Avian Influenza Viruses Detected in Birds in Sub-Saharan Africa: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Collection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Assessment of Quality and Risk Bias of the Included Studies
2.6. Statistical Analysis
3. Results
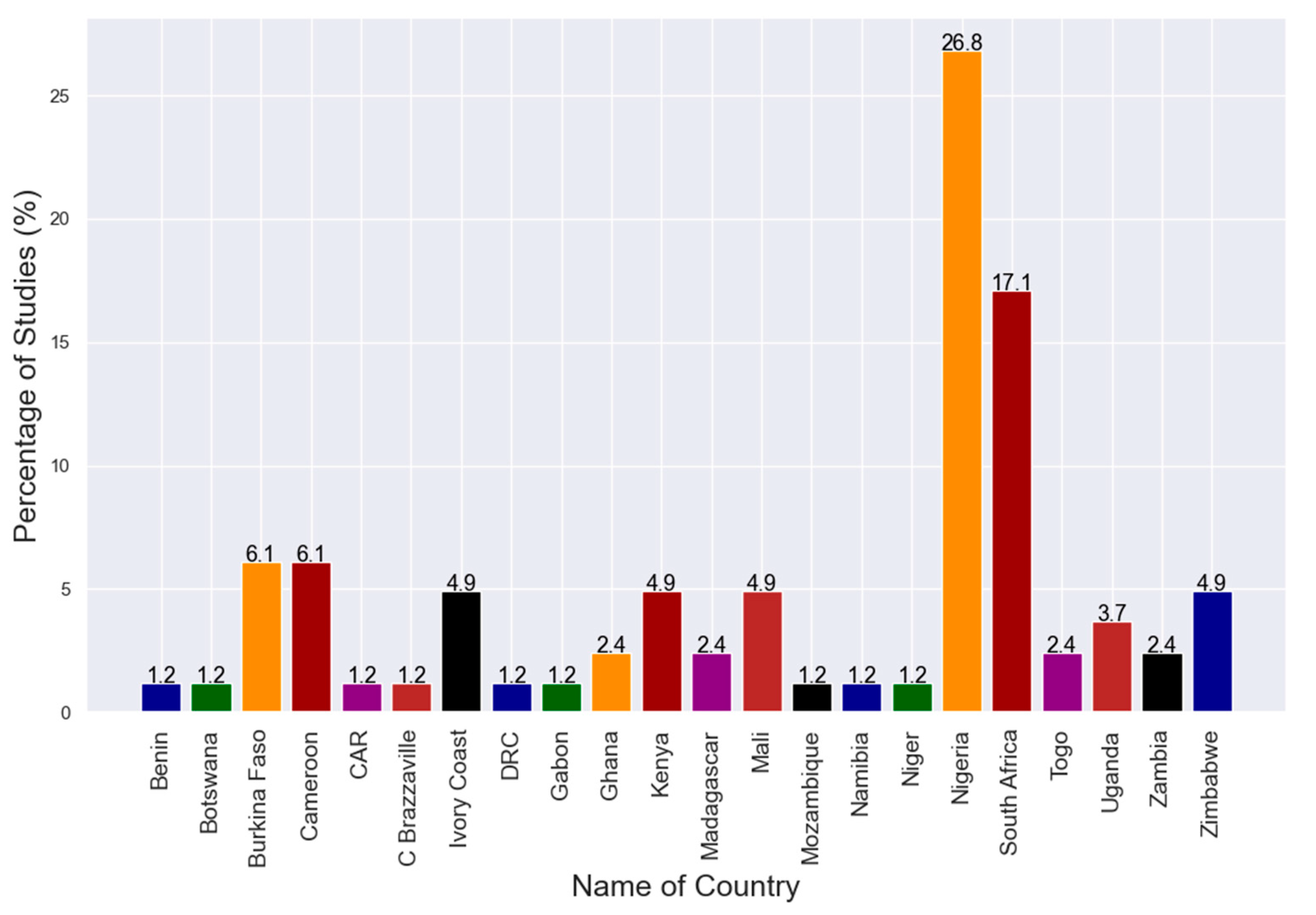
3.1. Search Results and Study Selection
3.2. Characteristics of the Included Studies
3.3. The Prevalence and Seasonality of AIV in Birds in Sub-Saharan Africa
3.4. Distribution of the AIV Subtypes and Avian Species
3.5. Distribution of AIVs among Different Avian Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peiris, J.S.; de Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelwhab, E.M.; Veits, J.; Mettenleiter, T.C. Prevalence and control of H7 avian influenza viruses in birds and humans. Epidemiol. Infect. 2014, 142, 896–920. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.R.; Yeddula, S.G.R.; Kim, S.H. Pathogenicity and Transmissibility of North American H7 Low Pathogenic Avian Influenza Viruses in Chickens and Turkeys. Viruses 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenstrom, J.; Osterhaus, A.D.; Fouchier, R.A. Global patterns of influenza a virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Landolt, G.A.; Olsen, C.W. Up to new tricks—A review of cross-species transmission of influenza A viruses. Anim. Health Res. Rev. 2007, 8, 1–21. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [Green Version]
- Inglis, S.C.; Carroll, A.R.; Lamb, R.A.; Mahy, B.W.J. Polypeptides specified by the influenza virus genome: I. Evidence for eight distinct gene products specified by fowl plague virus. Virology 1976, 74, 489–503. [Google Scholar] [CrossRef]
- Inglis, S.C.; McGeoch, D.J.; Mahy, B.W.J. Polypeptides specified by the influenza virus genome 2. Assignment of protein coding functions to individual genome segments by in vitro translation. Virology 1977, 78, 522–536. [Google Scholar] [CrossRef]
- Lamb, R.A.; Choppin, P.W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc. Natl. Acad. Sci. USA 1979, 76, 4908–4912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, R.A.; Choppin, P.W. Identification of a second protein (M2) encoded by RNA segment 7 of influenza virus. Virology 1981, 112, 729–737. [Google Scholar] [CrossRef]
- Lamb, R.A.; Choppin, P.W.; Chanock, R.M.; Lai, C.J. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc. Natl. Acad. Sci. USA 1980, 77, 1857–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, R.A.; Etkind, P.R.; Choppin, P.W. Evidence for a ninth influenza viral polypeptide. Virology 1978, 91, 60–78. [Google Scholar] [CrossRef]
- Shih, S.-R.; Suen, P.-C.; Chen, Y.-S.; Chang, S.-C. A Novel Spliced Transcript of Influenza A/WSN/33 Virus. Virus Genes 1998, 17, 179–183. [Google Scholar] [CrossRef]
- Chen, W.; Calvo, P.A.; Malide, D.; Gibbs, J.; Schubert, U.; Bacik, I.; Basta, S.; O’Neill, R.; Schickli, J.; Palese, P.; et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 2001, 7, 1306–1312. [Google Scholar] [CrossRef]
- Wise, H.M.; Barbezange, C.; Jagger, B.W.; Dalton, R.M.; Gog, J.R.; Curran, M.D.; Taubenberger, J.K.; Anderson, E.C.; Digard, P. Overlapping signals for translational regulation and packaging of influenza A virus segment 2. Nucleic Acids Res. 2011, 39, 7775–7790. [Google Scholar] [CrossRef] [Green Version]
- Wise, H.M.; Foeglein, A.; Sun, J.; Dalton, R.M.; Patel, S.; Howard, W.; Anderson, E.C.; Barclay, W.S.; Digard, P. A Complicated Message: Identification of a Novel PB1-Related Protein Translated from Influenza A Virus Segment 2 mRNA. J. Virol. 2009, 83, 8021–8031. [Google Scholar] [CrossRef] [Green Version]
- Wise, H.M.; Hutchinson, E.C.; Jagger, B.W.; Stuart, A.D.; Kang, Z.H.; Robb, N.; Schwartzman, L.M.; Kash, J.C.; Fodor, E.; Firth, A.E.; et al. Identification of a Novel Splice Variant Form of the Influenza A Virus M2 Ion Channel with an Antigenically Distinct Ectodomain. PLoS Pathog. 2012, 8, e1002998. [Google Scholar] [CrossRef] [Green Version]
- Selman, M.; Dankar, S.K.; Forbes, N.E.; Jia, J.-J.; Brown, E.G. Adaptive mutation in influenza A virus non-structural gene is linked to host switching and induces a novel protein by alternative splicing. Emerg. Microbes Infect. 2012, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, Y.; Noda, T.; Kawakami, E.; Akkina, R.; Kawaoka, Y. Identification of Novel Influenza A Virus Proteins Translated from PA mRNA. J. Virol. 2013, 87, 2455–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamayoshi, S.; Watanabe, M.; Goto, H.; Kawaoka, Y. Identification of a Novel Viral Protein Expressed from the PB2 Segment of Influenza A Virus. J. Virol. 2016, 90, 444–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagger, B.W.; Wise, H.M.; Kash, J.C.; Walters, K.-A.; Wills, N.M.; Xiao, Y.-L.; Dunfee, R.L.; Schwartzman, L.M.; Ozinsky, A.; Bell, G.L.; et al. An Overlapping Protein-Coding Region in Influenza A Virus Segment 3 Modulates the Host Response. Science 2012, 337, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, K.; Takada, A.; Ito, T.; Imai, M.; Takakuwa, H.; Hatta, M.; Ozaki, H.; Tanizaki, T.; Nagano, T.; Ninomiya, A.; et al. Precursor genes of future pandemic influenza viruses are perpetuated in ducks nesting in Siberia. Arch. Virol. 2000, 145, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ju, L.; Liu, P.; Zhou, J.; Lv, X.; Li, L.; Shen, H.; Su, H.; Jiang, L.; Jiang, Q. Serological and virological surveillance of avian influenza A virus H9N2 subtype in humans and poultry in Shanghai, China, between 2008 and 2010. Zoonoses Public Health 2015, 62, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Zaia, J. Why Glycosylation Matters in Building a Better Flu Vaccine. Mol. Cell. Proteom. 2019, 18, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Kuchipudi, S.V.; Nissly, R.H. Novel Flu Viruses in Bats and Cattle: “Pushing the Envelope” of Influenza Infection. Vet. Sci. 2018, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef]
- Nao, N.; Yamagishi, J.; Miyamoto, H.; Igarashi, M.; Manzoor, R.; Ohnuma, A.; Tsuda, Y.; Furuyama, W.; Shigeno, A.; Kajihara, M.; et al. Genetic Predisposition To Acquire a Polybasic Cleavage Site for Highly Pathogenic Avian Influenza Virus Hemagglutinin. MBio 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.M.; Arafa, A.; Hassan, M.K. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis. 2008, 52, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.G.; Amstislavski, P.; Greene, A.; Haseeb, M.A. The Landscape Epidemiology of Seasonal Clustering of Highly Pathogenic Avian Influenza (H5N1) in Domestic Poultry in Africa, Europe and Asia. Transbound. Emerg. Dis. 2017, 64, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Bahl, J.; Smith, G.J.; Wang, J.; Vijaykrishna, D.; Zhang, L.J.; Zhang, J.X.; Li, K.S.; Fan, X.H.; Cheung, C.L.; et al. The development and genetic diversity of H5N1 influenza virus in China, 1996–2006. Virology 2008, 380, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Fasanmi, O.G.; Odetokun, I.A.; Balogun, F.A.; Fasina, F.O. Public health concerns of highly pathogenic avian influenza H5N1 endemicity in Africa. Vet. World 2017, 10, 1194–1204. [Google Scholar] [CrossRef] [Green Version]
- Kayali, G.; Kandeil, A.; El-Shesheny, R.; Kayed, A.S.; Maatouq, A.M.; Cai, Z.; McKenzie, P.P.; Webby, R.J.; El-Refaey, S.; Kandeel, A.; et al. Avian Influenza A(H5N1) Virus in Egypt. Emerg. Infect. Dis. 2016, 22, 379–388. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, H.; Wu, P.; Uyeki, T.M.; Feng, L.; Lai, S.; Wang, L.; Huo, X.; Xu, K.; Chen, E.; et al. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–2017: An epidemiological study of laboratory-confirmed case series. Lancet Infect. Dis. 2017, 17, 822–832. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, J.; Deng, G.; Guo, J.; Zeng, X.; He, X.; Kong, H.; Gu, C.; Li, X.; Liu, J.; et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 2013, 341, 410–414. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. Pandemic influenza—Including a risk assessment of H5N1. Rev. Sci. Tech. 2009, 28, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef]
- Awuni, J.A.; Bianco, A.; Dogbey, O.J.; Fusaro, A.; Yingar, D.T.; Salviato, A.; Ababio, P.T.; Milani, A.; Bonfante, F.; Monne, I. Avian influenza H9N2 subtype in Ghana: Virus characterization and evidence of co-infection. Avian Pathol. 2019, 48, 470–476. [Google Scholar] [CrossRef]
- El-Houadfi, M.; Fellahi, S.; Nassik, S.; Guerin, J.L.; Ducatez, M.F. First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. Virol. J. 2016, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- You, L.; Diao, X. Assessing the Potential Impact of Avian Influenza on Poultry in West Africa: A Spatial Equilibrium Analysis. J. Agric. Econ. 2007, 58, 348–367. [Google Scholar] [CrossRef]
- Sonaiya, E.B. Family poultry, food security and the impact of HPAI. Worlds Poult. Sci. J. 2019, 63, 132–138. [Google Scholar] [CrossRef]
- Ofula, V.O.; Franklin, A.B.; Root, J.J.; Sullivan, H.J.; Gichuki, P.; Makio, A.; Bulimo, W.; Abong’o, B.O.; Muchai, M.; Schnabel, D. Detection of avian influenza viruses in wild waterbirds in the Rift Valley of Kenya using fecal sampling. Vector Borne Zoonotic Dis. 2013, 13, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Offeddu, V.; Cowling, B.J.; Peiris, J.S.M. Interventions in live poultry markets for the control of avian influenza: A systematic review. One Health 2016, 2, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodman, T. Waterbird Family Estimates in African Waterbird Population Estimates; Wetlands International: Wageningen, The Netherlands, 2006. [Google Scholar]
- Gaidet, N.; Dodman, T.; Caron, A.; Balanca, G.; Desvaux, S.; Goutard, F.; Cattoli, G.; Lamarque, F.; Hagemeijer, W.; Monicat, F. Avian influenza viruses in water birds, Africa. Emerg. Infect. Dis. 2007, 13, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Simulundu, E.; Mweene, A.S.; Tomabechi, D.; Hang’ombe, B.M.; Ishii, A.; Suzuki, Y.; Nakamura, I.; Sawa, H.; Sugimoto, C.; Ito, K.; et al. Characterization of H3N6 avian influenza virus isolated from a wild white pelican in Zambia. Arch. Virol. 2009, 154, 1517–1522. [Google Scholar] [CrossRef] [Green Version]
- Abolnik, C. Avian Influenza in South Africa: A Review. In Proceedings of the 9th Annual Congress of the Southern African Society For Veterinary Epidemiology and Preventive Medicine, Pretoria, South Africa, 18–20 August 2010; pp. 36–43. [Google Scholar]
- Simulundu, E.; Ishii, A.; Igarashi, M.; Mweene, A.S.; Suzuki, Y.; Hang’ombe, B.M.; Namangala, B.; Moonga, L.; Manzoor, R.; Ito, K.; et al. Characterization of influenza A viruses isolated from wild waterfowl in Zambia. J. Gen. Virol. 2011, 92, 1416–1427. [Google Scholar] [CrossRef]
- Khomenko, S.; Abolnik, C.; Roberts, L.; Waller, L.; Shaw, K.; Monne, I.; Taylor, J.; Dhingra, M.; Claudia, P.; Mugyeom, M.; et al. 2016–2018 Spread of H5N8 highly pathogenic avian influenza (HPAI) in sub-Saharan Africa: Epidemiological and ecological observations. Focus 2018, 12, 1–20. [Google Scholar]
- Twabela, A.T.; Tshilenge, G.M.; Sakoda, Y.; Okamatsu, M.; Bushu, E.; Kone, P.; Wiersma, L.; Zamperin, G.; Drago, A.; Zecchin, B.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus, Democratic Republic of the Congo, 2017. Emerg. Infect. Dis. 2018, 24, 1371–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouam, M.K.; Tchouankui, H.N.; Ngapagna, A.N. Epidemiological Features of Highly Pathogenic Avian Influenza in Cameroon. Vet. Med. Int. 2019, 2019, 3796369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letts, L.; Wilkins, S.; Law, M.; Stewart, D.; Bosch, J.; Westmorland, M. Critical Review form—Qualitative Studies (Version 2.0). 2007. Available online: https://www.unisa.edu.au/siteassets/episerver-6-files/global/health/sansom/documents/icahe/cats/mcmasters_qualreview_version2-0.pdf (accessed on 25 March 2020).
- Law, M.; Stewart, D.; Letts, L.; Pollock, N.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies. McMaster Univ. Occup. Ther. Evid. Based Pract. Res. Group. 1998. Available online: https://srs-mcmaster.ca/wp-content/uploads/2015/05/Guidelines-for-Critical-Review-Form-Quantitative-Studies.pdf (accessed on 25 March 2020).
- Njouom, R.; Aubin, J.T.; Bella, A.L.; Demsa, B.M.; Rouquet, P.; Gake, B.; Ngangnou, A.; Foupouapouognigni, Y.; Van Der Werf, S.; Rocourt, J.; et al. Highly pathogenic avian influenza virus subtype H5N1 in ducks in the Northern part of Cameroon. Vet. Microbiol. 2008, 130, 380–384. [Google Scholar] [CrossRef]
- Wade, A.; Jumbo, S.D.; Zecchin, B.; Fusaro, A.; Taiga, T.; Bianco, A.; Rodrigue, P.N.; Salomoni, A.; Kameni, J.M.F.; Zamperin, G.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus, Cameroon, 2017. Emerg. Infect. Dis. 2018, 24, 1367–1370. [Google Scholar] [CrossRef]
- Wade, A.; Taiga, T.; Fouda, M.A.; MaiMoussa, A.; Marc, F.K.J.; Njouom, R.; Vernet, M.A.; Djonwe, G.; Mballa, E.; Kazi, J.P.; et al. Highly pathogenic avian influenza A/H5N1 Clade 2.3.2.1c virus in poultry in Cameroon, 2016–2017. Avian Pathol. 2018, 47, 559–575. [Google Scholar] [CrossRef]
- Kirunda, H.; Erima, B.; Tumushabe, A.; Kiconco, J.; Tugume, T.; Mulei, S.; Mimbe, D.; Mworozi, E.; Bwogi, J.; Luswa, L.; et al. Prevalence of influenza A viruses in livestock and free-living waterfowl in Uganda. BMC Vet. Res. 2014, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Munyua, P.M.; Githinji, J.W.; Waiboci, L.W.; Njagi, L.M.; Arunga, G.; Mwasi, L.; Mbabu, R.M.; Macharia, J.M.; Breiman, R.F.; Njenga, M.K.; et al. Detection of influenza A virus in live bird markets in Kenya, 2009–2011. Influenza Other Respir. Viruses 2013, 7, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Ndumu, D.; Zecchin, B.; Fusaro, A.; Arinaitwe, E.; Erechu, R.; Kidega, E.; Kayiwa, J.; Muwanga, E.; Kirumira, M.; Kirembe, G.; et al. Highly pathogenic avian influenza H5N8 Clade 2.3.4.4B virus in Uganda, 2017. Infect. Genet. Evol. 2018, 66, 269–271. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Welch, C.N.; Ferreira, H.L.; Pusch, E.A.; Ateya, L.O.; Binepal, Y.S.; Apopo, A.A.; Dulu, T.D.; Afonso, C.L.; Suarez, D.L. Genetic characterization and pathogenesis of the first H9N2 low pathogenic avian influenza viruses isolated from chickens in Kenyan live bird markets. Infect. Genet. Evol. 2019. [Google Scholar] [CrossRef]
- Adamu, H.; Balami, A.; Abdu, P. Avian influenza, Gumboro and Newcastle disease antibodies and antigens in apparently healthy wild birds in Kano Metropolis, Nigeria. Niger. Vet. J. 2017, 38, 69–77. [Google Scholar]
- Cappelle, J.; de Almeida, R.S.; Fofana, B.; Dakouo, M.; Balanca, G.; Gil, P.; Albina, E.; Gaidet, N. Circulation of avian influenza viruses in wild birds in Inner Niger Delta, Mali. Influenza Other Respir. Viruses 2012, 6, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coker, T.; Meseko, C.; Odaibo, G.; Olaleye, D. Circulation of the low pathogenic avian influenza subtype H5N2 virus in ducks at a live bird market in Ibadan, Nigeria. Infect. Dis. Poverty 2014, 3, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couacy-Hymann, E.; Danho, T.; Keita, D.; Bodjo, S.C.; Kouakou, C.; Koffi, Y.M.; Beudje, F.; Tripodi, A.; de Benedictis, P.; Cattoli, G. The first specific detection of a highly pathogenic avian influenza virus (H5N1) in Ivory Coast. Zoonoses Public Health 2009, 56, 10–15. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Tarnagda, Z.; Tahita, M.C.; Sow, A.; de Landtsheer, S.; Londt, B.Z.; Brown, I.H.; Osterhaus, D.M.; Fouchier, R.A.; Ouedraogo, J.B.; et al. Genetic characterization of HPAI (H5N1) viruses from poultry and wild vultures, Burkina Faso. Emerg. Infect. Dis. 2007, 13, 611–613. [Google Scholar] [CrossRef]
- Durosinlorun, A.; Umoh, J.U.; Abdu, P.A.; Ajogi, I. Serologic evidence of infection with H5 subtype influenza virus in apparently healthy local chickens in Kaduna State, Nigeria. Avian Dis. 2010, 54, 365–368. [Google Scholar] [CrossRef]
- Fusade-Boyer, M.; Pato, P.S.; Komlan, M.; Dogno, K.; Jeevan, T.; Rubrum, A.; Kouakou, C.K.; Couacy-Hymann, E.; Batawui, D.; Go-Maro, E.; et al. Evolution of Highly Pathogenic Avian Influenza A(H5N1) Virus in Poultry, Togo, 2018. Emerg. Infect. Dis. 2019, 25, 2287–2289. [Google Scholar] [CrossRef]
- Gaidet, N.; Cattoli, G.; Hammoumi, S.; Newman, S.H.; Hagemeijer, W.; Takekawa, J.Y.; Cappelle, J.; Dodman, T.; Joannis, T.; Gil, P.; et al. Evidence of infection by H5N2 highly pathogenic avian influenza viruses in healthy wild waterfowl. PLoS Pathog. 2008, 4, e1000127. [Google Scholar] [CrossRef] [Green Version]
- Miko, R.; Abdu, P.; Assam, A.; Sai’du, L. Avian Influenza H5-Subtype Antibodies in Apparently Healthy Local Poultry in Live Bird Markets in Jigawa State, Nigeria. Bull. Anim. Health Prod. Afr. 2013, 61, 121–126. [Google Scholar]
- Molia, S.; Samake, K.; Diarra, A.; Sidibe, M.S.; Doumbia, L.; Camara, S.; Kante, S.; Kamissoko, B.; Diakite, A.; Gil, P.; et al. Avian influenza and Newcastle disease in three risk areas for H5N1 highly pathogenic avian influenza in Mali, 2007–2008. Avian Dis. 2011, 55, 650–658. [Google Scholar] [CrossRef]
- Molia, S.; Traore, A.; Gil, P.; Hammoumi, S.; Lesceu, S.; de Almeida, R.S.; Albina, E.; Chevalier, V. Avian influenza in backyard poultry of the Mopti region, Mali. Trop. Anim. Health Prod. 2010, 42, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Monne, I.; Joannis, T.M.; Fusaro, A.; De Benedictis, P.; Lombin, L.H.; Ularamu, H.; Egbuji, A.; Solomon, P.; Obi, T.U.; Cattoli, G.; et al. Reassortant avian influenza virus (H5N1) in poultry, Nigeria, 2007. Emerg. Infect. Dis. 2008, 14, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, I.O.; Faleke, O.O.; Garba, J. Avian influenza virus infection in apparently healthy domestic birds in Sokoto, Nigeria. Vet. Ital. 2012, 48, 309–312. [Google Scholar]
- Oluwayelu, D.O.; Omolanwa, A.; Adebiyi, A.I.; Aiki-Raji, O.C. Flock-Based Surveillance for Low Pathogenic Avian Influenza Virus in Commercial Breeders and Layers, Southwest Nigeria. Afr. J. Infect. Dis. 2017, 11, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owoade, A.A.; Gerloff, N.A.; Ducatez, M.F.; Taiwo, J.O.; Kremer, J.R.; Muller, C.P. Replacement of sublineages of avian influenza (H5N1) by reassortments, sub-Saharan Africa. Emerg. Infect. Dis. 2008, 14, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, C.J.; Adeyanju, A.T.; De Landtsheer, S.; Ottosson, U.; Manu, S.; Hagemeijer, W.; Mundkur, T.; Muller, C.P. Reassortant low-pathogenic avian influenza H5N2 viruses in African wild birds. J. Gen. Virol. 2011, 92, 1172–1183. [Google Scholar] [CrossRef]
- Tassoni, L.; Fusaro, A.; Milani, A.; Lemey, P.; Awuni, J.A.; Sedor, V.B.; Dogbey, O.; Commey, A.N.; Meseko, C.; Joannis, T.; et al. Genetically Different Highly Pathogenic Avian Influenza A(H5N1) Viruses in West Africa, 2015. Emerg. Infect. Dis. 2016, 22, 2132–2136. [Google Scholar] [CrossRef] [Green Version]
- Abolnik, C.; Cornelius, E.; Bisschop, S.P.; Romito, M.; Verwoerd, D. Phylogenetic analyses of genes from South African LPAI viruses isolated in 2004 from wild aquatic birds suggests introduction by Eurasian migrants. Dev. Biol. 2006, 124, 189–199. [Google Scholar]
- Abolnik, C.; Londt, B.Z.; Manvell, R.J.; Shell, W.; Banks, J.; Gerdes, G.H.; Akol, G.; Brown, I.H. Characterisation of a highly pathogenic influenza A virus of subtype H5N2 isolated from ostriches in South Africa in 2004. Influenza Other Respir. Viruses 2009, 3, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Caron, A.; Chiweshe, N.; Mundava, J.; Abolnik, C.; Dondona, A.C.; Scacchia, M.; Gaidet, N. Avian Viral Pathogens in Swallows, Zimbabwe: Infectious Diseases in Hirundinidae: A Risk to Swallow? Ecohealth 2017, 14, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Caron, A.; Grosbois, V.; Etter, E.; Gaidet, N.; de Garine-Wichatitsky, M. Bridge hosts for avian influenza viruses at the wildlife/domestic interface: An eco-epidemiological framework implemented in southern Africa. Prev. Vet. Med. 2014, 117, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Howerth, E.W.; Olivier, A.; Franca, M.; Stallknecht, D.E.; Gers, S. Pathobiology of highly pathogenic avian influenza virus H5N2 infection in juvenile ostriches from South Africa. Avian Dis. 2012, 56, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Pfitzer, S.; Verwoerd, D.J.; Gerdes, G.H.; Labuschagne, A.E.; Erasmus, A.; Manvell, R.J.; Grund, C. Newcastle disease and avian influenza A virus in wild waterfowl in South Africa. Avian Dis. 2000, 44, 655–660. [Google Scholar] [CrossRef]
- Poen, M.J.; Fouchier, R.A.M.; Webby, R.J.; Webster, R.G.; El-Zowalaty, M.E. Evidence of the Presence of Low Pathogenic Avian Influenza A Viruses in Wild Waterfowl in 2018 in South Africa. Pathogens 2019, 8, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molini, U.; Aikukutu, G.; Roux, J.P.; Kemper, J.; Ntahonshikira, C.; Marruchella, G.; Khaiseb, S.; Cattoli, G.; Dundon, W.G. Avian Influenza H5N8 Outbreak in African Penguins (Spheniscus demersus), Namibia, 2019. J. Wildl. Dis. 2020, 56, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Andriamanivo, H.R.; Lancelot, R.; Maminiaina, O.F.; Rakotondrafara, T.F.; Jourdan, M.; Renard, J.F.; Gil, P.; de Almeida, R.S.; Albina, E.; Martinez, D.; et al. Risk factors for avian influenza and Newcastle disease in smallholder farming systems, Madagascar highlands. Prev. Vet. Med. 2012, 104, 114–124. [Google Scholar] [CrossRef]
- Zecchin, B.; Minoungou, G.; Fusaro, A.; Moctar, S.; Ouedraogo-Kabore, A.; Schivo, A.; Salviato, A.; Marciano, S.; Monne, I. Influenza A (H9N2) Virus, Burkina Faso. Emerg. Infect. Dis. 2017, 23, 2118–2119. [Google Scholar] [CrossRef] [Green Version]
- Abolnik, C.; Bisschop, S.P.; Gerdes, G.H.; Olivier, A.J.; Horner, R.F. Phylogenetic analysis of low-pathogenicity avian influenza H6N2 viruses from chicken outbreaks (2001–2005) suggest that they are reassortants of historic ostrich low-pathogenicity avian influenza H9N2 and H6N8 viruses. Avian Dis. 2007, 51, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Abolnik, C.; Strydom, C.; Rauff, D.L.; Wandrag, D.B.R.; Petty, D. Continuing evolution of H6N2 influenza a virus in South African chickens and the implications for diagnosis and control. BMC Vet. Res. 2019, 15, 455. [Google Scholar] [CrossRef] [Green Version]
- Oluwayelu, D.O.; Aiki-Raji, C.O.; Adigun, O.T.; Olofintuyi, O.K.; Adebiyi, A.I. Serological Survey for Avian Influenza in Turkeys in Three States of Southwest Nigeria. Influenza Res. Treat. 2015, 2015, 787890. [Google Scholar] [CrossRef]
- Abolnik, C. Evolution of H5 highly pathogenic avian influenza: Sequence data indicate stepwise changes in the cleavage site. Arch. Virol. 2017, 162, 2219–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abolnik, C.; Gerdes, G.H.; Sinclair, M.; Ganzevoort, B.W.; Kitching, J.P.; Burger, C.E.; Romito, M.; Dreyer, M.; Swanepoel, S.; Cumming, G.S.; et al. Phylogenetic analysis of influenza A viruses (H6N8, H1N8, H4N2, H9N2, H10N7) isolated from wild birds, ducks, and ostriches in South Africa from 2007 to 2009. Avian Dis. 2010, 54, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Abolnik, C.; Olivier, A.; Reynolds, C.; Henry, D.; Cumming, G.; Rauff, D.; Romito, M.; Petty, D.; Falch, C. Susceptibility and Status of Avian Influenza in Ostriches. Avian Dis. 2016, 60, 286–295. [Google Scholar] [CrossRef]
- Abolnik, C.; Olivier, A.J.; Grewar, J.; Gers, S.; Romito, M. Molecular analysis of the 2011 HPAI H5N2 outbreak in ostriches, South Africa. Avian Dis. 2012, 56, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Bruckner, G.K.; Kotze, J.J. Avian influenza in ostriches: Epidemiological investigation in the Western Cape Province of South Africa. Vet. Ital. 2006, 42, 69–76. [Google Scholar] [PubMed]
- Fasina, F.O.; Bisschop, S.P.; Joannis, T.M.; Lombin, L.H.; Abolnik, C. Molecular characterization and epidemiology of the highly pathogenic avian influenza H5N1 in Nigeria. Epidemiol. Infect. 2009, 137, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Couacy-Hymann, E.; Kouakou, A.V.; Kouame, C.K.; Kouassi, A.L.; Koffi, Y.M.; Godji, P.; Nana, P.; Tarnagda, Z.; Akoua-Koffi, C. Surveillance for avian influenza and Newcastle disease in backyard poultry flocks in Cote d’Ivoire, 2007–2009. Rev. Sci. Tech. 2012, 31, 821–828. [Google Scholar] [CrossRef]
- Cumming, G.S.; Caron, A.; Abolnik, C.; Cattoli, G.; Bruinzeel, L.W.; Burger, C.E.; Cecchettin, K.; Chiweshe, N.; Mochotlhoane, B.; Mutumi, G.L.; et al. The ecology of influenza A viruses in wild birds in southern Africa. Ecohealth 2011, 8, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Fuller, T.L.; Ducatez, M.F.; Njabo, K.Y.; Couacy-Hymann, E.; Chasar, A.; Aplogan, G.L.; Lao, S.; Awoume, F.; Tehou, A.; Langeois, Q.; et al. Avian influenza surveillance in Central and West Africa, 2010–2014. Epidemiol. Infect. 2015, 143, 2205–2212. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, D.G.; Yoon, S.W. Recent outbreaks of highly pathogenic avian influenza viruses in South Korea. Clin. Exp. Vaccine Res. 2017, 6, 95–103. [Google Scholar] [CrossRef]
- Gaidet, N.; Caron, A.; Cappelle, J.; Cumming, G.S.; Balanca, G.; Hammoumi, S.; Cattoli, G.; Abolnik, C.; de Almeida, R.S.; Gil, P.; et al. Understanding the ecological drivers of avian influenza virus infection in wildfowl: A continental-scale study across Africa. Proc. Biol. Sci. 2012, 279, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Verhagen, J.H.; Samy, A.; Eriksson, P.; Fife, M.; Lundkvist, A.; Ellstrom, P.; Jarhult, J.D. Avian influenza viruses at the wild-domestic bird interface in Egypt. Infect. Ecol. Epidemiol. 2019, 9, 1575687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, C.; Sun, H.T.; Ma, J.; Qi, Y.; Qin, S.Y. Prevalence of avian influenza viruses and their associated antibodies in wild birds in China: A systematic review and meta-analysis. Microb. Pathog. 2019, 135, 103613. [Google Scholar] [CrossRef] [PubMed]
- Bergervoet, S.A.; Pritz-Verschuren, S.B.E.; Gonzales, J.L.; Bossers, A.; Poen, M.J.; Dutta, J.; Khan, Z.; Kriti, D.; van Bakel, H.; Bouwstra, R.; et al. Circulation of low pathogenic avian influenza (LPAI) viruses in wild birds and poultry in the Netherlands, 2006–2016. Sci. Rep. 2019, 9, 13681. [Google Scholar] [CrossRef] [PubMed]
- Diskin, E.R.; Friedman, K.; Krauss, S.; Nolting, J.M.; Poulson, R.L.; Slemons, R.D.; Stallknecht, D.E.; Webster, R.G.; Bowman, A.S. Subtype Diversity of Influenza A Virus in North American Waterfowl: A Multidecade Study. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Walker, D.; Pryor, S.P.; Niles, L.; Chenghong, L.; Hinshaw, V.S.; Webster, R.G. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 2004, 4, 177–189. [Google Scholar] [CrossRef]
- Suss, J.; Schafer, J.; Sinnecker, H.; Webster, R.G. Influenza virus subtypes in aquatic birds of eastern Germany. Arch. Virol. 1994, 135, 101–114. [Google Scholar] [CrossRef]
- Wallensten, A.; Munster, V.J.; Latorre-Margalef, N.; Brytting, M.; Elmberg, J.; Fouchier, R.A.; Fransson, T.; Haemig, P.D.; Karlsson, M.; Lundkvist, A.; et al. Surveillance of influenza A virus in migratory waterfowl in northern Europe. Emerg. Infect. Dis. 2007, 13, 404–411. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Olinger, C.M.; Owoade, A.A.; Tarnagda, Z.; Tahita, M.C.; Sow, A.; De Landtsheer, S.; Ammerlaan, W.; Ouedraogo, J.B.; Osterhaus, A.D.; et al. Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. J. Gen. Virol. 2007, 88, 2297–2306. [Google Scholar] [CrossRef]
- Joannis, T.; Lombin, L.H.; De Benedictis, P.; Cattoli, G.; Capua, I. Confirmation of H5N1 avian influenza in Africa. Vet. Rec. 2006, 158, 309–310. [Google Scholar] [CrossRef]
- Fasanmi, O.G.; Ahmed, S.S.U.; Oladele-Bukola, M.O.; El-Tahawy, A.S.; Elbestawy, A.R.; Fasina, F.O. An evaluation of biosecurity compliance levels and assessment of associated risk factors for highly pathogenic avian influenza H5N1 infection of live-bird-markets, Nigeria and Egypt. Acta Trop. 2016, 164, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abolnik, C.; Pieterse, R.; Peyrot, B.M.; Choma, P.; Phiri, T.P.; Ebersohn, K.; Heerden, C.J.V.; Vorster, A.A.; Zel, G.V.; Geertsma, P.J.; et al. The Incursion and Spread of Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4 Within South Africa. Avian Dis. 2019, 63, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Twabela, A.T.; Okamatsu, M.; Tshilenge, G.M.; Mpiana, S.; Masumu, J.; Nguyen, L.T.; Matsuno, K.; Monne, I.; Zecchin, B.; Sakoda, Y. Molecular, antigenic, and pathogenic characterization of H5N8 highly pathogenic avian influenza viruses isolated in the Democratic Republic of Congo in 2017. Arch. Virol. 2020, 165, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Abolnik, C.; Fehrsen, J.; Olivier, A.; van Wyngaardt, W.; Fosgate, G.; Ellis, C. Serological investigation of highly pathogenic avian influenza H5N2 in ostriches (Struthio camelus). Avian Pathol. 2013, 42, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Khamas, E.J. Avian influenza (H9N2) outbreak in Iraq. Iraqi J. Vet. Med. 2008, 32, 223–230. [Google Scholar]
- Lindh, E.; Ek-Kommonen, C.; Vaananen, V.M.; Vaheri, A.; Vapalahti, O.; Huovilainen, A. Molecular epidemiology of H9N2 influenza viruses in Northern Europe. Vet. Microbiol. 2014, 172, 548–554. [Google Scholar] [CrossRef]
- Monne, I.; Hussein, H.A.; Fusaro, A.; Valastro, V.; Hamoud, M.M.; Khalefa, R.A.; Dardir, S.N.; Radwan, M.I.; Capua, I.; Cattoli, G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir. Viruses 2013, 7, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Yaqub, T.; Mukhtar, N.; Imran, M.; Ghafoor, A.; Shahid, M.F.; Yaqub, S.; Smith, G.J.D.; Su, Y.C.F.; Naeem, M. Prevalence and Phylogenetics of H9n2 in Backyard and Commercial Poultry in Pakistan. Avian Dis. 2018, 62, 416–424. [Google Scholar] [CrossRef]
- Youk, S.S.; Lee, D.H.; Jeong, J.H.; Pantin-Jackwood, M.J.; Song, C.S.; Swayne, D.E. Live bird markets as evolutionary epicentres of H9N2 low pathogenicity avian influenza viruses in Korea. Emerg. Microbes Infect. 2020, 9, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Xu, D.; Yang, X.; Zhang, J.; Wang, S.; Shi, H.; Liu, X. Genetic and biological characterization of H9N2 avian influenza viruses isolated in China from 2011 to 2014. PLoS ONE 2018, 13, e0199260. [Google Scholar] [CrossRef] [Green Version]
- Peiris, M.; Yuen, K.Y.; Leung, C.W.; Chan, K.H.; Ip, P.L.; Lai, R.W.; Orr, W.K.; Shortridge, K.F. Human infection with influenza H9N2. Lancet 1999, 354, 916–917. [Google Scholar] [CrossRef]
- Caron, A.; Abolnik, C.; Mundava, J.; Gaidet, N.; Burger, C.E.; Mochotlhoane, B.; Bruinzeel, L.; Chiweshe, N.; de Garine-Wichatitsky, M.; Cumming, G.S. Persistence of low pathogenic avian influenza virus in waterfowl in a Southern African ecosystem. Ecohealth 2011, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]




| Study Region | No. of Studies | No. Samples | No. Positive | Prevalence (%) |
|---|---|---|---|---|
| Central Africa a | 4 | 4868 | 344 | 7.1 |
| East Africa b | 6 | 13,875 | 146 | 1.1 |
| West Africa c | 19 | 38,203 | 1269 | 3.3 |
| Southern Africa d | 12 | 12,518 | 349 | 2.8 |
| Total | 41 | 69,464 | 2108 | Overall prevalence: 3.0 |
| Study Regions | No. of Studies | No. of Samples | No. Samples | Seroprevalence (%) |
|---|---|---|---|---|
| Central Africa a | 0 | - | - | - |
| East Africa b | 2 | 3517 | 77 | 2.2 |
| West Africa c | 11 | 16,669 | 875 | 5.2 |
| Southern Africa d | 4 | 235,084 | 9605 | 4.1 |
| Total | 18 | 255,270 | 10,557 | Overall seroprevalence: 4.1 |
| Study Region | No. of Studies | No. of Samples | Dry Season (%) | Wet Season (%) | Reference |
|---|---|---|---|---|---|
| Central Africa | 4 | 4868 | 325 (6.7%) | 19 (0.4%) | [53,58,59,60] |
| East Africa | 5 | 9556 | 71 (0.7%) | 46 (0.5%) | [45,61,62,63,64] |
| West Africa | 18 | 11,680 | 319 (2.7%) | 158 (1.4%) | [41,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81] |
| Southern Africa | 11 | 6009 | 165 (2.7%) | 48 (0.7%) | [49,51,82,83,84,85,86,87,88,89,90] |
| Total (Overall Prevalence%) | 38 | 32,113 | 880 (2.7%) | 271 (0.8%) |
| Study Region | Central Africa | East Africa | Southern Africa | West Africa |
|---|---|---|---|---|
| HA Subtypes Detected | H5 | H4, H5, H9 | H1, H3, H4, H5, H6, H7, H9, H10, H11 | H3, H5, H7, H9 |
| Most Prevalent HA Subtypes | H5 | H5 | H5 | H5 |
| HA Subtypes not Detected | H1–H4, H6–H16 | H1–H3, H6–H8, H10–H16 | H2, H8, H12–H16 | H1–H2, H4, H6, H8, H10–H16 |
| Prevalent NA Subtypes | N1, N8 | N2, N6, N8 | N1, N2, N6, N7, N8, N9 | N1, N2, N7, N8 |
| NA Subtypes not Detected | N2, N3, N4, N5, N6, N7, N9 | N1, N3, N4, N5, N7, N9 | N3, N4, N5 | N3, N4, N5, N6, N9 |
| Prevalent Subtype Combinations | H5N1, H5N8, H5Nx | H4N6, H5N8, H9N2, H5Nx | H1N2, H1N8, H3N6, H3N8, H4N2, H4N6, H4N8, H5N1, H5N2, H5N8, H6N2, H6N8, H9N2, H10N7, H10N9, H11N9, H5Nx, H6Nx, H7Nx | H3N8, H5N1, H5N2, H7N7, H9N2, H5Nx, H7Nx |
| Most Prevalent Subtype Combinations | H5N1 | H5N8 | H5N2, H5N8, H6N2 | H5N1, H9N2 |
| Host | Number of Publications * | Number of Countries | Names of Countries |
|---|---|---|---|
| Domestic Birds | |||
| Chicken | 35 | 12 | Burkina Faso, Cameroon, Cote d’Ivoire, Ghana, Kenya, Madagascar, Mali, Nigeria, South Africa, Togo, Uganda |
| Domestic duck | 20 | 10 | Cameroon, Cote d’Ivoire, DRC, Ghana, Kenya, Madagascar, Mali, Nigeria, South Africa, Uganda |
| Ostrich | 8 | 1 | South Africa |
| Turkey | 7 | 7 | Cameroon, Cote d’Ivoire, Kenya, Mali, Nigeria, South Africa, Uganda |
| Domestic guinea fowl | 7 | 5 | Burkina Faso, Cameroon, Mali, Nigeria, South Africa |
| Pigeon | 5 | 3 | Cameroon, Nigeria, South Africa |
| Domestic geese | 4 | 4 | Cameroon, Kenya, Madagascar, South Africa |
| Poultry a | 3 | 3 | Nigeria |
| Indian peafowl | 2 | 1 | Cameroon |
| Wild Birds | |||
| Egyptian goose | 6 | 3 | Kenya, South Africa, Zambia |
| Wild species a | 4 | 3 | Nigeria, Africa, South Africa |
| White-faced whistling duck | 3 | 3 | Kenya, Nigeria, Mali |
| Yellow-billed duck | 3 | 2 | Kenya, South Africa |
| Hooded vulture | 3 | 1 | Burkina Faso |
| Red-billed quelea | 2 | 1 | Mali, Zimbabwe |
| Red-billed teal | 2 | 1 | South Africa |
| Great white pelican | 2 | 1 | Zambia |
| Spur-winged goose | 2 | 1 | Nigeria |
| Duck | 2 | 2 | Zambia, Zimbabwe |
| Cattle egret | 2 | 2 | Nigeria, Zimbabwe |
| Barn swallow | 2 | 1 | Zimbabwe |
| African sacred ibis | 2 | 1 | South Africa |
| Turtle dove | 2 | 1 | Cote d’Ivoire |
| Cape teal | 2 | 2 | Kenya, South Africa |
| African penguin b | 1 | 1 | Namibia |
| Sparrowhawk | 1 | 1 | Cote d’Ivoire |
| Dove | 1 | 1 | Cote d’Ivoire |
| Crow | 1 | 1 | Cote d’Ivoire |
| Weaver | 1 | 1 | Cote d’Ivoire |
| Hottentot teal | 1 | 1 | Kenya |
| Red-knobbed coot | 1 | 1 | Kenya |
| Garganey | 1 | 1 | Mali |
| Ruff | 1 | 1 | Mali |
| Northern pintail | 1 | 1 | Mali |
| Purple swamphen | 1 | 1 | Mali |
| Common moorhen | 1 | 1 | Mali |
| Comb duck | 1 | 1 | Mali |
| Gull-billed tern | 1 | 1 | Mali |
| Spotted redshank | 1 | 1 | Mali |
| Speckled pigeon | 1 | 1 | Nigeria |
| Canada goose | 1 | 1 | Nigeria |
| Gray crown crane | 1 | 1 | Nigeria |
| African gray parrot | 1 | 1 | Nigeria |
| Cape shoveler | 1 | 1 | South Africa |
| Swift tern | 1 | 1 | South Africa |
| White-winged black tern | 1 | 1 | South Africa |
| Hadada ibis | 1 | 1 | South Africa |
| Shelduck | 1 | 1 | South Africa |
| Brown-throated martin | 1 | 1 | Kenya |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalonda, A.; Saasa, N.; Nkhoma, P.; Kajihara, M.; Sawa, H.; Takada, A.; Simulundu, E. Avian Influenza Viruses Detected in Birds in Sub-Saharan Africa: A Systematic Review. Viruses 2020, 12, 993. https://doi.org/10.3390/v12090993
Kalonda A, Saasa N, Nkhoma P, Kajihara M, Sawa H, Takada A, Simulundu E. Avian Influenza Viruses Detected in Birds in Sub-Saharan Africa: A Systematic Review. Viruses. 2020; 12(9):993. https://doi.org/10.3390/v12090993
Chicago/Turabian StyleKalonda, Annie, Ngonda Saasa, Panji Nkhoma, Masahiro Kajihara, Hirofumi Sawa, Ayato Takada, and Edgar Simulundu. 2020. "Avian Influenza Viruses Detected in Birds in Sub-Saharan Africa: A Systematic Review" Viruses 12, no. 9: 993. https://doi.org/10.3390/v12090993
APA StyleKalonda, A., Saasa, N., Nkhoma, P., Kajihara, M., Sawa, H., Takada, A., & Simulundu, E. (2020). Avian Influenza Viruses Detected in Birds in Sub-Saharan Africa: A Systematic Review. Viruses, 12(9), 993. https://doi.org/10.3390/v12090993








