African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa
Abstract
:1. Introduction
2. Methodology
3. The African Swine Fever Virus Biology
4. African Swine Fever Virus Characterisation, Genomics and Genotyping
Molecular Epidemiology of African Swine Fever Virus in Sub-Saharan Africa
5. ASF Prevention, Control and Vaccine Development
6. Emerging Gaps and Future Research Focus
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African Swine Fever Virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.G.; Netherton, C.L.; Upton, C. African Swine Fever Virus Replication and Genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission Routes of African Swine Fever Virus to Domestic Pigs: Current Knowledge and Future Research Directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, M.C.; de la Torre Reoyo, A.; Fernández-Pinero, J.; Iglesias, I.; Muñoz, M.J.; Arias, M.L. African Swine Fever: A Global View of the Current Challenge. Porc. Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penrith, M.-L.; Vosloo, W. Review of African Swine Fever : Transmission, Spread and Control: Review Article. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The Persistence of African Swine Fever Virus in Field-Infected Ornithodoros Erraticus during the ASF Endemic Period in Portugal. PLoS ONE 2011, 6, e20383. [Google Scholar] [CrossRef] [PubMed]
- Ravaomanana, J.; Jori, F.; Vial, L.; Pérez-Sánchez, R.; Blanco, E.; Michaud, V.; Roger, F. Assessment of Interactions between African Swine Fever Virus, Bushpigs (Potamochoerus Larvatus), Ornithodoros Ticks and Domestic Pigs in North-Western Madagascar: ASFV and Its Potential Reservoir Hosts in Madagascar. Transbound. Emerg. Dis. 2011, 58, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L. History of ‘Swine Fever’ in Southern Africa. J. S. Afr. Vet. Assoc. 2013, 84, 6. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.D.S.; Penrith, M.-L.; Crucière, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping Field Strains of African Swine Fever Virus by Partial P72 Gene Characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrín, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia. Transbound. Emerg. Dis. 2017, 64, 1393–1404. [Google Scholar] [CrossRef]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic Characterization of African Swine Fever Virus Isolates from Soft Ticks at the Wildlife/Domestic Interface in Mozambique and Identification of a Novel Genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.L.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African Swine Fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouakou, K.V.; Michaud, V.; Biego, H.G.; Gnabro, H.P.G.; Kouakou, A.V.; Mossoun, A.M.; Awuni, J.A.; Minoungou, G.L.; Aplogan, G.L.; Awoumé, F.K.; et al. African and Classical Swine Fever Situation in Ivory-Coast and Neighboring Countries, 2008–2013. Acta Trop. 2017, 166, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Luka, P.D.; Achenbach, J.E.; Mwiine, F.N.; Lamien, C.E.; Shamaki, D.; Unger, H.; Erume, J. Genetic Characterization of Circulating African Swine Fever Viruses in Nigeria (2007–2015). Transbound. Emerg. Dis. 2017, 64, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.-A.; Penrith, M.L.; Fasina, F.O.; Beltran-Alcrudo, D. The African Swine Fever Epidemic in West Africa, 1996–2002. Transbound. Emerg. Dis. 2018, 65, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, J.N.; Nyabongo, L.; Ntirandekura, J.B.; Yona, C.; Ntakirutimana, D.; Kamana, O.; Nauwynck, H.; Misinzo, G. Genetic Analysis of African Swine Fever Virus From the 2018 Outbreak in South-Eastern Burundi. Front. Vet. Sci. 2020, 7, 578474. [Google Scholar] [CrossRef]
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African Swine Fever: How Can Global Spread Be Prevented? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubisi, B.A.; Bastos, A.D.S.; Dwarka, R.M.; Vosloo, W. Molecular Epidemiology of African Swine Fever in East Africa. Arch. Virol. 2005, 150, 2439–2452. [Google Scholar] [CrossRef]
- Misinzo, G.; Magambo, J.; Masambu, J.; Yongolo, M.G.; Van Doorsselaere, J.; Nauwynck, H.J. Genetic Characterization of African Swine Fever Viruses from a 2008 Outbreak in Tanzania: Genotyping of 2008 Tanzanian ASFV. Transbound. Emerg. Dis. 2011, 58, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Misinzo, G.; Jumapili, F.; Ludosha, M.; Mafie, E.; Silialis, J.; Mushi, R.; Viaene, W.; Doorsselaere, J. Genotyping of African Swine Fever Virus from a 2009 Outbreak in Tanzania. Res. Opin. Anim. Vet. Sci. 2012, 2, 334–338. [Google Scholar]
- Wambura, P.N.; Masambu, J.; Msami, H. Molecular Diagnosis and Epidemiology of African Swine Fever Outbreaks in Tanzania. Vet. Res. Commun. 2006, 30, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Atuhaire, D.K.; Afayoa, M.; Ochwo, S.; Mwesigwa, S.; Okuni, J.B.; Olaho-Mukani, W.; Ojok, L. Molecular Characterization and Phylogenetic Study of African Swine Fever Virus Isolates from Recent Outbreaks in Uganda (2010–2013). Virol. J. 2013, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, C.; Okoth, E.; Pelayo, V.; Anchuelo, R.; Martin, E.; Simon, A.; Llorente, A.; Nieto, R.; Soler, A.; Martin, R.; et al. African Swine Fever Viruses with Two Different Genotypes, Both of Which Occur in Domestic Pigs, Are Associated with Ticks and Adult Warthogs, Respectively, at a Single Geographical Site. J. Gen. Virol. 2011, 92, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Malogolovkin, A.; Kolbasov, D. Genetic and Antigenic Diversity of African Swine Fever Virus. Virus Res. 2019, 271, 197673. [Google Scholar] [CrossRef]
- Mulumba–Mfumu, L.; Achenbach, J.; Mauldin, M.; Dixon, L.; Tshilenge, C.; Thiry, E.; Moreno, N.; Blanco, E.; Saegerman, C.; Lamien, C.; et al. Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of P72 Genotypes I, IX and XIV, Including 19 Variants. Viruses 2017, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, M.; Jurado, C.; Gallardo, C.; Fernández-Pinero, J.; Sánchez-Vizcaíno, J.M. Gaps in African Swine Fever: Analysis and Priorities. Transbound. Emerg. Dis. 2018, 65, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.L.; Netherton, C.; Dixon, L.K. Unraveling the Armor of a Killer: Evasion of Host Defenses by African Swine Fever Virus. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Meng, G. An Integrative Resource of African Swine Fever for Genomics and Proteomics Analysis. BioRxiv 2019, 670109. [Google Scholar] [CrossRef] [Green Version]
- Inmaculada, G.; Covadonga, A. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.M.; Moreno, L.T.; Alejo, A.; Lacasta, A.; Rodríguez, F.; Salas, M.L. Genome Sequence of African Swine Fever Virus BA71, the Virulent Parental Strain of the Nonpathogenic and Tissue-Culture Adapted BA71V. PLoS ONE 2015, 10, e0142889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jori, F.; Bastos, A.D.S. Role of Wild Suids in the Epidemiology of African Swine Fever. EcoHealth 2009, 6, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Wozniakowski, G.; Fraczyk, M.; Niemczuk, K.; Pejsak, Z. Selected aspects related to epidemiology, pathogenesis, immunity, and control of African swine fever. J. Vet. Res. 2016, 60, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Pikalo, J.; Zani, L.; Hühr, J.; Beer, M.; Blome, S. Pathogenesis of African Swine Fever in Domestic Pigs and European Wild Boar—Lessons Learned from Recent Animal Trials. Virus Res. 2019, 271, 197614. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African Swine Fever: A Re-Emerging Viral Disease Threatening the Global Pig Industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Gethmann, J.; Amler, S.; Globig, A.; Knoll, B.; Conraths, F.J. The Potential Role of Scavengers in Spreading African Swine Fever among Wild Boar. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Thomson, G.R. The Epidemiologyof African Swine Fever: The Role of Free-Living Hosts in Africa. Onderstepoort J. Vet. Res. 1985, 209, 201–209. [Google Scholar]
- Conley, Y.P.; Biesecker, L.G.; Gonsalves, S.; Merkle, C.J.; Kirk, M.; Aouizerat, B.E. Current and Emerging Technology Approaches in Genomics: Technology Approaches in Genomics. J. Nurs. Scholarsh. 2013, 45, 5–14. [Google Scholar] [CrossRef] [Green Version]
- De León, P.; Bustos, M.J.; Carrascosa, A.L. Laboratory Methods to Study African Swine Fever Virus. Virus Res. 2013, 173, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K. The Structure and Function of the African Swine Fever Virus Genome. Rev. Sci. Tech. 1986, 7, 469–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbery, J.; Upton, C. Organization of the Multigene Families of African Swine Fever Virus. Fine Focus 2017, 9. [Google Scholar] [CrossRef]
- Misinzo, G.; Kwavi, D.E.; Sikombe, C.D.; Makange, M.; Peter, E.; Muhairwa, A.P.; Madege, M.J. Molecular Characterization of African Swine Fever Virus from Domestic Pigs in Northern Tanzania during an Outbreak in 2013. Trop. Anim. Health Prod. 2014, 46, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, V.; Holinka, L.G.; Sanford, B.; Krug, P.W.; Carlson, J.; Pacheco, J.M.; Reese, B.; Risatti, G.R.; Gladue, D.P.; Borca, M.V. African Swine Fever Virus Georgia Isolate Harboring Deletions of 9GL and MGF360/505 Genes Is Highly Attenuated in Swine but Does Not Confer Protection against Parental Virus Challenge. Virus Res. 2016, 221, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.D.S.; Penrith, M.-L.; Macome, F.; Pinto, F.; Thomson, G.R. Co-Circulation of Two Genetically Distinct Viruses in an Outbreak of African Swine Fever in Mozambique: No Evidence for Individual Co-Infection. Vet. Microbiol. 2004, 103, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Epifano, C.; Krijnse-Locker, J.; Salas, M.L.; Rodríguez, J.M.; Salas, J. The African Swine Fever Virus Nonstructural Protein PB602L Is Required for Formation of the Icosahedral Capsid of the Virus Particle. J. Virol. 2006, 80, 12260–12270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, C.; Mwaengo, D.M.; Macharia, J.M.; Arias, M.; Taracha, E.A.; Soler, A.; Okoth, E.; Martín, E.; Kasiti, J.; Bishop, R.P. Enhanced Discrimination of African Swine Fever Virus Isolates through Nucleotide Sequencing of the P54, P72, and PB602L (CVR) Genes. Virus Genes 2009, 38, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Nix, R.J.; Gallardo, C.; Hutchings, G.; Blanco, E.; Dixon, L.K. Molecular Epidemiology of African Swine Fever Virus Studied by Analysis of Four Variable Genome Regions. Arch. Virol. 2006, 151, 2475–2494. [Google Scholar] [CrossRef] [PubMed]
- Boshoff, C.I.; Bastos, A.D.S.; Gerber, L.J.; Vosloo, W. Genetic Characterisation of African Swine Fever Viruses from Outbreaks in Southern Africa (1973–1999). Vet. Microbiol. 2007, 121, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubisi, B.A.; Dwarka, R.M.; Meenowa, D.; Jaumally, R. An Investigation into the First Outbreak of African Swine Fever in the Republic of Mauritius. Transbound. Emerg. Dis. 2009, 56, 178–188. [Google Scholar] [CrossRef]
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic Variation among African Swine Fever Genotype II Viruses, Eastern and Central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef] [Green Version]
- Wade, A.; Achenbach, J.E.; Gallardo, C.; Settypalli, T.B.K.; Souley, A.; Djonwe, G.; Loitsch, A.; Dauphin, G.; Ngang, J.J.E.; Boyomo, O.; et al. Genetic Characterization of African Swine Fever Virus in Cameroon, 2010–2018. J. Microbiol. 2019, 57, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ngu Ngwa, V.; Abouna, A.; Zoli, A.P.; Attili, A.-R. Epidemiology of African Swine Fever in Piggeries in the Center, South and South-West of Cameroon. Vet. Sci. 2020, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Patrick, B.N.; Machuka, E.M.; Githae, D.; Banswe, G.; Amimo, J.O.; Ongus, J.R.; Masembe, C.; Bishop, R.P.; Steinaa, L.; Djikeng, A.; et al. Evidence for the Presence of African Swine Fever Virus in Apparently Healthy Pigs in South-Kivu Province of the Democratic Republic of Congo. Vet. Microbiol. 2020, 240, 108521. [Google Scholar] [CrossRef] [PubMed]
- Patrick, B.N.; Dieudonné, W.S.; Théophile, N.M.; Ahadi, B.B.; Espoir, B.B.; Gustave, M.N.; Karume, K.; Bajope, B. Evidence of African Swine Fever Virus in Pigs Slaughtered at Muhanzi Municipal Abattoir in Bukavu City, Eastern of Democratic Republic of Congo. Int. J. Microbiol. Biotechnol. 2019, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Couacy-Hymann, E.; Kouakou, K.V.; Achenbach, J.E.; Kouadio, L.; Koffi, Y.M.; Godji, H.P.; Adjé, K.E.; Oulaï, J.; Pell-Minhiaud, H.J.; Lamien, C.E. Re-emergence of Genotype I of African Swine Fever Virus in Ivory Coast. Transbound. Emerg. Dis. 2019, 66, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.F.; Bishop, R.P.; Onzere, C.; Mcintosh, M.T.; Lemire, K.A.; de Glanville, W.A.; Cook, E.A.J.; Fèvre, E.M. Evidence for the Presence of African Swine Fever Virus in an Endemic Region of Western Kenya in the Absence of Any Reported Outbreak. BMC Vet. Res. 2016, 12, 192. [Google Scholar] [CrossRef] [Green Version]
- Okoth, E.; Gallardo, C.; Macharia, J.M.; Omore, A.; Pelayo, V.; Bulimo, D.W.; Arias, M.; Kitala, P.; Baboon, K.; Lekolol, I.; et al. Comparison of African Swine Fever Virus Prevalence and Risk in Two Contrasting Pig-Farming Systems in South-West and Central Kenya. Prev. Vet. Med. 2013, 110, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.P.; Fleischauer, C.; de Villiers, E.P.; Okoth, E.A.; Arias, M.; Gallardo, C.; Upton, C. Comparative Analysis of the Complete Genome Sequences of Kenyan African Swine Fever Virus Isolates within P72 Genotypes IX and X. Virus Genes 2015, 50, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Onzere, C.K.; Bastos, A.D.; Okoth, E.A.; Lichoti, J.K.; Bochere, E.N.; Owido, M.G.; Ndambuki, G.; Bronsvoort, M.; Bishop, R.P. Multi-Locus Sequence Typing of African Swine Fever Viruses from Endemic Regions of Kenya and Eastern Uganda (2011–2013) Reveals Rapid B602L Central Variable Region Evolution. Virus Genes 2018, 54, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Abworo, E.O.; Onzere, C.; Oluoch Amimo, J.; Riitho, V.; Mwangi, W.; Davies, J.; Blome, S.; Peter Bishop, R. Detection of African Swine Fever Virus in the Tissues of Asymptomatic Pigs in Smallholder Farming Systems along the Kenya–Uganda Border: Implications for Transmission in Endemic Areas and ASF Surveillance in East Africa. J. Gen. Virol. 2017, 98, 1806–1814. [Google Scholar] [CrossRef]
- Ravaomanana, J.; Michaud, V.; Jori, F.; Andriatsimahavandy, A.; Roger, F.; Albina, E.; Vial, L. First Detection of African Swine Fever Virus in Ornithodoros Porcinus in Madagascar and New Insights into Tick Distribution and Taxonomy. Parasites Vectors 2010, 3, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molini, U.; Mushonga, B.; Settypalli, T.B.K.; Dundon, W.G.; Khaiseb, S.; Jago, M.; Cattoli, G.; Lamien, C.E. Molecular Characterization of African Swine Fever Virus from Outbreaks in Namibia in 2018. Transbound. Emerg. Dis. 2020, 67, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Samkange, A.; Mushonga, B.; Mudimba, D.; Chiwome, B.A.; Jago, M.; Kandiwa, E.; Bishi, A.S.; Molini, U. African Swine Fever Outbreak at a Farm in Central Namibia. Case Rep. Vet. Med. 2019, 7, 3619593. [Google Scholar] [CrossRef] [PubMed]
- Arnot, L.F.; du Toit, J.T.; Bastos, A.D.S. Molecular Monitoring of African Swine Fever Virus Using Surveys Targeted at Adult Ornithodoros Ticks: A Re-Evaluation of Mkuze Game Reserve, South Africa. Onderstepoort J. Vet. Res. 2009, 76, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical Diagnosis of African Swine Fever in Contact-Exposed Swine by a Real-Time PCR Assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Van Rensburg, L.J.; Etter, E.; Heath, L.; Penrith, M.-L.; van Heerden, J. Understanding African Swine Fever Outbreaks in Domestic Pigs in a Sylvatic Endemic Area: The Case of the South African Controlled Area between 1977–2017. Transbound. Emerg. Dis. 2020, 67, 2753–2769. [Google Scholar] [CrossRef]
- Yona, C.M.; Vanhee, M.; Simulundu, E.; Makange, M.; Nauwynck, H.J.; Misinzo, G. Persistent Domestic Circulation of African Swine Fever Virus in Tanzania, 2015–2017. BMC Vet. Res. 2020, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.; Machuka, E.; Githae, D.; Okoth, E.; Cleaveland, S.; Shirima, G.; Kusiluka, L.; Pelle, R. Detection of African Swine Fever Virus Genotype XV in a Sylvatic Cycle in Saadani National Park, Tanzania. Transbound. Emerg. Dis. 2020, 68, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Njau, E.P.; Entfellner, J.-B.D.; Machuka, E.M.; Bochere, E.N.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Upton, C.; Bishop, R.P.; Pelle, R.; et al. The First Genotype II African Swine Fever Virus Isolated in Africa Provides Insight into the Current Eurasian Pandemic. Sci. Rep. 2021, 11, 13081. [Google Scholar] [CrossRef]
- Chang’a, J.S.; Mayenga, C.; Settypalli, T.B.K.; Achenbach, J.E.; Mwanandota, J.J.; Magidanga, B.; Cattoli, G.; Jeremiah, M.; Kamigwe, A.; Guo, S.; et al. Symptomatic and Asymptomatic Cases of African Swine Fever in Tanzania. Transbound. Emerg. Dis. 2019, 66, 2402–2410. [Google Scholar] [CrossRef]
- Masembe, C.; Sreenu, V.B.; Filipe, A.D.S.; Wilkie, G.S.; Ogweng, P.; Mayega, F.J.; Muwanika, V.B.; Biek, R.; Palmarini, M.; Davison, A.J. Genome Sequences of Five African Swine Fever Virus Genotype IX Isolates from Domestic Pigs in Uganda. Microbiol. Resour. Announc. 2018, 7, e01018-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norbert Mwiine, F.; Nkamwesiga, J.; Ndekezi, C.; Ochwo, S. Molecular Characterization of African Swine Fever Viruses from Outbreaks in Peri-Urban Kampala, Uganda. Adv. Virol. 2019, 2019, 1463245. [Google Scholar] [CrossRef] [PubMed]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative Analysis of African Swine Fever Virus Genotypes and Serogroups. Emerg. Infect. Dis. 2015, 21, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, S.; Williamson, A.-L.; Malesa, R.; van Heerden, J.; Boshoff, C.I.; Bastos, A.D.S.; Heath, L.; Carulei, O. Genome Sequences of Three African Swine Fever Viruses of Genotypes I, III, and XXII from South Africa and Zambia, Isolated from Ornithodoros Soft Ticks. Microbiol. Resour. Announc. 2020, 9, e01376-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoromo, J.; Simulundu, E.; Chambaro, H.M.; Mataa, L.; Lubaba, C.H.; Pandey, G.S.; Takada, A.; Misinzo, G.; Mweene, A.S. Diagnosis and Genotyping of African Swine Fever Viruses from 2015 Outbreaks in Zambia. Onderstepoort J. Vet. Res. 2016, 83, a1095. [Google Scholar] [CrossRef] [PubMed]
- Simulundu, E.; Chambaro, H.M.; Sinkala, Y.; Kajihara, M.; Ogawa, H.; Mori, A.; Ndebe, J.; Dautu, G.; Mataa, L.; Lubaba, C.H.; et al. Co-Circulation of Multiple Genotypes of African Swine Fever Viruses among Domestic Pigs in Zambia (2013–2015). Transbound. Emerg. Dis. 2018, 65, 114–122. [Google Scholar] [CrossRef]
- Simulundu, E.; Sinkala, Y.; Chambaro, H.M.; Chinyemba, A.; Banda, F.; Mooya, L.E.; Ndebe, J.; Chitanga, S.; Makungu, C.; Munthali, G.; et al. Genetic Characterisation of African Swine Fever Virus from 2017 Outbreaks in Zambia: Identification of P72 Genotype II Variants in Domestic Pigs. Onderstepoort J. Vet. Res. 2018, 85, a1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Heerden, J.; Malan, K.; Gadaga, B.M.; Spargo, R.M. Reemergence of African Swine Fever in Zimbabwe, 2015. Emerg. Infect. Dis. J. 2017, 23. [Google Scholar] [CrossRef]
- Ogweng, P. The Role of the Bushpigs in the Epidemiology of African Swine Fever at the Wildlife-Livestock Interfaces in Uganda. Master’s Thesis, Makerere University, Kampala, Uganda, 2017. [Google Scholar]
- Bastos, A.D.S.; Arnot, L.F.; Jacquier, M.D.; Maree, S. A Host Species-Informative Internal Control for Molecular Assessment of African Swine Fever Virus Infection Rates in the African Sylvatic Cycle Ornithodoros Vector. Med. Vet. Entomol. 2009, 23, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Katale, B.; Fyumagwa, R.; Mdaki, M.; Hoare, R. Prevalence of African Swine Fever Virus in Warthogs in the Serengeti Ecosystem, Tanzania. Res. Opin. Anim. Vet. Sci. 2012, 2, 339–343. [Google Scholar]
- Etter, E.M.C.; Seck, I.; Grosbois, V.; Jori, F.; Blanco, E.; Vial, L.; Akakpo, A.J.; Bada-Alhambedji, R.; Kone, P.; Roger, F.L. Seroprevalence of African Swine Fever in Senegal, 2006. Emerg. Infect. Dis. J. 2011, 17. [Google Scholar] [CrossRef]
- Penrith, M.L.; Thomson, G.R.; Bastos, A.D.S.; Phiri, O.C.; Lubisi, B.A.; Du Plessis, E.C.; Macome, F.; Pinto, F.; Botha, B.; Esterhuysen, J.J. An Investigation into Natural Resistance to African Swine Fever in Domestic Pigs from an Endemic Area in Southern Africa. Rev. Sci. Tech. 2004, 23, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, K.; Sternberg-Lewerin, S.; Blome, S.; Viltrop, A.; Penrith, M.-L.; Chenais, E. Lack of Evidence for Long Term Carriers of African Swine Fever Virus—A Systematic Review. Virus Res. 2019, 272, 197725. [Google Scholar] [CrossRef]
- OIE—World Organisation for Animal Health. African Swine Fever. Available online: http://www.oie.int/en/animal-health-in-the-world/animal-diseases/african-swine-fever/ (accessed on 26 June 2019).
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E.; Dietze, K. Transboundary Spread of Pig Diseases: The Role of International Trade and Travel. BMC Vet. Res. 2019, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Penrith, M. African Swine Fever. Onderstepoort J. Vet. Res. 2009, 95, 91–95. [Google Scholar]
- Simulundu, E.; Lubaba, C.H.; van Heerden, J.; Kajihara, M.; Mataa, L.; Chambaro, H.M.; Sinkala, Y.; Munjita, S.M.; Munang’andu, H.M.; Nalubamba, K.S.; et al. The Epidemiology of African Swine Fever in “Nonendemic” Regions of Zambia (1989–2015): Implications for Disease Prevention and Control. Viruses 2017, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Alcrudo, D.; Gallardo, M.A.A.C.; Kramer, S.A.; Penrith, M.L.; Kamata, A.; Wiersma, L. African swine fever: Detection and diagnosis—A manual for veterinarians. FAO Anim. Prod. Health Man. 2017, 19, 88. [Google Scholar]
- Chenais, E.; Sternberg-Lewerin, S.; Boqvist, S.; Liu, L.; LeBlanc, N.; Aliro, T.; Masembe, C.; Ståhl, K. African Swine Fever Outbreak on a Medium-Sized Farm in Uganda: Biosecurity Breaches and within-Farm Virus Contamination. Trop. Anim. Health Prod. 2017, 49, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nantima, N.; Davies, J.; Dione, M.; Ocaido, M.; Okoth, E.; Mugisha, A.; Bishop, R. Enhancing Knowledge and Awareness of Biosecurity Practices for Control of African Swine Fever among Smallholder Pig Farmers in Four Districts along the Kenya–Uganda Border. Trop. Anim. Health Prod. 2016, 48, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, B.; Perez, A.M.; Feliziani, F.; Rolesu, S.; Mur, L.; Sánchez-Vizcaíno, J.M. Evaluation of the Risk Factors Contributing to the African Swine Fever Occurrence in Sardinia, Italy. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Mur, L.; Martínez-López, B.; Sánchez-Vizcaíno, J.M. Risk of African Swine Fever Introduction into the European Union through Transport-Associated Routes: Returning Trucks and Waste from International Ships and Planes. BMC Vet. Res. 2012, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artois, M. The role of wildlife in the control of domestic animal diseases. OIE Comm. Régionale Eur. 2012, 8. [Google Scholar]
- Barongo, M.B.; Bishop, R.P.; Fèvre, E.M.; Knobel, D.L.; Ssematimba, A. A Mathematical Model That Simulates Control Options for African Swine Fever Virus (ASFV). PLoS ONE 2016, 11, e0158658. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Edwards, L.; Batten, C.A. Virological Diagnosis of African Swine Fever—Comparative Study of Available Tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, M.J.; Brooks, T.J.G.; Pollock, N.R. Diagnosis of Ebola Virus Disease: Past, Present, and Future. Clin. Microbiol. Rev. 2016, 29, 773–793. [Google Scholar] [CrossRef] [Green Version]
- Pollock, N.R.; Wonderly, B. Evaluating Novel Diagnostics in an Outbreak Setting: Lessons Learned from Ebola. J. Clin. Microbiol. 2017, 55, 1255–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, J.; O’Donnell, V.; Alfano, M.; Velazquez Salinas, L.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Higgs, S.; Borca, M.V. Association of the Host Immune Response with Protection Using a Live Attenuated African Swine Fever Virus Model. Viruses 2016, 8, 291. [Google Scholar] [CrossRef]
- Krug, P.W.; Holinka, L.G.; O’Donnell, V.; Reese, B.; Sanford, B.; Fernandez-Sainz, I.; Gladue, D.P.; Arzt, J.; Rodriguez, L.; Risatti, G.R.; et al. The Progressive Adaptation of a Georgian Isolate of African Swine Fever Virus to Vero Cells Leads to a Gradual Attenuation of Virulence in Swine Corresponding to Major Modifications of the Viral Genome. J. Virol. 2015, 89, 2324–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanford, B.; Holinka, L.G.; O’Donnell, V.; Krug, P.W.; Carlson, J.; Alfano, M.; Carrillo, C.; Wu, P.; Lowe, A.; Risatti, G.R.; et al. Deletion of the Thymidine Kinase Gene Induces Complete Attenuation of the Georgia Isolate of African Swine Fever Virus. Virus Res. 2016, 213, 165–171. [Google Scholar] [CrossRef]
- Gallardo, C.; Nurmoja, I.; Soler, A.; Delicado, V.; Simón, A.; Martin, E.; Perez, C.; Nieto, R.; Arias, M. Evolution in Europe of African Swine Fever Genotype II Viruses from Highly to Moderately Virulent. Vet. Microbiol. 2018, 219, 70–79. [Google Scholar] [CrossRef]
- King, K.; Chapman, D.; Argilaguet, J.M.; Fishbourne, E.; Hutet, E.; Cariolet, R.; Hutchings, G.; Oura, C.A.L.; Netherton, C.L.; Moffat, K.; et al. Protection of European Domestic Pigs from Virulent African Isolates of African Swine Fever Virus by Experimental Immunisation. Vaccine 2011, 29, 4593–4600. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.L.; Abrams, C.C.; Goatley, L.C.; Netherton, C.; Chapman, D.G.; Sanchez-Cordon, P.; Dixon, L.K. Deletion of African Swine Fever Virus Interferon Inhibitors from the Genome of a Virulent Isolate Reduces Virulence in Domestic Pigs and Induces a Protective Response. Vaccine 2016, 34, 4698–4705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Cordón, P.J.; Chapman, D.; Jabbar, T.; Reis, A.L.; Goatley, L.; Netherton, C.L.; Taylor, G.; Montoya, M.; Dixon, L. Different Routes and Doses Influence Protection in Pigs Immunised with the Naturally Attenuated African Swine Fever Virus Isolate OURT88/3. Antivir. Res. 2017, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, E.; van Heerden, J.; Bosch-Camós, L.; Accensi, F.; Navas, M.J.; López-Monteagudo, P.; Argilaguet, J.; Gallardo, C.; Pina-Pedrero, S.; Salas, M.L.; et al. Live Attenuated African Swine Fever Viruses as Ideal Tools to Dissect the Mechanisms Involved in Cross-Protection. Viruses 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Steinaa, L.; Bishop, R.; Okoth, E.; Svitek, N.; Riitho, V. Pig Vaccines and Diagnostics for African Swine Fever: The Case of Uganda. Res. Program Livest. Fish 2016, 26, 4. [Google Scholar]
- Gaudreault, N.N.; Richt, J.A. Subunit Vaccine Approaches for African Swine Fever Virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goatley, L.C.; Reis, A.L.; Portugal, R.; Goldswain, H.; Shimmon, G.L.; Hargreaves, Z.; Ho, C.-S.; Montoya, M.; Sánchez-Cordón, P.J.; Taylor, G.; et al. A Pool of Eight Virally Vectored African Swine Fever Antigens Protect Pigs against Fatal Disease. Vaccines 2020, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C.; et al. Identification and Immunogenicity of African Swine Fever Virus Antigens. Front. Immunol. 2019, 10, 1318. [Google Scholar] [CrossRef] [Green Version]
- Jancovich, J.K.; Chapman, D.; Hansen, D.T.; Robida, M.D.; Loskutov, A.; Craciunescu, F.; Borovkov, A.; Kibler, K.; Goatley, L.; King, K.; et al. Immunization of Pigs by DNA Prime and Recombinant Vaccinia Virus Boost To Identify and Rank African Swine Fever Virus Immunogenic and Protective Proteins. J. Virol. 2018, 92, e02219-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokhandwala, S.; Petrovan, V.; Popescu, L.; Sangewar, N.; Elijah, C.; Stoian, A.; Olcha, M.; Ennen, L.; Bray, J.; Bishop, R.P.; et al. Adenovirus-Vectored African Swine Fever Virus Antigen Cocktails Are Immunogenic but Not Protective against Intranasal Challenge with Georgia 2007/1 Isolate. Vet. Microbiol. 2019, 235, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.-Y.; Pérez-Núñez, D.; Morozov, I.; Sánchez, E.G.; Gaudreault, N.N.; Trujillo, J.D.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-Protein Vaccination Strategy Does Not Protect from Challenge with African Swine Fever Virus Armenia 2007 Strain. Vaccines 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Penrith, M.; Bastos, A.D.; Etter, E.M.C.; Beltrán-Alcrudo, D. Epidemiology of African Swine Fever in Africa Today: Sylvatic Cycle versus Socio-economic Imperatives. Transbound. Emerg. Dis. 2019, 66, 672–686. [Google Scholar] [CrossRef]
- Jori, F.; Vial, L.; Penrith, M.L.; Pérez-Sánchez, R.; Etter, E.; Albina, E.; Michaud, V.; Roger, F. Review of the Sylvatic Cycle of African Swine Fever in Sub-Saharan Africa and the Indian Ocean. Virus Res. 2013, 173, 212–227. [Google Scholar] [CrossRef] [PubMed]
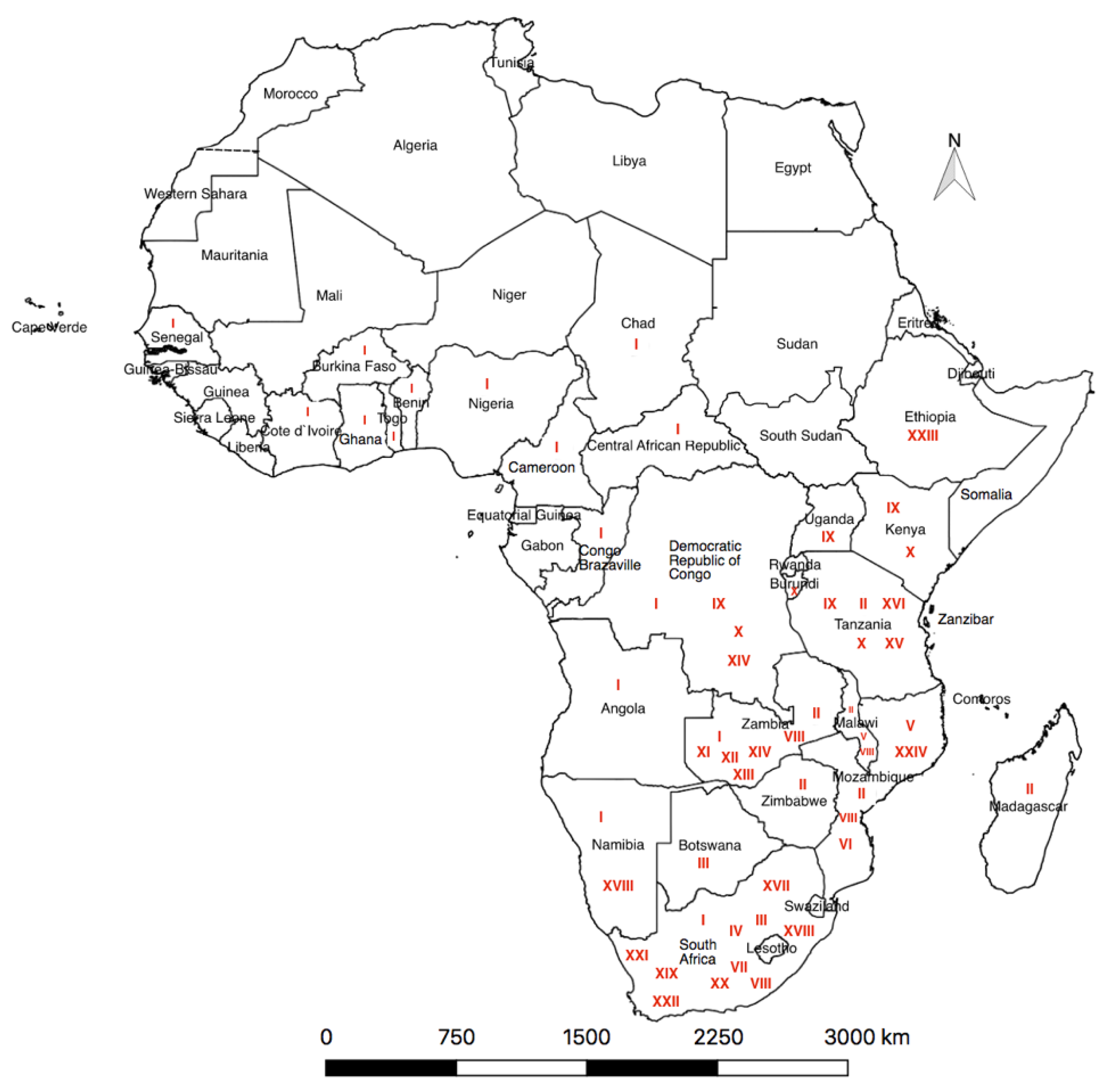


| Country | Genotype | Sample Source(s) | Year of Study/ Collection | Reference(s) |
|---|---|---|---|---|
| Burundi | X | Domestic pigs | 2018 | [16] |
| Cameroon | I | Domestic pigs | 2010–2018 | [51] |
| Cameroon | I | Domestic pigs | 2018 | [52] |
| Democratic Republic of Congo (DRC) | IX | Domestic pigs | 2009 | [23] |
| DRC | X | Domestic pigs | 2016 | [53] |
| DRC | IX | Domestic pigs | 2011 | [54] |
| DRC | I, IX, XIV | Domestic pigs | 2005–2012 | [25] |
| Ethiopia | XXIII | Domestic pigs | 2011 | [10] |
| Ivory Coast | I | Domestic pigs | 2014 | [55] |
| Ivory Coast | I | Domestic pigs | 2008–2013 | [13] |
| Kenya | IX | Domestic pigs | 2006–2007 | [56] |
| Kenya | IX | Domestic pigs | 2007 | [46] |
| Kenya | X, IX | Domestic pigs, soft ticks and warthogs | 2005 | [23] |
| Kenya | IX, X | Domestic pigs | 2008–2009 | [57] |
| Kenya | IX, X | Soft tick, Domestic pigs | 2006 | [58] |
| Kenya, Uganda | IX | Domestic pigs | 2011–2013 | [59] |
| Kenya-Uganda | IX | Domestic pigs | Not mentioned | [60] |
| Madagascar | Not mentioned | Soft ticks | 2007–2008 | [61] |
| Madagascar, West Africa, and Mozambique | I, VIII | Domestic pigs | 2000 | [9] |
| Malawi | I, II | Domestic pigs | 2019 | [44] |
| Malawi | V, VIII | Warthog, Domestic pigs | 1960 | |
| Malawi, Mozambique, Zambia | VIII, V | Domestic pigs | 2001–2003 | [18] |
| Mauritius | II | Domestic pigs | 2007–2008 | [49] |
| Mozambique | II, V, XXIV | Soft ticks | 2007 | [11] |
| Mozambique | II, VIII, V, VI | Domestic pigs | 1998 | [44] |
| Namibia | I and XVIII | Domestic pigs | 2018 | [62] |
| Namibia | Not mentioned | Domestic pigs | 2018 | [63] |
| Nigeria | I | Domestic pigs | 2007–2015 | [14] |
| South Africa | III, XX | Soft ticks | 1985–1987 | [64] |
| South Africa | III, XIX, XX, XXI | Soft ticks | 1987–1996 | [65] |
| South Africa | I, III, IV, VII, VIII, XIX, XX, XXI and XXII | Soft ticks, Warthogs | 1987–2003 | [66] |
| Swaziland, South Africa | XIX, VII, XVII–XXII | Soft ticks | 1973–1999 | [48] |
| Tanzania | II, IX, X | Domestic pigs | 2015–2017 | [67] |
| Tanzania | XV | Soft ticks | 2017 | [68] |
| Tanzania | II, IX | Domestic pigs | 2015, 2017 | [69,70] |
| Tanzania | X | Domestic pigs | 2013 | [42] |
| Tanzania | XV | Domestic pigs | 2008 | [19] |
| Tanzania | X | Domestic pig | 2009 | [20] |
| Tanzania | XVI | Domestic pigs | 2001 | [21] |
| Uganda | IX | Domestic pigs | 2007 | [23] |
| Uganda | IX | Domestic Pigs | 2015 | [71] |
| Uganda | IX | Domestic pigs | 2015 | [72] |
| Uganda | IX | Domestic pigs | 2010–2013 | [22] |
| Zaire (DRC) | I | Domestic pigs | 1974–1989 | [73] |
| Zaire, South Africa | IV, XX | Domestic pigs, warthogs | 1977—Zaire 1999—S. Africa | [74] |
| Zambia | I | Domestic pigs | 2015 | [75] |
| Zambia | I, II, XI, XII, XIII, XIV | Domestic pigs | 2013–2015 | [76] |
| Zambia | II | Domestic pigs | 2017 | [77] |
| Zambia | VIII | Domestic pig | 1988 | [44] |
| Zambia, South Africa | I, III, XXII | Soft ticks | 1983—Zambia 2008—S. Africa | [74] |
| Zimbabwe | II | Domestic pigs | 2015 | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses 2021, 13, 2285. https://doi.org/10.3390/v13112285
Njau EP, Machuka EM, Cleaveland S, Shirima GM, Kusiluka LJ, Okoth EA, Pelle R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses. 2021; 13(11):2285. https://doi.org/10.3390/v13112285
Chicago/Turabian StyleNjau, Emma P., Eunice M. Machuka, Sarah Cleaveland, Gabriel M. Shirima, Lughano J. Kusiluka, Edward A. Okoth, and Roger Pelle. 2021. "African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa" Viruses 13, no. 11: 2285. https://doi.org/10.3390/v13112285
APA StyleNjau, E. P., Machuka, E. M., Cleaveland, S., Shirima, G. M., Kusiluka, L. J., Okoth, E. A., & Pelle, R. (2021). African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses, 13(11), 2285. https://doi.org/10.3390/v13112285






