Imaging of Virus-Infected Cells with Soft X-ray Tomography
Abstract
:1. Introduction
2. Soft X-ray Tomography
2.1. Method Basics
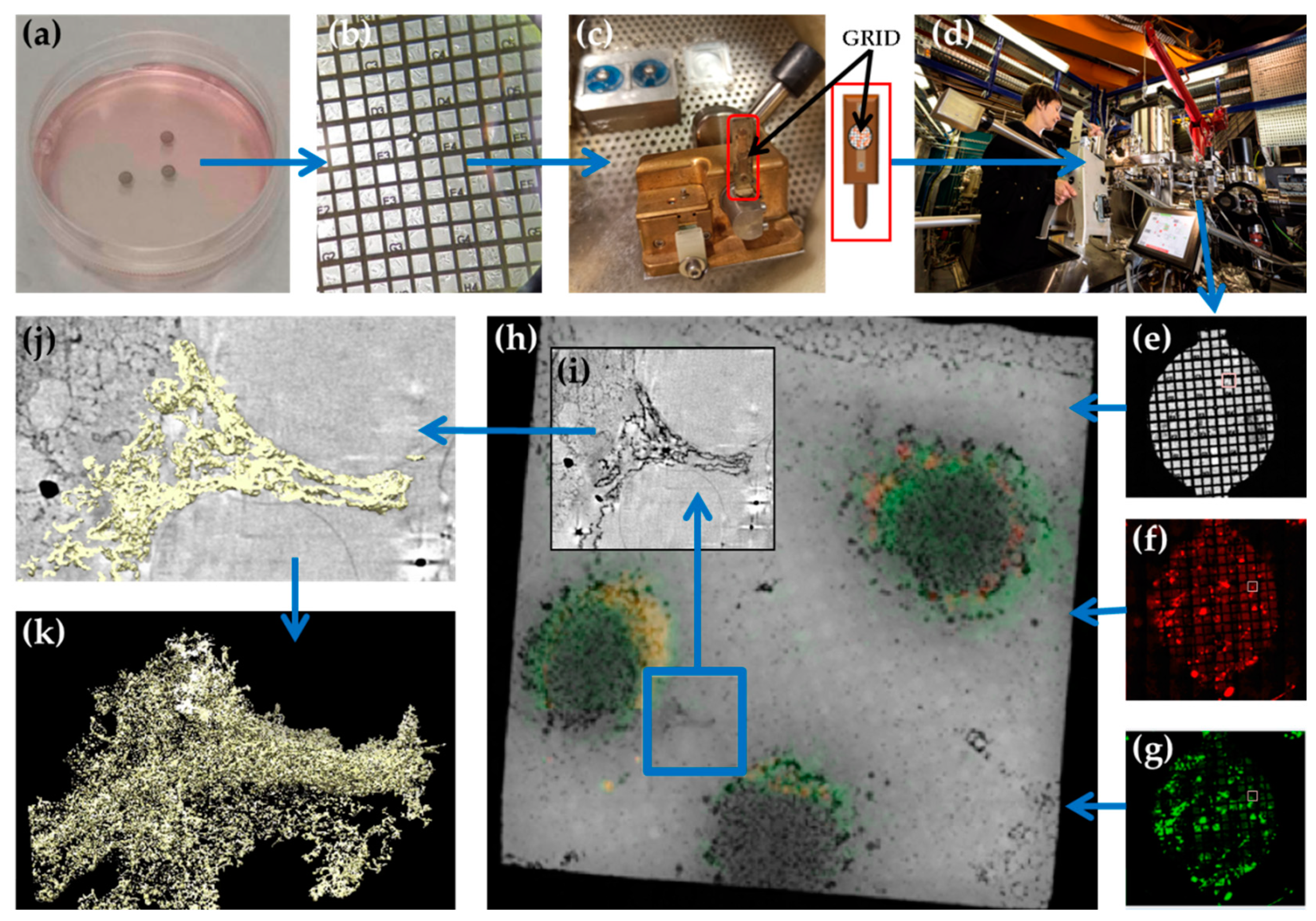
2.2. Sample Preparation for Cryo-SXT
2.3. Imaging at the Transmission X-ray Microscope
3. Examples of Virus Research with Cryo-SXT
3.1. Cryo-SXT Analysis of Ultrastructural Alterations in Liver Cells upon HCV Infection and after Antiviral Treatment
3.2. X-ray Tomography Analysis of Vaccinia Virus Morphogenesis
3.3. Ultrastructure Alterations Associated with Zika Virus Replication
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, R.E.F. The Origin of Viruses from Cells. In Aspects of Cell Regulation; Danielli, J.F.B., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 245–280. ISBN 978-0-12-364376-6. [Google Scholar]
- Hernandez-Gonzalez, M.; Larocque, G.; Way, M. Viral use and subversion of membrane organization and trafficking. J. Cell Sci. 2021, 134, 1–14. [Google Scholar] [CrossRef]
- De la Torre, J.C.; Borrow, P. Virus-Induced Alterations in Cells. In Principles of Medical Biology; JAI Press Inc.: Stamford, CT, USA, 1997; Volume 9B, pp. 365–379. ISBN 1559388145. [Google Scholar]
- Novoa, R.R.; Calderita, G.; Arranz, R.; Fontana, J.; Granzow, H.; Risco, C. Virus factories: Associations of cell organelles for viral replication and morphogenesis. Biol. Cell 2005, 97, 147–172. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Krijnse-Locker, J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; Wileman, T. Virus factories, double membrane vesicles and viroplasm generated in animal cells. Curr. Opin. Virol. 2011, 1, 381–387. [Google Scholar] [CrossRef]
- Strating, J.R.; van Kuppeveld, F.J. Viral rewiring of cellular lipid metabolism to create membranous replication compartments. Curr. Opin. Cell Biol. 2017, 47, 24–33. [Google Scholar] [CrossRef]
- Okano, K.; Mikhailov, V.; Maeda, S. Colocalization of Baculovirus IE-1 and Two DNA-Binding Proteins, DBP and LEF-3, to Viral Replication Factories. J. Virol. 1999, 73, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.; Liu, J.J.; Yeh, K.-C.; Knipe, D.M. Herpes Simplex Virus Infection Blocks Events in the G1 Phase of the Cell Cycle. Virology 2000, 267, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, L.D.; Schaffer, P.A.; Dorsky, D.I.; Crumpacker, C.S.; Parris, D.S. Localization of the herpes simplex virus type 1 65-kilodalton DNA-binding protein and DNA polymerase in the presence and absence of viral DNA synthesis. J. Virol. 1990, 64, 5738–5749. [Google Scholar] [CrossRef] [Green Version]
- Nagaraju, T.; Sugden, A.U.; Sugden, B. Four-dimensional analyses show that replication compartments are clonal factories in which Epstein–Barr viral DNA amplification is coordinated. Proc. Natl. Acad. Sci. USA 2019, 116, 24630–24638. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.D.; Bouchet-Marquis, C.; Heiser, K.; Szomolanyi-Tsuda, E.; Mishra, R.; Lamothe, B.; Hoenger, A.; Garcea, R.L. Virion Assembly Factories in the Nucleus of Polyomavirus-Infected Cells. PLoS Pathog. 2012, 8, e1002630. [Google Scholar] [CrossRef]
- Koji, I.; Bernard, M. Role of Vaccinia Virus A20R Protein in DNA Replication: Construction and Characterization of Temperature-Sensitive Mutants. J. Virol. 2001, 75, 1656–1663. [Google Scholar] [CrossRef] [Green Version]
- Tilsner, J.; Linnik, O.; Wright, K.M.; Bell, K.; Roberts, A.G.; Lacomme, C.; Santa Cruz, S.; Oparka, K.J. The TGB1 Movement Protein of Potato virus X Reorganizes Actin and Endomembranes into the X-Body, a Viral Replication Factory. Plant Physiol. 2012, 158, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Wang, Z.; Liu, Y.; Han, Y.; Miao, M.; Qi, N.; Yang, J.; Xia, H.; Li, X.; Qin, C.-F.; et al. The Self-Interaction of a Nodavirus Replicase Is Enhanced by Mitochondrial Membrane Lipids. PLoS ONE 2014, 9, e89628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megias, E.; do Carmo, L.S.T.; Nicolini, C.; Silva, L.P.; Blawid, R.; Nagata, T.; Mehta, A. Chloroplast Proteome of Nicotiana benthamiana Infected by Tomato Blistering Mosaic Virus. Protein J. 2018, 37, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Inaba, J.; Nagy, P.D. Tombusvirus RNA replication depends on the TOR pathway in yeast and plants. Virology 2018, 519, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; López-Montero, N.; Elliott, R.M.; Fernández, J.J.; Risco, C. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cell. Microbiol. 2008, 10, 2012–2028. [Google Scholar] [CrossRef]
- Suzan-Monti, M.; La Scola, B.; Barrassi, L.; Espinosa, L.; Raoult, D. Ultrastructural characterization of the giant volcano-like virus factory of Acanthamoeba polyphaga Mimivirus. PLoS ONE 2007, 2, e328. [Google Scholar] [CrossRef]
- Moshe, A.; Gorovits, R. Virus-Induced Aggregates in Infected Cells. Viruses 2012, 4, 2218–2232. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Oliva, A.; Ortega-González, P.; Risco, C. Targeting host lipid flows: Exploring new antiviral and antibiotic strategies. Cell. Microbiol. 2019, 21, e12996. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Berná, A.J.; Rodríguez, M.J.; Chichón, F.J.; Friesland, M.F.; Sorrentino, A.; Carrascosa, J.L.; Pereiro, E.; Gastaminza, P. Structural Changes in Cells Imaged by Soft X-ray Cryo-Tomography during Hepatitis C Virus Infection. ACS Nano 2016, 10, 6597–6611. [Google Scholar] [CrossRef]
- Sorrentino, A.; Nicolás, J.; Valcárcel, R.; Chichón, F.J.; Rosanes, M.; Avila, J.; Tkachuk, A.; Irwin, J.; Ferrer, S.; Pereiro, E. MISTRAL: A transmission soft X-ray microscopy beamline for cryo nano-tomography of biological samples and magnetic domains imaging. J. Synchrotron Radiat. 2015, 22, 1112–1117. [Google Scholar] [CrossRef]
- Groen, J.; Sorrentino, A.; Aballe, L.; Oliete, R.; Valcárcel, R.; Okolo, C.; Kounatidis, I.; Harkiolaki, M.; Pérez-Berná, A.J.; Pereiro, E. A 3D cartographic description of the cell by cryo soft x-ray tomography. J. Vis. Exp. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Guttmann, P.; Werner, S.; Rehbein, S.; Habel, C.; Schneider, G. First Results from the X-Ray Microscopy Beamline U41-PGM1-XM at BESSY II. Microsc. Microanal. 2018, 24, 204–205. [Google Scholar] [CrossRef] [Green Version]
- Le Gros, M.A.; Mcdermott, G.; Cinquin, B.P.; Smith, E.A.; Do, M.; Chao, W.L.; Naulleau, P.P.; Larabell, C.A. Biological soft X-ray tomography on beamline 2.1 at the Advanced Light Source. J. Synchrotron Radiat. 2014, 21, 1370–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkiolaki, M.; Darrow, M.C.; Spink, M.C.; Kosior, E.; Dent, K.; Duke, E. Cryo-soft X-ray tomography: Using soft X-rays to explore the ultrastructure of whole cells. Emerg. Top. Life Sci. 2018, 2, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natterer, F. The Mathematics of Computerized Tomography; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2001; ISBN 978-0-89871-493-7. [Google Scholar]
- Weiß, D.; Schneider, G.; Niemann, B.; Guttmann, P.; Rudolph, D.; Schmahl, G. Computed tomography of cryogenic biological specimens based on X-ray microscopic images. Ultramicroscopy 2000, 84, 185–197. [Google Scholar] [CrossRef]
- Groen, J.; Conesa, J.J.; Valcárcel, R.; Pereiro, E. The cellular landscape by cryo soft X-ray tomography. Biophys. Rev. 2019, 11, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, G.; Le Gros, M.A.; Knoechel, C.G.; Uchida, M.; Larabell, C.A. Soft X-ray tomography and cryogenic light microscopy: The cool combination in cellular imaging. Trends Cell Biol. 2009, 19, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Möller, L.; Schünadel, L.; Nitsche, A.; Schwebke, I.; Hanisch, M.; Laue, M. Evaluation of Virus Inactivation by Formaldehyde to Enhance Biosafety of Diagnostic Electron Microscopy. Viruses 2015, 7, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Korogod, N.; Petersen, C.C.H.; Knott, G.W. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Kellenberger, E.; Johansen, R.; Maeder, M.; Bohrmann, B.; Stauffer, E.; Villiger, W. Artefacts and morphological changes during chemical fixation. J. Microsc. 1992, 168, 181–201. [Google Scholar] [CrossRef]
- Blancard, C.; Salin, B. Plunge Freezing: A Tool for the Ultrastructural and Immunolocalization Studies of Suspension Cells in Transmission Electron Microscopy. J. Vis. Exp. 2017, 123, e54874. [Google Scholar] [CrossRef]
- Pereiro, E.; Nicolás, J.; Ferrer, S.; Howells, M.R. A soft X-ray beamline for transmission X-ray microscopy at ALBA. J. Synchrotron Radiat. 2009, 16, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer Visualization of Three-Dimensional Image Data Using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard Heymann, J.; Cardone, G.; Winkler, D.C.; Steven, A.C. Computational resources for cryo-electron tomography in Bsoft. J. Struct. Biol. 2008, 161, 232–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messaoudi, C.; Aschman, N.; Cunha, M.; Oikawa, T.; Sorzano, C.O.S.; Marco, S. Three-Dimensional Chemical Mapping by EFTEM-TomoJ Including Improvement of SNR by PCA and ART Reconstruction of Volume by Noise Suppression. Microsc. Microanal. 2013, 19, 1669–1677. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Belevich, I.; Joensuu, M.; Kumar, D.; Vihinen, H.; Jokitalo, E. Microscopy Image Browser: A Platform for Segmentation and Analysis of Multidimensional Datasets. PLoS Biol. 2016, 14, e1002340. [Google Scholar] [CrossRef]
- Pruggnaller, S.; Mayr, M.; Frangakis, A.S. A visualization and segmentation toolbox for electron microscopy. J. Struct. Biol. 2008, 164, 161–165. [Google Scholar] [CrossRef]
- Mendonça, L.; Howe, A.; Gilchrist, J.B.; Sheng, Y.; Sun, D.; Knight, M.L.; Zanetti-Domingues, L.C.; Bateman, B.; Krebs, A.-S.; Chen, L.; et al. Correlative multi-scale cryo-imaging unveils SARS-CoV-2 assembly and egress. Nat. Commun. 2021, 12, 4629. [Google Scholar] [CrossRef]
- Aho, V.; Myllys, M.; Ruokolainen, V.; Hakanen, S.; Mäntylä, E.; Virtanen, J.; Hukkanen, V.; Kühn, T.; Timonen, J.; Mattila, K.; et al. Chromatin organization regulates viral egress dynamics. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.; Mäntylä, E.; Ekman, A.; Hakanen, S.; Mattola, S.; Chen, J.H.; Weinhardt, V.; Ruokolainen, V.; Sodeik, B.; Larabell, C.; et al. Quantitative microscopy reveals stepwise alteration of chromatin structure during herpesvirus infection. Viruses 2019, 11, 935. [Google Scholar] [CrossRef] [Green Version]
- Myllys, M.; Ruokolainen, V.; Aho, V.; Smith, E.A.; Hakanen, S.; Peri, P.; Salvetti, A.; Timonen, J.; Hukkanen, V.; Larabell, C.A.; et al. Herpes simplex virus 1 induces egress channels through marginalized host chromatin. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Kounatidis, I.; Stanifer, M.L.; Phillips, M.A.; Paul-Gilloteaux, P.; Heiligenstein, X.; Wang, H.; Okolo, C.A.; Fish, T.M.; Spink, M.C.; Stuart, D.I.; et al. 3D Correlative Cryo-Structured Illumination Fluorescence and Soft X-ray Microscopy Elucidates Reovirus Intracellular Release Pathway. Cell 2020, 182, 515–530.e17. [Google Scholar] [CrossRef]
- Hagen, C.; Guttmann, P.; Klupp, B.; Werner, S.; Rehbein, S.; Mettenleiter, T.C.; Schneider, G.; Grünewald, K. Correlative VIS-fluorescence and soft X-ray cryo-microscopy/tomography of adherent cells. J. Struct. Biol. 2012, 177, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggins, S.W.; Bambha, K.M.; Terrault, N.A.; Inadomi, J.; Shiboski, S.; Dodge, J.L.; Gralla, J.; Rosen, H.R.; Roberts, J.P. Projected future increase in aging hepatitis C virus–infected liver transplant candidates: A potential effect of hepatocellular carcinoma. Liver Transplant. 2012, 18, 1471–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasallo, C.; Gastaminza, P. Cellular stress responses in hepatitis C virus infection: Mastering a two-edged sword. Virus Res. 2015, 209, 100–117. [Google Scholar] [CrossRef]
- Jones, C.T.; Catanese, M.T.; Law, L.M.J.; Khetani, S.R.; Syder, A.J.; Ploss, A.; Oh, T.S.; Schoggins, J.W.; MacDonald, M.R.; Bhatia, S.N.; et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat. Biotechnol. 2010, 28, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, E.; Brohm, C.; Kallis, S.; Bartenschlager, R.; Pietschmann, T. Efficient trans-Encapsidation of Hepatitis C Virus RNAs into Infectious Virus-Like Particles. J. Virol. 2008, 82, 7034–7046. [Google Scholar] [CrossRef] [Green Version]
- Romero-Brey, I.; Bartenschlager, R. Membranous Replication Factories Induced by Plus-Strand RNA Viruses. Viruses 2014, 6, 2826–2857. [Google Scholar] [CrossRef]
- Mottola, G.; Cardinali, G.; Ceccacci, A.; Trozzi, C.; Bartholomew, L.; Torrisi, M.R.; Pedrazzini, E.; Bonatti, S.; Migliaccio, G. Hepatitis C Virus Nonstructural Proteins Are Localized in a Modified Endoplasmic Reticulum of Cells Expressing Viral Subgenomic Replicons. Virology 2002, 293, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M. Treatment of Chronic Hepatitis C: Current and Future BT—Hepatitis C Virus: From Molecular Virology to Antiviral Therapy. In Hepatitis C Virus: From Molecular Virology to Antiviral Therapy; Bartenschlager, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 321–342. ISBN 978-3-642-27340-7. [Google Scholar]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, B. Reflections on the early development of poxvirus vectors. Vaccine 2013, 31, 4220–4222. [Google Scholar] [CrossRef] [Green Version]
- Chichón, F.J.; Rodríguez, M.J.; Pereiro, E.; Chiappi, M.; Perdiguero, B.; Guttmann, P.; Werner, S.; Rehbein, S.; Schneider, G.; Esteban, M.; et al. Cryo X-ray nano-tomography of vaccinia virus infected cells. J. Struct. Biol. 2012, 177, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Luo, Q.; Zhang, Z.-W.; Li, Z.-L. Commentary: Teratogenic effects of the Zika virus and the role of the placenta. Front. Cell. Infect. Microbiol. 2017, 7, 62. [Google Scholar] [CrossRef]
- Broutet, N.; Krauer, F.; Riesen, M.; Khalakdina, A.; Almiron, M.; Aldighieri, S.; Espinal, M.; Low, N.; Dye, C. Zika Virus as a Cause of Neurologic Disorders. N. Engl. J. Med. 2016, 374, 1506–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, M.; Goellner, S.; Acosta, E.G.; Neufeldt, C.J.; Oleksiuk, O.; Lampe, M.; Haselmann, U.; Funaya, C.; Schieber, N.; Ronchi, P.; et al. Ultrastructural Characterization of Zika Virus Replication Factories. Cell Rep. 2017, 18, 2113–2123. [Google Scholar] [CrossRef] [Green Version]
- Gangodkar, S.; Jain, P.; Dixit, N.; Ghosh, K.; Basu, A. Dengue virus-induced autophagosomes and changes in endomembrane ultrastructure imaged by electron tomography and whole-mount grid-cell culture techniques. J. Electron. Microsc. 2010, 59, 503–511. [Google Scholar] [CrossRef]
- Huang, B. Super-resolution optical microscopy: Multiple choices. Curr. Opin. Chem. Biol. 2010, 14, 10–14. [Google Scholar] [CrossRef]
- Grünewald, K.; Cyrklaff, M. Structure of complex viruses and virus-infected cells by electron cryo tomography. Curr. Opin. Microbiol. 2006, 9, 437–442. [Google Scholar] [CrossRef]
- Leis, A.; Rockel, B.; Andrees, L.; Baumeister, W. Visualizing cells at the nanoscale. Trends Biochem. Sci. 2009, 34, 60–70. [Google Scholar] [CrossRef]
- Ekman, A.A.; Chen, J.-H.; Guo, J.; McDermott, G.; Le Gros, M.A.; Larabell, C.A. Mesoscale imaging with cryo-light and X-rays: Larger than molecular machines, smaller than a cell. Biol. Cell 2017, 109, 24–38. [Google Scholar] [CrossRef] [Green Version]
- The chaperonin CCT controls T cell receptor–driven 3D configuration of centrioles. Sci. Adv. 2021, 6, eabb7242. [CrossRef]
- Xu, C.S.; Hayworth, K.J.; Lu, Z.; Grob, P.; Hassan, A.M.; García-Cerdán, J.G.; Niyogi, K.K.; Nogales, E.; Weinberg, R.J.; Hess, H.F. Enhanced FIB-SEM systems for large-volume 3D imaging. Elife 2017, 6, e25916. [Google Scholar] [CrossRef]
- Spehner, D.; Steyer, A.M.; Bertinetti, L.; Orlov, I.; Benoit, L.; Pernet-Gallay, K.; Schertel, A.; Schultz, P. Cryo-FIB-SEM as a promising tool for localizing proteins in 3D. J. Struct. Biol. 2020, 211, 107528. [Google Scholar] [CrossRef] [PubMed]
- Otón, J.; Pereiro, E.; Conesa, J.J.; Chichón, F.J.; Luque, D.; Rodríguez, J.M.; Pérez-Berná, A.J.; Sorzano, C.O.S.; Klukowska, J.; Herman, G.T.; et al. XTEND: Extending the depth of field in cryo soft X-ray tomography. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otón, J.; Pereiro, E.; Pérez-Berná, A.J.; Millach, L.; Sorzano, C.O.S.; Marabini, R.; Carazo, J.M. Characterization of transfer function, resolution and depth of field of a soft X-ray microscope applied to tomography enhancement by Wiener deconvolution. Biomed. Opt. Express 2016, 7, 5092–5103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankertz, A.; Domingo, M.; Folch, J.M.; LeCann, P.; Jestin, A.; Segalés, J.; Chmielewicz, B.; Plana-Durán, J.; Soike, D. Characterisation of PCV-2 isolates from Spain, Germany and France. Virus Res. 2000, 66, 65–77. [Google Scholar] [CrossRef]
- Bharat, T.A.M.; Noda, T.; Riches, J.D.; Kraehling, V.; Kolesnikova, L.; Becker, S.; Kawaoka, Y.; Briggs, J.A.G. Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography. Proc. Natl. Acad. Sci. USA 2012, 109, 4275–4280. [Google Scholar] [CrossRef] [Green Version]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudy, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C.; et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl. Acad. Sci. USA 2014, 111, 4274–4279. [Google Scholar] [CrossRef] [Green Version]
- Conesa, J.J.; Sevilla, E.; Terrón, M.C.; González, L.M.; Gray, J.; Pérez-Berná, A.J.; Carrascosa, J.L.; Pereiro, E.; Chichón, F.J.; Luque, D.; et al. Four-Dimensional Characterization of the Babesia divergens Asexual Life Cycle, from the Trophozoite to the Multiparasite Stage. mSphere 2021, 5, e00928-20. [Google Scholar] [CrossRef]
- Reineck, P.; Abraham, A.N.; Poddar, A.; Shukla, R.; Abe, H.; Ohshima, T.; Gibson, B.C.; Dekiwadia, C.; Conesa, J.J.; Pereiro, E.; et al. Multimodal Imaging and Soft X-Ray Tomography of Fluorescent Nanodiamonds in Cancer Cells. Biotechnol. J. 2021, 16, 2000289. [Google Scholar] [CrossRef] [PubMed]
- Conesa, J.J.; Carrasco, A.C.; Rodríguez-Fanjul, V.; Yang, Y.; Carrascosa, J.L.; Cloetens, P.; Pereiro, E.; Pizarro, A.M. Unambiguous Intracellular Localization and Quantification of a Potent Iridium Anticancer Compound by Correlative 3D Cryo X-Ray Imaging. Angew. Chemie Int. Ed. 2020, 59, 1270–1278. [Google Scholar] [CrossRef]
- Okolo, C.A.; Kounatidis, I.; Groen, J.; Nahas, K.L.; Balint, S.; Fish, T.M.; Koronfel, M.A.; Cortajarena, A.L.; Dobbie, I.M.; Pereiro, E.; et al. Sample preparation strategies for efficient correlation of 3D SIM and soft X-ray tomography data at cryogenic temperatures. Nat. Protoc. 2021, 16, 2851–2885. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Perry, N.; Okolo, C.A.; Kounatidis, I.; Fish, T.M.; Nahas, K.L.; Jadhav, A.; Koronfel, M.A.; Groen, J.; Pereiro, E.; et al. Cryo-Structured Illumination Microscopic Data Collection from Cryogenically Preserved Cells. J. Vis. Exp. 2021, e62274. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garriga, D.; Chichón, F.J.; Calisto, B.M.; Ferrero, D.S.; Gastaminza, P.; Pereiro, E.; Pérez-Berna, A.J. Imaging of Virus-Infected Cells with Soft X-ray Tomography. Viruses 2021, 13, 2109. https://doi.org/10.3390/v13112109
Garriga D, Chichón FJ, Calisto BM, Ferrero DS, Gastaminza P, Pereiro E, Pérez-Berna AJ. Imaging of Virus-Infected Cells with Soft X-ray Tomography. Viruses. 2021; 13(11):2109. https://doi.org/10.3390/v13112109
Chicago/Turabian StyleGarriga, Damià, Francisco Javier Chichón, Bárbara M. Calisto, Diego S. Ferrero, Pablo Gastaminza, Eva Pereiro, and Ana Joaquina Pérez-Berna. 2021. "Imaging of Virus-Infected Cells with Soft X-ray Tomography" Viruses 13, no. 11: 2109. https://doi.org/10.3390/v13112109
APA StyleGarriga, D., Chichón, F. J., Calisto, B. M., Ferrero, D. S., Gastaminza, P., Pereiro, E., & Pérez-Berna, A. J. (2021). Imaging of Virus-Infected Cells with Soft X-ray Tomography. Viruses, 13(11), 2109. https://doi.org/10.3390/v13112109








