Chloroquine and Sulfadoxine Derivatives Inhibit ZIKV Replication in Cervical Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Virus
2.3. Compounds
2.4. Cytotoxicity Assay
2.5. Infection and Treatment
2.6. Antiviral Activity Assessments
2.6.1. Plaque-Forming Reduction Assay
2.6.2. Viral Genome Detection
2.6.3. Viral Protein Expression Detection
2.7. Statistical Analysis
3. Results
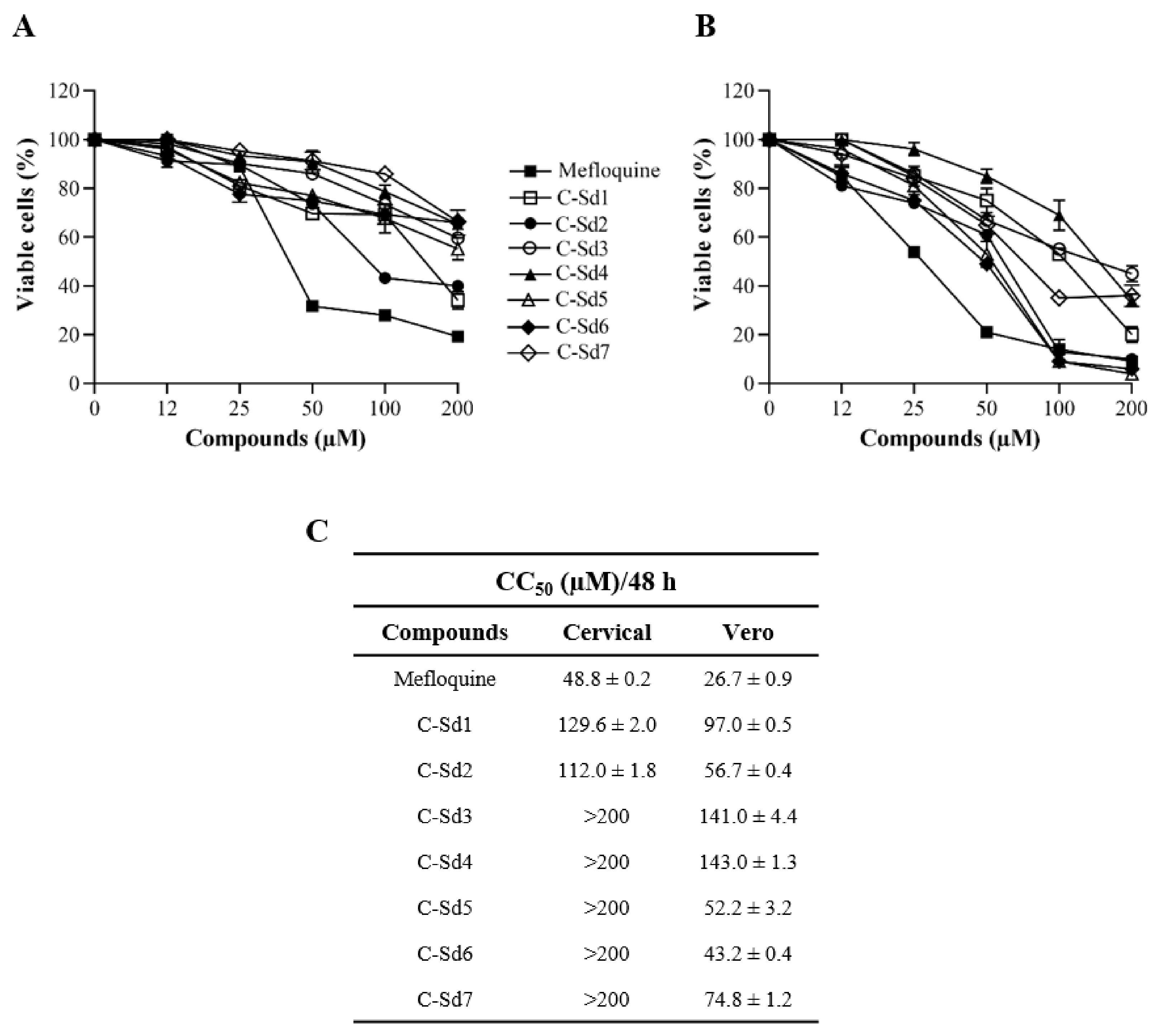
3.1. Cytotoxic Effect of C-Sds in Cervical and Vero Cell Lines
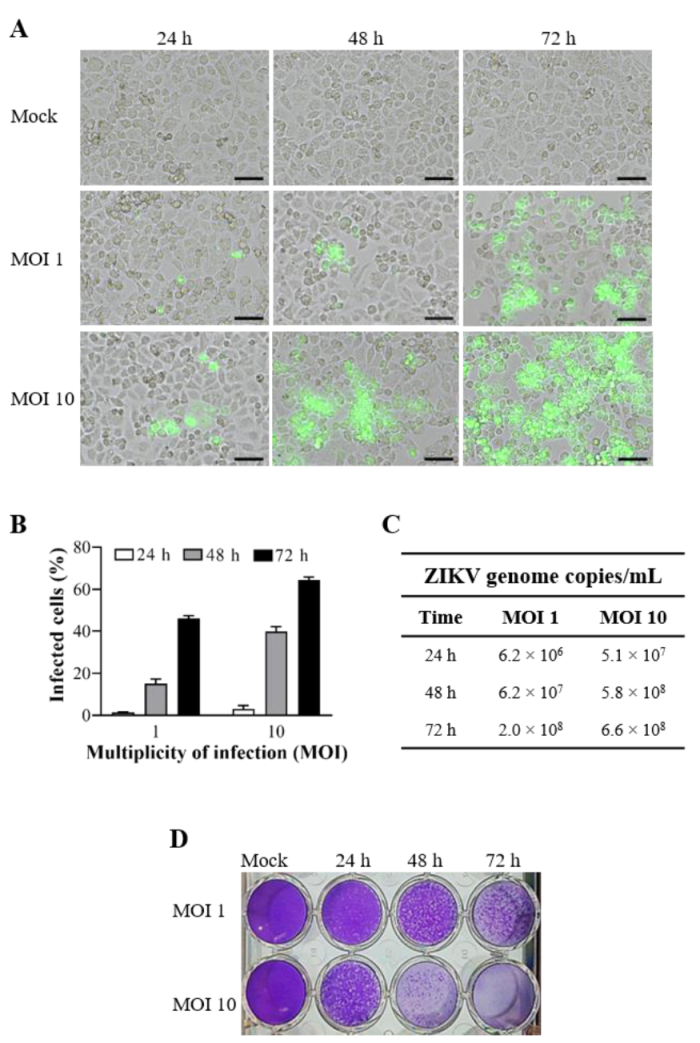
3.2. ZIKV Infection Kinetics in Cervical Cell Line
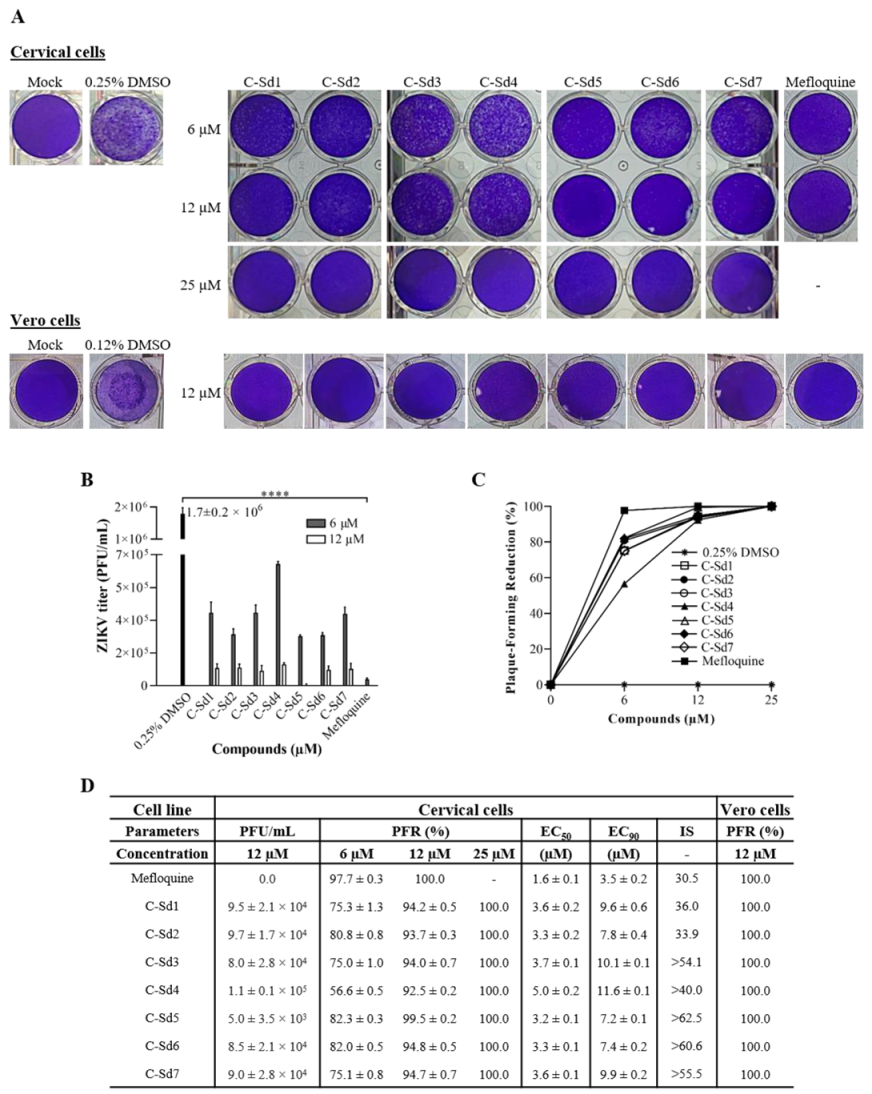
3.3. C-Sds Inhibit ZIKV Growth in Cervical and Vero Cell Lines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, T.; Li, J.; Carr, M.J.; Duchêne, S.; Shi, W. The Asian Lineage of Zika Virus: Transmission and Evolution in Asia and the Americas. Virol. Sin. 2019, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization/World Health Organization. Zika Cumulative Cases. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=12390:zika-cumulati-ve-cases&Itemid=42090&lang=en (accessed on 29 October 2020).
- Rozé, B.; Najioullah, F.; Fergé, J.-L.; Dorléans, F.; Apetse, K.; Barnay, J.-L.; Daudens-Vaysse, E.; Brouste, Y.; Césaire, R.; Fagour, L.; et al. Guillain-Barré Syndrome Associated With Zika Virus Infection in Martinique in 2016: A Prospective Study. Clin. Infect. Dis. 2017, 65, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Barbi, L.; Coelho, A.V.C.; De Alencar, L.C.A.; Crovella, S. Prevalence of Guillain-Barré syndrome among Zika virus infected cases: A systematic review and meta-analysis. Braz. J. Infect. Dis. 2018, 22, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Nogueira, R.M.R.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Polonio, C.M.; De Freitas, C.L.; Zanluqui, N.G.; Peron, J.P.S. Zika virus congenital syndrome: Experimental models and clinical aspects. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.A.; Dantas, D.N.A.; Carvalho, G.A.F.D.L.; Da Silva, A.N.; Lira, A.L.B.D.C.; Enders, B.C. Analysis of the concept of the Zika Virus congenital syndrome. Cien Saude Colet 2020, 25, 567–574. (In Portuguese) [Google Scholar] [CrossRef]
- Counotte, M.J.; Kim, C.R.; Wang, J.; Bernstein, K.; Deal, C.D.; Broutet, N.J.N.; Low, N. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med. 2018, 15, e1002611. [Google Scholar] [CrossRef]
- Tobar, P.; Vega, M.; Ordoñez, C.; Rivera, L.; Landivar, J.; Zambrano, H. Detection of Zika Virus and Human Papilloma Virus in Cervical Cytology Samples using Two Real Time PCR Based Techniques in Ecuadorian Women diagnosed with ASCUS. Puerto Rico Health Sci. J. 2018, 37, S96–S98. [Google Scholar]
- Peregrine, J.; Gurung, S.; Lindgren, M.C.; Husain, S.; Zavy, M.T.; Myers, D.A.; Papin, J.F. Zika Virus Infection, Reproductive Organ Targeting, and Semen Transmission in the Male Olive Baboon. J. Virol. 2019, 94, 01434-19. [Google Scholar] [CrossRef]
- Mead, P.; Duggal, N.K.; Hook, S.A.; DeLorey, M.; Fischer, M.; McGuire, D.O.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. N. Engl. J. Med. 2018, 378, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, T.E.; Souza, R.P.; Pelloso, S.M.; Morelli, F.; Suehiro, T.T.; Damke, E.; Bonfim-Mendonça, P.D.S.; Da Silva, V.R.S.; Consolaro, M.E.L. Case Reports: Prolonged Detection of Zika Virus RNA in Vaginal and Endocervical Samples from a Brazilian Woman, 2018. Am. J. Trop. Med. Hyg. 2019, 100, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Vojtech, L.; Wagoner, J.; Slivinski, N.S.J.; Jackson, K.J.; Wang, R.; Khadka, S.; Luthra, P.; Basler, C.F.; Polyak, S.J. The Antiviral Drug Arbidol Inhibits Zika Virus. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Robinson, C.L.; Chong, A.C.N.; Ashbrook, A.W.; Jeng, G.; Jin, J.; Chen, H.; Tang, E.I.; Martin, L.A.; Kim, R.S.; Kenyon, R.M.; et al. Male germ cells support long-term propagation of Zika virus. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Strange, D.P.; Jiyarom, B.; Zarandi, N.P.; Xie, X.; Baker, C.; Sadri-Ardekani, H.; Shi, P.-Y.; Verma, S. Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells. mBio 2019, 10, 01372-19. [Google Scholar] [CrossRef]
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Pol, A.V.D.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.e4. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.-J.J.; Bowen, R.A.; Brault, A.C. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef]
- Caine, E.A.; Scheaffer, S.M.; Arora, N.; Zaitsev, K.; Artyomov, M.N.; Coyne, C.B.; Moley, K.H.; Diamond, M.S. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Gurung, S.; Nadeau, H.; Maxted, M.; Peregrine, J.; Reuter, D.; Norris, A.; Edwards, R.; Hyatt, K.; Singleton, K.; Papin, J.F.; et al. Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons. J. Virol. 2020, 94, 00058-20. [Google Scholar] [CrossRef]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef]
- Almeida, R.D.N.; Braz-De-Melo, H.A.; Santos, I.D.O.; Corrêa, R.; Kobinger, G.P.; Magalhães, K.G. The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology. Cells 2020, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Teramoto, T.; Kulkarni, A.A.; Bhattacharjee, A.K.; Padmanabhan, R. Antiviral activities of selected antimalarials against dengue virus type 2 and Zika virus. Antivir. Res. 2017, 137, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, X.; Ji, X.; Quanquin, N.; Deng, Y.-Q.; Tian, M.; Aliyari, R.; Zuo, X.; Yuan, L.; Afridi, S.K.; et al. Chloroquine, a FDA-approved Drug, Prevents Zika Virus Infection and its Associated Congenital Microcephaly in Mice. EBioMedicine 2017, 24, 189–194. [Google Scholar] [CrossRef]
- Han, Y.; Pham, H.T.; Xu, H.; Quan, Y.; Mesplede, T. Antimalarial drugs and their metabolites are potent Zika virus inhibitors. J. Med. Virol. 2019, 91, 1182–1190. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, C.; Li, C.; Zhang, F.; Peng, J.; Wang, Q.; Liu, X.; Ye, X.; Li, P.; Wu, M.; et al. Chloroquine inhibits endosomal viral RNA release and autophagy-dependent viral replication and effectively prevents maternal to fetal transmission of Zika virus. Antivir. Res. 2019, 169, 104547. [Google Scholar] [CrossRef]
- Mishra, A.; Batchu, H.; Srivastava, K.; Singh, P.; Shukla, P.K.; Batra, S. Synthesis and evaluation of new diaryl ether and quinoline hybrids as potential antiplasmodial and antimicrobial agents. Bioorg. Med. Chem. Lett. 2014, 24, 1719–1723. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, R.; Ali, R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The Use of Antimalarial Drugs against Viral Infection. Microorganisms 2020, 8, 85. [Google Scholar] [CrossRef]
- DelVecchio, R.; Higa, L.M.; Pezzuto, P.; Valadão, A.L.; Garcez, P.P.; Monteiro, F.L.L.; Loiola, E.C.; Dias, A.A.; Silva, F.J.M.; Aliota, M.T.; et al. Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. Viruses 2016, 8, 322. [Google Scholar] [CrossRef]
- Barbosa-Lima, G.; Pinto, L.S.D.S.; Kaiser, C.R.; Wardell, J.L.; De Freitas, C.S.; Vieira, Y.R.; Marttorelli, A.; Neto, J.C.; Bozza, P.T.; Wardell, S.M.S.V.; et al. N-(2-(arylmethylimino)ethyl)-7-chloroquinolin-4-amine derivatives, synthesized by thermal and ultrasonic means, are endowed with anti-Zika virus activity. Eur. J. Med. Chem. 2017, 127, 434–441. [Google Scholar] [CrossRef]
- Shiryaev, S.A.; Mesci, P.; Pinto, A.; Fernandes, I.; Sheets, N.; Shresta, S.; Farhy, C.; Huang, C.-T.; Strongin, A.Y.; Muotri, A.R.; et al. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Mesplède, T.; Xu, H.; Quan, Y.; Wainberg, M.A. The antimalarial drug amodiaquine possesses anti-ZIKA virus activities. J. Med. Virol. 2018, 90, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Parnell, L.A.; Diamond, M.S.; Mysorekar, I.U. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med. 2017, 214, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Liang, B.; Aarthy, M.; Singh, S.K.; Garg, N.; Mysorekar, I.U.; Giri, R. Hydroxychloroquine Inhibits Zika Virus NS2B-NS3 Protease. ACS Omega 2018, 3, 18132–18141. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Powell, S.T.; Prasanth, K.R.; Schott-Lerner, G.; Soto-Acosta, R.; Galarza-Muñoz, G.; McGrath, E.L.; Urrabaz-Garza, R.; Gao, J.; et al. A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe 2016, 20, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bawa, S.; Gupta, H. Biological Activities of Quinoline Derivatives. Mini-Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef]
- Ramharack, P.; Soliman, M.E.S. ChemInform Abstract: Zika Virus Drug Targets: A Missing Link in Drug Design and Discovery—A Route Map to Fill the Gap. ChemInform 2016, 47, 68719–68731. [Google Scholar] [CrossRef]
- Barbosa-Lima, G.; Moraes, A.M.; Araújo, A.D.S.; Da Silva, E.T.; De Freitas, C.S.; Vieira, Y.R.; Marttorelli, A.; Neto, J.C.; Bozza, P.T.; De Souza, M.V.; et al. 2,8-bis(trifluoromethyl)quinoline analogs show improved anti-Zika virus activity, compared to mefloquine. Eur. J. Med. Chem. 2017, 127, 334–340. [Google Scholar] [CrossRef]
- Wang, L.; Liang, R.; Gao, Y.; Li, Y.; Deng, X.; Xiang, R.; Zhang, Y.; Ying, T.; Jiang, S.; Yu, F. Development of Small-Molecule Inhibitors Against Zika Virus Infection. Front. Microbiol. 2019, 10, 2725. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Boechat, N.; Ferreira, M.D.L.G.; Júnior, C.C.; Jesus, A.M.; Leite, M.M.; Souza, N.B.; Krettli, A.U. Anti- Plasmodium falciparum activity of quinoline–sulfonamide hybrids. Bioorg. Med. Chem. 2015, 23, 5979–5984. [Google Scholar] [CrossRef]
- Agbulos, D.S.; Barelli, L.; Giordano, B.V.; Hunter, F.F. Zika Virus: Quantification, Propagation, Detection, and Storage. Curr. Protoc. Microbiol. 2016, 43, 15D.4.1–15D.4.16. [Google Scholar] [CrossRef]
- Yamamoto, K.A.; Galhardi, L.C.F.; Rincão, V.P.; Soares, S.D.A.; Vieira, Í.G.P.; Ricardo, N.M.P.S.; Nozawa, C.M.; Linhares, R.E.C. Antiherpetic activity of an Agaricus brasiliensis polysaccharide, its sulfated derivative and fractions. Int. J. Biol. Macromol. 2013, 52, 9–13. [Google Scholar] [CrossRef]
- Corman, V.M.; Rasche, A.; Baronti, C.; Aldabbagh, S.; Cadar, D.; Reusken, C.B.; Pas, S.D.; Goorhuis, A.; Schinkel, J.; Molenkamp, R.; et al. Assay optimization for molecular detection of Zika virus. Bull. World Health Organ. 2016, 94, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Vieira, D.F.; Jácome, F.C.; Da Silva, M.A.N.; Caldas, G.C.; De Filippis, A.M.B.; De Sequeira, P.C.; De Souza, E.M.; Andrade, A.A.; Manso, P.P.D.A.; Trindade, G.F.; et al. Structural investigation of C6/36 and Vero cell cultures infected with a Brazilian Zika virus. PLoS ONE 2017, 12, e0184397. [Google Scholar] [CrossRef]
- Farias, K.J.S.; Machado, P.R.L.; Da Fonseca, B.A.L. Chloroquine Inhibits Dengue Virus Type 2 Replication in Vero Cells but Not in C6/36 Cells. Sci. World J. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Boonyasuppayakorn, S.; Reichert, E.D.; Manzano, M.; Nagarajan, K.; Padmanabhan, R. Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antivir. Res. 2014, 106, 125–134. [Google Scholar] [CrossRef]
- Wang, L.-F.; Lin, Y.-S.; Huang, N.-C.; Mayumi, M.; Tsai, W.-L.; Chen, J.-J.; Kubota, T.; Matsuoka, M.; Chen, S.-R.; Yang, C.-S.; et al. Hydroxychloroquine-Inhibited Dengue Virus Is Associated with Host Defense Machinery. J. Interf. Cytokine Res. 2015, 35, 143–156. [Google Scholar] [CrossRef]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Rao, P.V.L.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against chikungunya virus in vero cells. J. Med. Virol. 2010, 82, 817–824. [Google Scholar] [CrossRef]
- Roques, P.; Thiberville, S.-D.; Dupuis-Maguiraga, L.; Lum, F.-M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; Ng, L.F.P.; De Lamballerie, X.; et al. Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef]
- Pena, L.J.; Guarines, K.M.; Silva, A.J.D.; Leal, L.R.S.; Félix, D.M.; Silva, A.; De Oliveira, S.A.; Ayres, C.F.J.; Silva-Júnior, A.; De Freitas, A.C. In vitro and in vivo models for studying Zika virus biology. J. Gen. Virol. 2018, 99, 1529–1550. [Google Scholar] [CrossRef]
- Luo, H.; Winkelmann, E.R.; Fernandez-Salas, I.; Li, L.; Mayer, S.V.; Danis-Lozano, R.; Sánchez-Casas, R.M.; Vasilakis, N.; Tesh, R.; Barrett, A.D.; et al. Zika, dengue and yellow fever viruses induce differential anti-viral immune responses in human monocytic and first trimester trophoblast cells. Antivir. Res. 2018, 151, 55–62. [Google Scholar] [CrossRef]
- Beam, E.; Razonable, R.R. Cytomegalovirus in Solid Organ Transplantation: Epidemiology, Prevention, and Treatment. Curr. Infect. Dis. Rep. 2012, 14, 633–641. [Google Scholar] [CrossRef]
- Vegvari, C.; Hadjichrysanthou, C.; Cauët, E.; Lawrence, E.; Cori, A.; De Wolf, F.; Anderson, R.M. How Can Viral Dynamics Models Inform Endpoint Measures in Clinical Trials of Therapies for Acute Viral Infections? PLoS ONE 2016, 11, e0158237. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]

| C-Sds | Formula | Nomenclature | Structure | Molecular Weight |
|---|---|---|---|---|
| C-Sd1 | C19H20ClN3O2S | N-(3-((7-chloroquinolin-4-yl)amino)propyl)-4-methyl-benzenesulfonamide |  | 389.0965 |
| C-Sd2 | C18H17BrClN3O2S | 4-Bromo-N-(3-((7-chloroquinolin-4-yl)amino)propyl)-benzenesulfonamide |  | 452.9913 |
| C-Sd3 | C18H17ClFN3O2S | N-(3-((7-chloroquinolin-4-yl)amino)propyl)-4-fluorobenzenesulfonamide |  | 393.0714 |
| C-Sd4 | C19H20ClN3O2S | N-(4-((7-chloroquinolin-4-yl)amino)butyl)-benzenesulfonamide |  | 389.0965 |
| C-Sd5 | C19H19Cl2N3O2S | 4-Chloro-N-(4-((7-chloroquinolin-4-yl)amino)butyl)-benzenesulfonamide |  | 423.0575 |
| C-Sd6 | C19H19BrClN3O2S | 4-Bromo-N-(4-((7-chloroquinolin-4-yl)amino)butyl)-benzenesulfonamide |  | 467.0070 |
| C-Sd7 | C19H19ClFN3O2S | N-(4-((7-chloroquinolin-4-yl)amino)butyl)-4-fluorobenzenesulfonamide |  | 407.0871 |
| Mefloquine hydrochloride C17H17ClF6N2O | (R)-(2.8-bis(trifluoromethyl)quinolin-4-yl)((S)-piperidin-2-yl)methanol hydrochloride |  | 414.0934 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.A.A.; Torres, L.R.; Capobianco, L.R.P.L.; de Paula, V.S.; Cascabulho, C.M.; Salomão, K.; Bonecini-Almeida, M.d.G.; Ferreira, M.d.L.G.; Boechat, N.; Pinheiro, L.C.d.S.; et al. Chloroquine and Sulfadoxine Derivatives Inhibit ZIKV Replication in Cervical Cells. Viruses 2021, 13, 36. https://doi.org/10.3390/v13010036
de Souza AAA, Torres LR, Capobianco LRPL, de Paula VS, Cascabulho CM, Salomão K, Bonecini-Almeida MdG, Ferreira MdLG, Boechat N, Pinheiro LCdS, et al. Chloroquine and Sulfadoxine Derivatives Inhibit ZIKV Replication in Cervical Cells. Viruses. 2021; 13(1):36. https://doi.org/10.3390/v13010036
Chicago/Turabian Stylede Souza, Audrien Alves Andrade, Lauana Ribas Torres, Lyana Rodrigues Pinto Lima Capobianco, Vanessa Salete de Paula, Cynthia Machado Cascabulho, Kelly Salomão, Maria da Gloria Bonecini-Almeida, Maria de Lourdes Garcia Ferreira, Nubia Boechat, Luiz Carlos da Silva Pinheiro, and et al. 2021. "Chloroquine and Sulfadoxine Derivatives Inhibit ZIKV Replication in Cervical Cells" Viruses 13, no. 1: 36. https://doi.org/10.3390/v13010036
APA Stylede Souza, A. A. A., Torres, L. R., Capobianco, L. R. P. L., de Paula, V. S., Cascabulho, C. M., Salomão, K., Bonecini-Almeida, M. d. G., Ferreira, M. d. L. G., Boechat, N., Pinheiro, L. C. d. S., & de Souza, E. M. (2021). Chloroquine and Sulfadoxine Derivatives Inhibit ZIKV Replication in Cervical Cells. Viruses, 13(1), 36. https://doi.org/10.3390/v13010036







