, e1047582. [Google Scholar] [CrossRef]
- Kraus, B.; Fischer, K.; Sliva, K.; Schnierle, B.S. Vaccination directed against the human endogenous retrovirus-K (HERV-K) gag protein slows HERV-K gag expressing cell growth in a murine model system. Virol. J. 2014, 11, 58. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Krone, B.; Fadda, E.; Buja, A.; Grange, J.M.; Rausa, G.; de Vries, E.; Koelmel, K.F. Does yellow fever 17D vaccine protect against melanoma? Vaccine 2009, 27, 588–591. [Google Scholar] [CrossRef]
- Tran, T.; Burt, D.; Eapen, L.; Keller, O.R. Spontaneous regression of metastatic melanoma after inoculation with tetanus-diphtheria-pertussis vaccine. Curr. Oncol. 2013, 20, 270. [Google Scholar] [CrossRef]
- Schiavetti, F.; Thonnard, J.; Colau, D.; Boon, T.; Coulie, P.G. A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res. 2002, 62, 5510–5516. [Google Scholar]
- Büscher, K.; Hahn, S.; Hofmann, M.; Trefzer, U.; Özel, M.; Sterry, W.; Löwer, J.; Löwer, R.; Kurth, R.; Denner, J. Expression of the human endogenous retrovirus-K transmembrane envelope, Rec and Np9 proteins in melanomas and melanoma cell lines. Melanoma Res. 2006, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Humer, J.; Waltenberger, A.; Grassauer, A.; Kurz, M.; Valencak, J.; Rapberger, R.; Hahn, S.; Löwer, R.; Wolff, K.; Bergmann, M.; et al. Identification of a Melanoma Marker Derived from Melanoma-Associated Endogenous Retroviruses. Cancer Res. 2006, 66, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Ugurel, S.; Hanschmann, K.-M.; Strobel, H.; Tondera, C.; Schadendorf, D.; Löwer, J.; Löwer, R. Serological Response to Human Endogenous Retrovirus K in Melanoma Patients Correlates with Survival Probability. AIDS Res. Hum. Retrovir. 2008, 24, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Rabinovich, B.A.; Mi, T.; Switzer, K.C.; Olivares, S.; Maiti, S.N.; Plummer, J.B.; Singh, H.; Kumaresan, P.R.; Huls, H.M.; et al. Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin. Cancer Res. 2015, 21, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Jungbluth, A.A.; Frosina, D.; Holz, M.; Ritter, E.; Nakayama, E.; Ishida, T.; Obata, Y.; Carver, B.; Scher, H.; et al. Prostate Cancer Progression Correlates with Increased Humoral Immune Response to a Human Endogenous Retrovirus GAG Protein. Clin. Cancer Res. 2013, 19, 6112–6125. [Google Scholar] [CrossRef]
- Wallace, T.A.; Downey, R.F.; Seufert, C.J.; Schetter, A.; Dorsey, T.H.; Johnson, C.A.; Goldman, R.; Loffredo, C.A.; Yan, P.; Sullivan, F.J.; et al. Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis 2014, 35, 2074–2083. [Google Scholar] [CrossRef]
- Rastogi, A.; Ali, A.; Tan, S.-H.; Banerjee, S.; Chen, Y.; Cullen, J.; Xavier, C.P.; Mohamed, A.A.; Ravindranath, L.; Srivastav, J.; et al. Autoantibodies against oncogenic ERG protein in prostate cancer: Potential use in diagnosis and prognosis in a panel with C-MYC, AMACR and HERV-K Gag. Genes Cancer 2016, 7, 394–413. [Google Scholar] [CrossRef][Green Version]
- Kleiman, A.; Senyuta, N.; Tryakin, A.; Sauter, M.; Karseladze, A.; Tjulandin, S.; Gurtsevitch, V.; Mueller-Lantzsch, N. HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in patients with germ cell tumors. Int. J. Cancer 2004, 110, 459–461. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Galindo-Escobedo, L.V.; Rimoldi, D.; Geng, W.; Romero, P.; Koch, M.; Weitz, J.; Krempien, R.; Niethammer, A.G.; Beckhove, P.; et al. Potential target antigens for immunotherapy in human pancreatic cancer. Cancer Lett. 2007, 252, 290–298. [Google Scholar] [CrossRef]
- Ma, W.; Hong, Z.; Liu, H.; Chen, X.; Ding, L.; Liu, Z.; Zhou, F.; Yuan, Y. Human Endogenous Retroviruses-K (HML-2) Expression Is Correlated with Prognosis and Progress of Hepatocellular Carcinoma. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pérot, P.; Cheynet, V.; Decaussin-Petrucci, M.; Oriol, G.; Mugnier, N.; Rodriguez-Lafrasse, C.; Ruffion, A.; Mallet, F. Microarray-based Identification of Individual HERV Loci Expression: Application to Biomarker Discovery in Prostate Cancer. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.A. Does HERV-K represent a potential therapeutic target for prostate cancer? Expert Opin. Ther. Targets 2017, 21, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Obata, Y.; Ohara, N.; Matsushita, H.; Sato, S.; Uenaka, A.; Saika, T.; Miyamura, T.; Chayama, K.; Nakamura, Y.; et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008, 8, 15. [Google Scholar]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Azerou, R.; Lu, D.W.; Chen, D.-T.; Johanning, G.L. Detecting the expression of human endogenous retrovirus Eenvelope transcripts in human prostate adenocarcinoma. Cancer 2003, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.J.; Kuebler, P.J.; Heymann, J.E.; Sheehy, M.M.; Ortiz, G.S.; Ogg, G.; Barbour, J.D.; Lenz, J.; Steinfeld, A.D.; Nixon, D.F. Detection of T Lymphocytes Specific for Human Endogenous Retrovirus K (HERV-K) in Patients with Seminoma. AIDS Res. Hum. Retrovir. 2006, 22, 52–56. [Google Scholar] [CrossRef]
- Casau, A.E.; Vaughan, J.E.; Lozano, G.; Levine, A.J. Germ Cell Expression of an Isolated Human Endogenous Retroviral Long Terminal Repeat of the HERV-K/HTDV Family in Transgenic Mice. J. Virol. 1999, 73, 9976–9983. [Google Scholar] [CrossRef]
- Mueller, T.; Hantsch, C.; Volkmer, I.; Staege, M.S. Differentiation-Dependent Regulation of Human Endogenous Retrovirus K Sequences and Neighboring Genes in Germ Cell Tumor Cells. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Schommer, S.; Kremmer, E.; Remberger, K.; Dölken, G.; Lemm, I.; Buck, M.; Best, B.; Neumann-Haefelin, D.; Mueller-Lantzsch, N. Human endogenous retrovirus K10: Expression of Gag protein and detection of antibodies in patients with seminomas. J. Virol. 1995, 69, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Liu, J.; Rycaj, K.; Huang, M.; Tsai, K.; Rosen, D.G.; Chen, D.-T.; Lu, D.W.; Barnhart, K.F.; Johanning, G.L. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer 2007, 120, 81–90. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).