Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling
Abstract
:1. Introduction
2. Materials and Methods
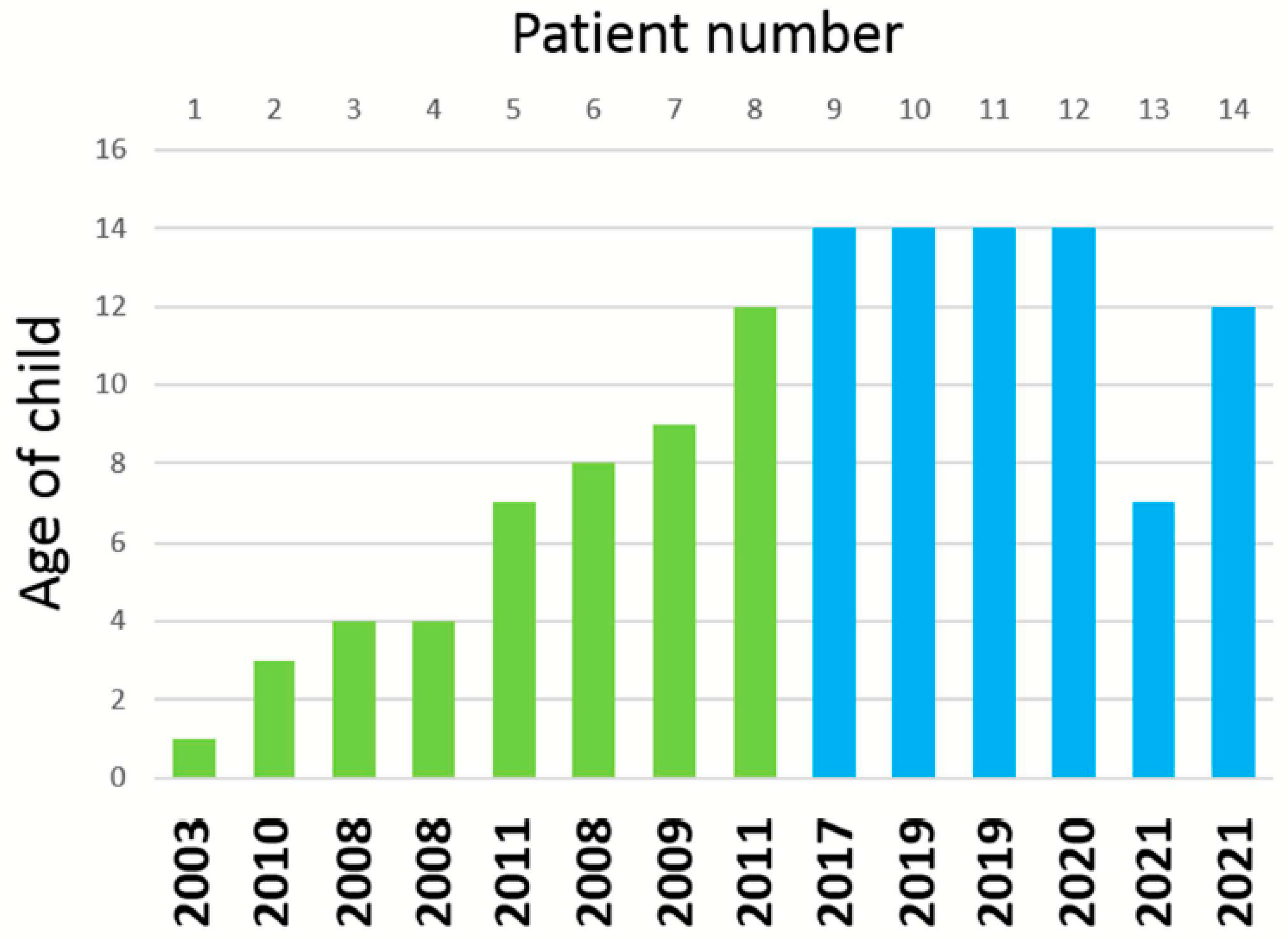
2.1. Patients and Ethics Statement
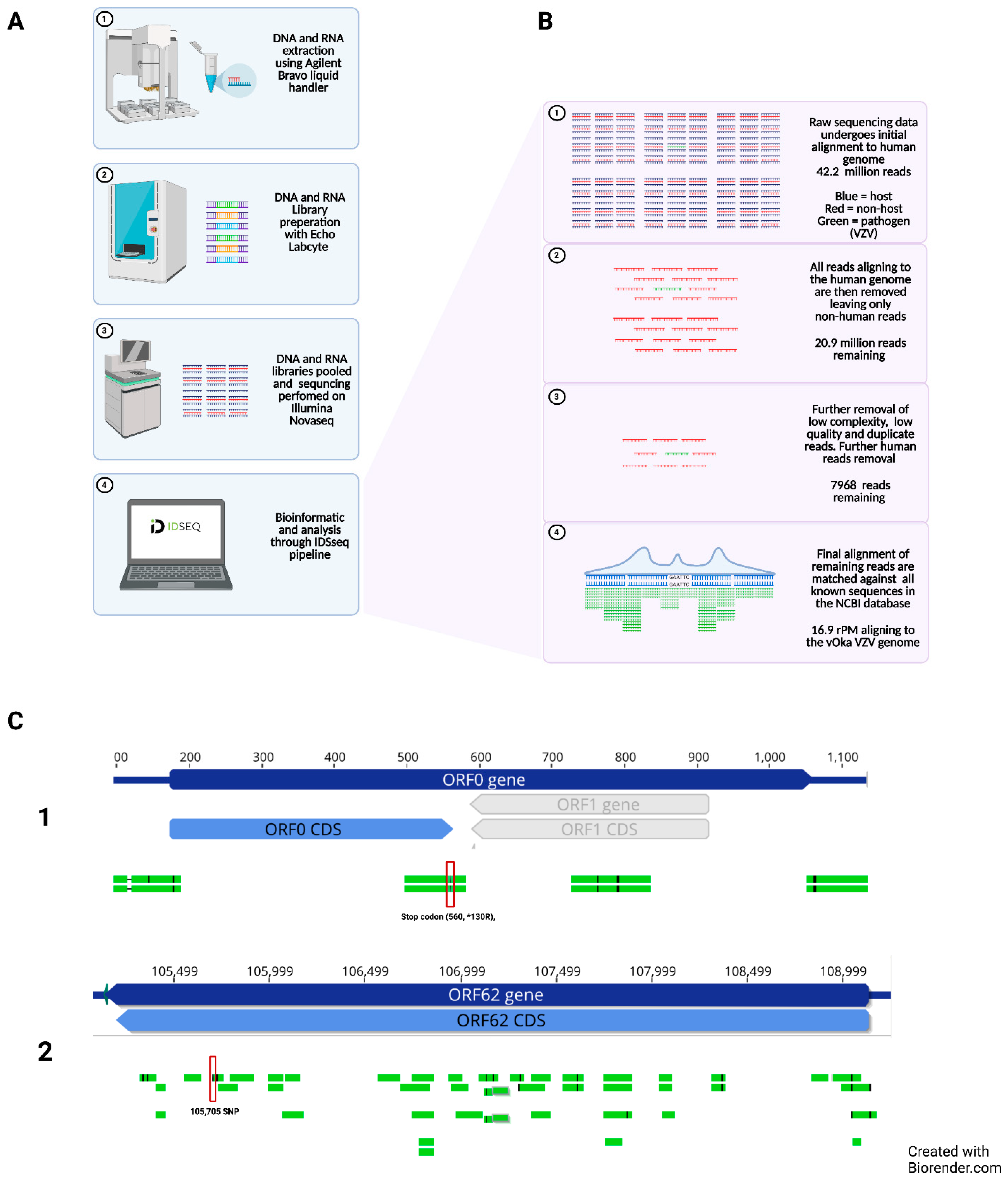
2.2. Next Generation Sequencing of Cerebrospinal Fluid
2.3. Sanger Sequencing
2.4. Immunology Exome Analysis
2.5. Measurement of Viral Antibody
2.6. Measurement of Chemokines and Interleukins
3. Results
3.1. Varicella Vaccine Meningitis Case 13
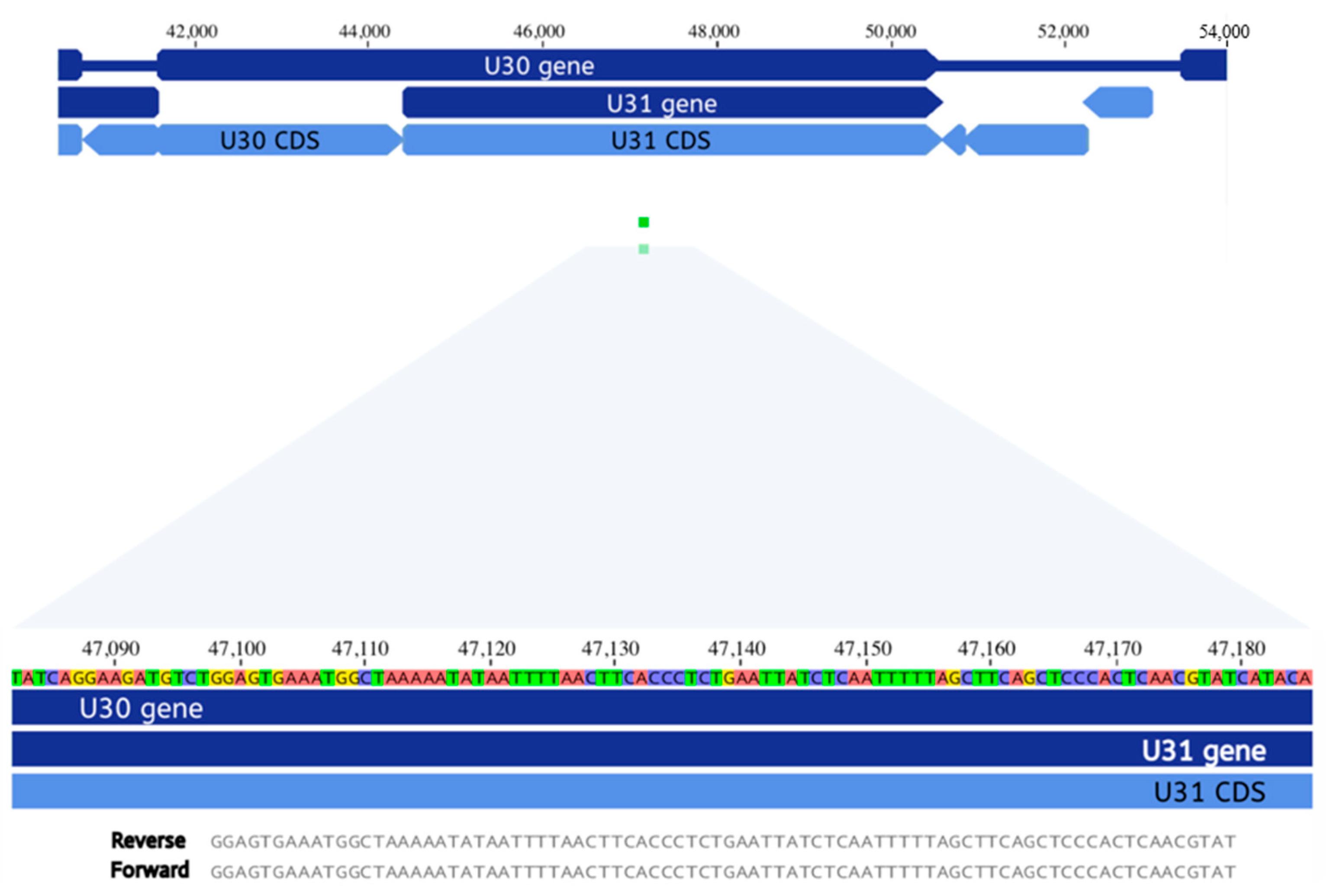
3.2. Metagenomic Next Generation Sequencing of Cerebrospinal Fluid
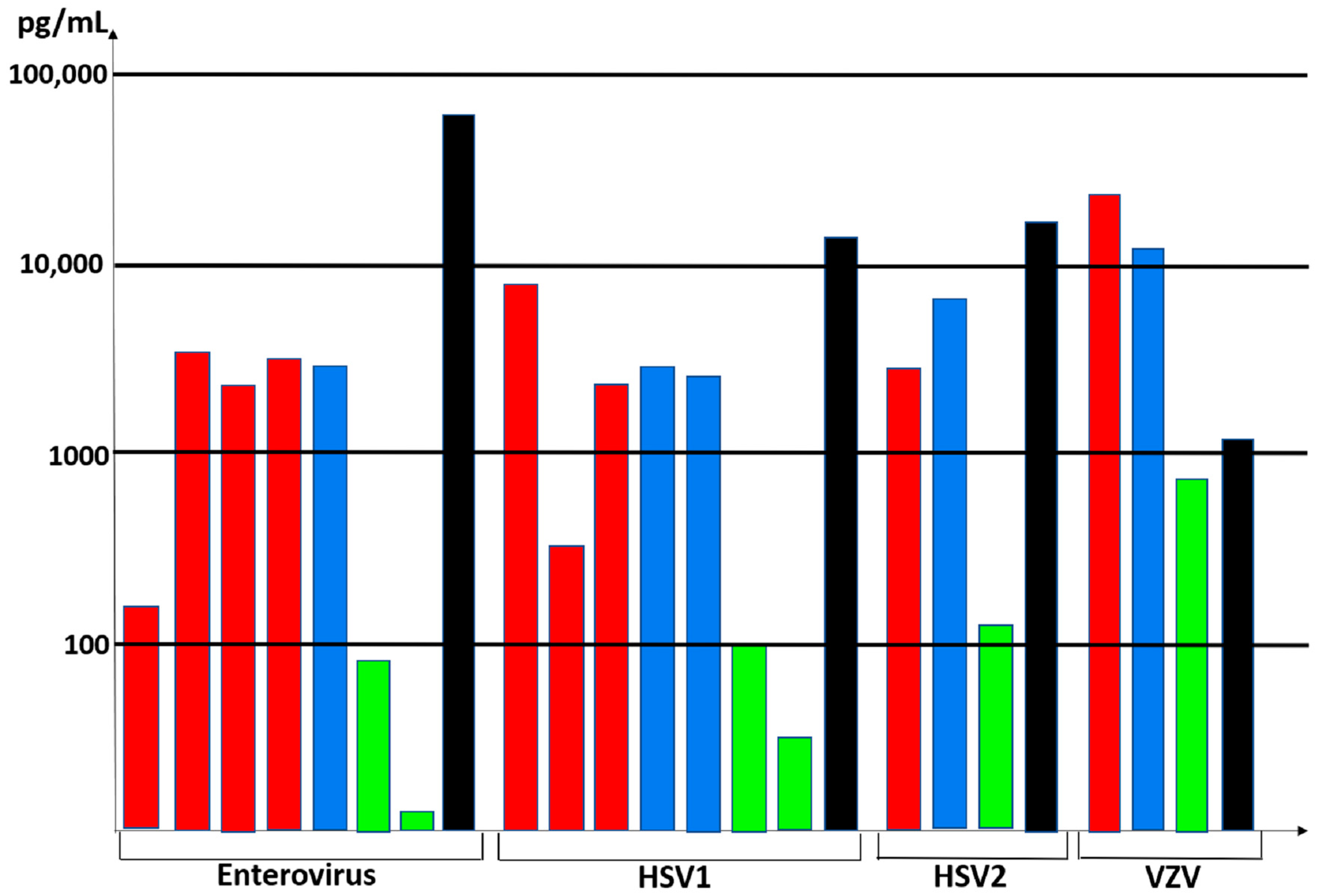
3.3. Viral Antibody Studies
3.4. Corticosteroid Burst Treatment Prior to Varicella Vaccine Meningitis
3.5. Cytokine Profiling in the Cerebrospinal Fluid
3.6. Case 14 and Immunology Exome Sequencing
4. Discussion
4.1. Corticosteroids and VZV Reactivation
4.2. Specificity of mNGS and the Biofire Filmarray Encephalitis Panel
4.3. Cytokine Profiles in CSF after Neurotropic Virus Infection
4.4. Elevated CXCL10 in CSF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Krause, P.R.; Klinman, D.M. Varicella vaccination: Evidence for frequent reactivation of the vaccine strain in healthy children. Nat. Med. 2000, 6, 451–454. [Google Scholar] [CrossRef]
- Harpaz, R.; Leung, J.W. The Epidemiology of Herpes Zoster in the United States during the Era of Varicella and Herpes Zoster Vaccines: Changing Patterns among Children. Clin. Infect. Dis. 2019, 69, 345–347. [Google Scholar] [CrossRef] [Green Version]
- Pahud, B.A.; Glaser, C.A.; Dekker, C.L.; Arvin, A.M.; Schmid, D.S. Varicella zoster disease of the central nervous system: Epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J. Infect. Dis. 2011, 203, 316–323. [Google Scholar] [CrossRef]
- Horien, C.; Grose, C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin. Pediatr. Neurol. 2012, 19, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusel, E.H.; Grose, C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses 2020, 12, 1078. [Google Scholar] [CrossRef]
- Ramachandran, V.; Elliott, S.C.; Rogers, K.L.; Cohrs, R.J.; Weinberger, M.; Jackson, W.; Carpenter, J.E.; Grose, C.; Bonthius, D.J. Varicella Vaccine Meningitis as a Complication of Herpes Zoster in Twice-Immunized Immunocompetent Adolescents. J. Child Neurol. 2020, 35, 889–895. [Google Scholar] [CrossRef]
- Wilson, M.R.; Sample, H.A.; Zorn, K.C.; Arevalo, S.; Yu, G.; Neuhaus, J.; Federman, S.; Stryke, D.; Briggs, B.; Langelier, C.; et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 2019, 380, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; O’Donovan, B.D.; Gelfand, J.M.; Sample, H.A.; Chow, F.C.; Betjemann, J.P.; Shah, M.P.; Richie, M.B.; Gorman, M.P.; Hajj-Ali, R.A.; et al. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol. 2018, 75, 947–955. [Google Scholar] [CrossRef]
- Quinlivan, M.L.; Jensen, N.J.; Radford, K.W.; Schmid, D.S. Novel genetic variation identified at fixed loci in ORF62 of the Oka varicella vaccine and in a case of vaccine-associated herpes zoster. J. Clin. Microbiol. 2012, 50, 1533–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, D.S. Varicella-zoster virus vaccine: Molecular genetics. Curr. Top. Microbiol. Immunol. 2010, 342, 323–340. [Google Scholar] [CrossRef]
- Baleydier, F.; Ranza, E.; Schappi, M.; Rougemont, A.L.; Merlini, L.; Ansari, M.; Blanchard-Rohner, G. Activated Phosphoinositide 3 Kinase Delta Syndrome (APDS): A Primary Immunodeficiency Mimicking Lymphoma. J. Pediatr. Hematol. Oncol. 2019, 41, e521–e524. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [Green Version]
- Williams, V.; Gershon, A.; Brunell, P.A. Serologic response to Varicella-zoster membrane antigens measured by direct immunofluorescence. J. Infect. Dis. 1974, 130, 669–672. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Asano, Y.; Kobayashi, I.; Nakashima, T.; Yazaki, T.; Suga, S.; Ozaki, T.; Wyatt, L.S.; Frenkel, N. Seroepidemiology of human herpesvirus 7 in healthy children and adults in Japan. J. Med. Virol. 1993, 41, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; McLucas, B.C.; Montoya, L.A.; Brotski, C.M.; Das, S.; Miholits, M.; Sebata, T.H. Multiplexing protein and gene level measurements on a single Luminex platform. Methods 2019, 158, 27–32. [Google Scholar] [CrossRef]
- Gomi, Y.; Sunamachi, H.; Mori, Y.; Nagaike, K.; Takahashi, M.; Yamanishi, K. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 2002, 76, 11447–11459. [Google Scholar] [CrossRef] [Green Version]
- Peters, G.A.; Tyler, S.D.; Carpenter, J.E.; Jackson, W.; Mori, Y.; Arvin, A.M.; Grose, C. The attenuated genotype of Varicella-Zoster virus includes an ORF0 transitional stop codon mutation. J. Virol. 2012, 86, 10695–10703. [Google Scholar] [CrossRef] [Green Version]
- Depledge, D.P.; Yamanishi, K.; Gomi, Y.; Gershon, A.A.; Breuer, J. Deep Sequencing of Distinct Preparations of the Live Attenuated Varicella-Zoster Virus Vaccine Reveals a Conserved Core of Attenuating Single-Nucleotide Polymorphisms. J. Virol. 2016, 90, 8698–8704. [Google Scholar] [CrossRef] [Green Version]
- Kotani, N.; Kudo, R.; Sakurai, Y.; Sawamura, D.; Sessler, D.I.; Okada, H.; Nakayama, H.; Yamagata, T.; Yasujima, M.; Matsuki, A. Cerebrospinal fluid interleukin 8 concentrations and the subsequent development of postherpetic neuralgia. Am. J. Med. 2004, 116, 318–324. [Google Scholar] [CrossRef]
- Fukushima, K.; Ishiguro, A.; Shimbo, T. Transient elevation of granulocyte colony-stimulating factor levels in cerebrospinal fluid at the initial stage of aseptic meningitis in children. Pediatr. Res. 1995, 37, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Carter-Timofte, M.E.; Paludan, S.R.; Mogensen, T.H. RNA Polymerase III as a Gatekeeper to Prevent Severe VZV Infections. Trends Mol. Med. 2018, 24, 904–915. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Jouanguy, E.; Ugolini, S.; Smahi, A.; Elain, G.; Romero, P.; Segal, D.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A.; et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007, 317, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Tomlinson, A.H. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J. Neurol. Sci. 1972, 15, 35–48. [Google Scholar] [CrossRef]
- LaGuardia, J.J.; Cohrs, R.J.; Gilden, D.H. Prevalence of Varicella-Zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J. Virol. 1999, 73, 8571–8577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddman, R.; Edvinsson, L.; Hara, H. Axonal tracing of autonomic nerve fibers to the superficial temporal artery in the rat. Cell Tissue Res. 1989, 256, 559–565. [Google Scholar] [CrossRef]
- Levin, M.J.; Dahl, K.M.; Weinberg, A.; Giller, R.; Patel, A.; Krause, P.R. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of Varicella-Zoster virus, in an immunosuppressed child. J. Infect. Dis. 2003, 188, 954–959. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; James, A.; Kung, E.; Madhavan, V. A Case of Herpes Zoster and Meningitis in a Twice-Vaccinated Healthy Adolescent. J. Pediatr. Infect. Dis. 2017, 12, 142–144. [Google Scholar] [CrossRef] [Green Version]
- Harrington, W.E.; Mato, S.; Burroughs, L.; Carpenter, P.A.; Gershon, A.; Schmid, D.S.; Englund, J.A. Vaccine Oka Varicella Meningitis in Two Adolescents. Pediatrics 2019, 144, e20191522. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.C.; Wang, J.Y.; Chang, S.M.; Chang, Y.C.; Tsai, Y.F.; Wu, A.C.; Huang, J.L.; Tsai, H.J. Association of Oral Corticosteroid Bursts with Severe Adverse Events in Children. JAMA Pediatr. 2021, 175, 723–729. [Google Scholar] [CrossRef]
- Shee, J.C.; Fehrsen, P. Reactivation of varicella virus by cortisone therapy. Br. Med. J. 1953, 2, 82. [Google Scholar] [CrossRef] [Green Version]
- Good, R.A.; Smith, R.T.; Vernier, R.L. Serious untoward reactions to therapy with cortisone and adrenocorticotropin in pediatric practice. II. Pediatrics 1957, 19, 272–284. [Google Scholar]
- Price, N.B.; Grose, C. Corticosteroids Contribute to Serious Adverse Events Following Live Attenuated Varicella Vaccination and Live Attenuated Zoster Vaccination. Vaccines 2021, 9, 23. [Google Scholar] [CrossRef]
- Leber, A.L.; Everhart, K.; Balada-Llasat, J.M.; Cullison, J.; Daly, J.; Holt, S.; Lephart, P.; Salimnia, H.; Schreckenberger, P.C.; DesJarlais, S.; et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J. Clin. Microbiol. 2016, 54, 2251–2261. [Google Scholar] [CrossRef] [Green Version]
- Pandey, U.; Greninger, A.L.; Levin, G.R.; Jerome, K.R.; Anand, V.C.; Dien Bard, J. Pathogen or Bystander: Clinical Significance of Detecting Human Herpesvirus 6 in Pediatric Cerebrospinal Fluid. J. Clin. Microbiol. 2020, 58, 5. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Pereira, M.; Miko, B.; Radmard, S.; Whittier, S.; Thakur, K. Clinical Significance of Human Herpesvirus 6 Positivity on the FilmArray Meningitis/Encephalitis Panel. Clin. Infect. Dis. 2018, 67, 1125–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, J. Determination and analysis of the complete nucleotide sequence of human herpesvirus. J. Virol. 1996, 70, 5975–5989. [Google Scholar] [CrossRef] [Green Version]
- Parisi, S.G.; Basso, M.; Del Vecchio, C.; Andreis, S.; Franchin, E.; Bello, F.D.; Pagni, S.; Biasolo, M.A.; Manganelli, R.; Barzon, L.; et al. Virological testing of cerebrospinal fluid in children aged less than 14 years with a suspected central nervous system infection: A retrospective study on 304 consecutive children from January 2012 to May 2015. Eur. J. Paediatr. Neurol. 2016, 20, 588–596. [Google Scholar] [CrossRef]
- Maric, L.S.; Lepej, S.Z.; Gorenec, L.; Grgic, I.; Trkulja, V.; Rode, O.D.; Roglic, S.; Grmoja, T.; Barisic, N.; Tesovic, G. Chemokines CXCL10, CXCL11, and CXCL13 in acute disseminated encephalomyelitis, non-polio enterovirus aseptic meningitis, and neuroborreliosis: CXCL10 as initial discriminator in diagnostic algorithm? Neurol. Sci. 2018, 39, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Studahl, M.; Persson Berg, L.; Eriksson, K. CXCL11 production in cerebrospinal fluid distinguishes herpes simplex meningitis from herpes simplex encephalitis. J. Neuroinflamm. 2017, 14, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.E.; Song, D.; Shin, K.; Nam, S.O.; Ko, A.; Kong, J.; Kim, Y.M.; Yeon, G.M.; Lee, Y.J. Prospective research of human parechovirus and cytokines in cerebrospinal fluid of young children less than one year with sepsis-like illness: Comparison with enterovirus. J. Clin. Virol. 2019, 119, 11–16. [Google Scholar] [CrossRef]
- Wang, S.M.; Lei, H.Y.; Su, L.Y.; Wu, J.M.; Yu, C.K.; Wang, J.R.; Liu, C.C. Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin. Microbiol. Infect. 2007, 13, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, S.; Cai, C.; Liu, J.; Wang, Y.; Jiang, Y.; Du, L.; Chen, Z. Characterization of inflammatory cytokine profiles in cerebrospinal fluid of hand, foot, and mouth disease children with enterovirus 71-related encephalitis in Hangzhou, Zhejiang, China. Medicine 2019, 98, e18464. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hsia, S.H.; Huang, Y.C.; Wu, C.T.; Chang, L.Y. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin. Infect. Dis. 2003, 36, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Kamei, S.; Taira, N.; Ishihara, M.; Sekizawa, T.; Morita, A.; Miki, K.; Shiota, H.; Kanno, A.; Suzuki, Y.; Mizutani, T.; et al. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine 2009, 46, 187–193. [Google Scholar] [CrossRef]
- Rosler, A.; Pohl, M.; Braune, H.J.; Oertel, W.H.; Gemsa, D.; Sprenger, H. Time course of chemokines in the cerebrospinal fluid and serum during herpes simplex type 1 encephalitis. J. Neurol. Sci. 1998, 157, 82–89. [Google Scholar] [CrossRef]
- Aurelius, E.; Andersson, B.; Forsgren, M.; Skoldenberg, B.; Strannegard, O. Cytokines and other markers of intrathecal immune response in patients with herpes simplex encephalitis. J. Infect. Dis. 1994, 170, 678–681. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Carpenter, J.E.; Buckingham, E.M.; Jackson, W.; Knudtson, K.; Moffat, J.F.; Kita, H.; Grose, C. Cellular Stress Response to Varicella-Zoster Virus Infection of Human Skin Includes Highly Elevated Interleukin-6 Expression. Open Forum Infect. Dis. 2018, 5, ofy118. [Google Scholar] [CrossRef] [Green Version]
- Steain, M.; Gowrishankar, K.; Rodriguez, M.; Slobedman, B.; Abendroth, A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural Varicella-Zoster virus infection. J. Virol. 2011, 85, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Lind, L.; Eriksson, K.; Grahn, A. Chemokines and matrix metalloproteinases in cerebrospinal fluid of patients with central nervous system complications caused by Varicella-Zoster virus. J. Neuroinflamm. 2019, 16, 42. [Google Scholar] [CrossRef]
- Ouwendijk, W.J.; Abendroth, A.; Traina-Dorge, V.; Getu, S.; Steain, M.; Wellish, M.; Andeweg, A.C.; Osterhaus, A.D.; Gilden, D.; Verjans, G.M.; et al. T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J. Virol. 2013, 87, 2979–2982. [Google Scholar] [CrossRef] [Green Version]
- Gershon, A.A.; Arvin, A.M.; Levin, M.J.; Seward, J.F.; Schmid, D.S. Varicella vaccine in the United States: A decade of prevention and the way forward. J. Infect. Dis. 2008, 197 (Suppl. 2), S39–S40. [Google Scholar] [CrossRef] [Green Version]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Primers 2015, 1, 15016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.; Marin, M. Update on trends in varicella mortality during the varicella vaccine era-United States, 1990–2016. Hum. Vaccin Immunother. 2018, 14, 2460–2463. [Google Scholar] [CrossRef] [Green Version]
- Woodward, M.; Marko, A.; Galea, S.; Eagel, B.; Straus, W. Varicella Virus Vaccine Live: A 22-Year Review of Postmarketing Safety Data. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2019; Volume 6. [Google Scholar] [CrossRef] [Green Version]
- Sadaoka, T.; Depledge, D.P.; Rajbhandari, L.; Venkatesan, A.; Breuer, J.; Cohen, J.I. In vitro system using human neurons demonstrates that Varicella-Zoster vaccine virus is impaired for reactivation, but not latency. Proc. Natl. Acad. Sci. USA 2016, 113, E2403–E2412. [Google Scholar] [CrossRef] [Green Version]
- Hope-Simpson, R.E. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grose, C. Pangaea and the Out-of-Africa Model of Varicella-Zoster Virus Evolution and Phylogeography. J. Virol. 2012, 86, 9558–9565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M. Effectiveness of live varicella vaccine. Expert Opin. Biol. Ther. 2004, 4, 199–216. [Google Scholar] [CrossRef]
| Category | Patient 13 | Patient 12 |
|---|---|---|
| Gender | Male | Female |
| 1st Vaccine | 1 Year | 1 Year |
| 2nd Vaccine | 4 Years | 5 Years |
| Meningitis | 7 Years | 14 Years |
| Head Image | Normal | Normal |
| Prior Zoster | No | Yes |
| VZV type | Vaccine | Vaccine |
| Corticosteroids | Prednisolone | Prednisone |
| Time Before Meningitis | 3 Weeks | 4 Weeks |
| Daily Dosage | 20 mg | 20 mg |
| Mg/Kg/Day | 0.8 mg/kg | 0.3 mg/kg |
| Duration | 7 Days | 5 days |
| Reason for Steroids | Poison Ivy | Asthma |
| Follow-up | Recovered | Recovered |
| Cytokine | PT. 13 | PT. 12 | Cytokine | PT. 13 | PT. 12 |
|---|---|---|---|---|---|
| IL-1A | 3.19 | 1.02 | IFNa | 104.44 | 38.79 |
| IL-1RA | 184.63 | 1620.65 | IFNg | 75.76 | 865.85 |
| IL-1b | 6.14 | 3.70 | MIP1a | 39.27 | 117.88 |
| IL-2 | 8.74 | 12.63 | CXCL10 | 1178.58 | 507.83 |
| IL-2R | 104.40 | 79.92 | CCL11 | 3.91 | 2.86 |
| IL-3 | 10.42 | 11.61 | Rantes | 115.95 | 30.76 |
| IL-4 | 16.42 | 23.54 | GM-CSF | 1.74 | 3.21 |
| IL-5 | 43.76 | 11.55 | TNFa | 75.76 | 1.07 |
| IL-6 | 90.57 | 31,816.58 | HGF | 93.61 | 187.21 |
| IL-7 | 10.22 | 11.99 | MIP-1b | 91.13 | 156.86 |
| IL-8 | 4034.30 | 11,604.79 | CCL2 | 616.79 | 3025.46 |
| IL-9 | 8.83 | 6.50 | FGF2 | 28.21 | 15.03 |
| IL-10 | 75.39 | 846.40 | VEGF | 2.35 | 4.36 |
| IL-12 | 72.32 | 39.31 | G-CSF | 55.79 | 171.57 |
| IL-13 | 15.48 | 18.67 | CXCL9 | 81.16 | 273.94 |
| IL-15 | 56.41 | 26.00 | EGF | 48.25 | 42.61 |
| IL-17A | 7.22 | 3.67 | |||
| IL-17F | 348.68 | 72.26 | |||
| IL-22 | 22.12 | 70.20 |
| ACD | C8B | CFP | FADD | IL10RA | LPIN2 | NSMCE3 | RSGRP1 | SPINK5 | TRAC |
| ACP5 | C9 | CFTR | FAS | IL10RB | LRBA | OAS1 | RBCK1 | SPPL2A | TRAF3IP2 |
| ADA | CARD11 | CHD7 | FASLG | IL12B | LYST | ORAI1 | RC3H1 | SRP54 | TREX1 |
| ADA2 | CARD14 | CIB1 | FAT4 | IL12RB1 | MAGT1 | OTULIN | RECQL4 | STAT1 | TRIM22 |
| ADAM17 | CARD9 | CIITA | FCGR3A | IL17F | MALT1 | PARN | RELA | STAT2 | TRNT1 |
| ADAR | CARMIL2 | CLPB | FCHO1 | IL17RA | MAP3K14 | PAX1 | RFX5 | STAT3 | TTC37 |
| AICDA | CASP10 | COPA | FERMT3 | IL17RC | MBL2 | PEPD | RFXANK | STAT5B | TTC7A |
| AIRE | CASP8 | CORO1A | FNIP1 | IL1RN | MCM4 | PGM3 | RFXAP | STIM1 | TYK2 |
| AK2 | CCBE1 | CSF2RA | FOXN1 | IL21 | MEFV | PIK3CD | RHOH | STING1 | UNC13D |
| ALPI | CD19 | CSF2RB | FOXP3 | IL21R | MOGS | PIK3CG | RIPK1 | STK4 | UNC93B1 |
| AP1S3 | CD247 | CSF3R | FPR1 | IL2RA | MPO | PIK3R1 | RNASEH2A | STX11 | UNG |
| AP3B1 | CD27 | CTC1 | G6PC3 | IL2RB | MRTFA | PLCG2 | RNASEH2B | STXBP2 | USB1 |
| AP3D1 | CD3D | CTLA4 | G6PD | IL2RG | MSN | PMS2 | RNASEH2C | TAP1 | USP18 |
| ARPC1B | CD3E | CTPS1 | GATA1 | IL36RN | MTHFD1 | PNP | RNF168 | TAP2 | VPS13B |
| ATM | CD3G | CTSC | GATA2 | IL6R | MVK | POLA1 | RNF31 | TAPBP | VPS45 |
| ATP6AP1 | CD4 | CXCR4 | GFI1 | IL6ST | MYD88 | POLD1 | RORC | TAZ | WAS |
| B2M | CD40 | CYBA | GINS1 | IL7R | MYO5B | POLE | RPSA | TBK1 | WDR1 |
| BACH2 | CD40LG | CYBB | GUCY2C | INO80 | MYSM1 | POLR3A | RTEL1 | TBX1 | WIPF1 |
| BCL10 | CD46 | CYBC1 | HAVCR2 | IRAK4 | NBN | POLR3C | SAMD9 | TCF3 | XIAP |
| BCL11B | CD55 | DBR1 | HAX1 | IRF3 | MCF1 | POMP | SAMD9L | TCN2 | ZAP70 |
| BLM | CD59 | DCLRE1B | HELLS | IRF7 | NCF2 | PRF1 | SAMHD1 | TERT | ZBTB24 |
| BLNK | CD70 | DCLRE1C | HPS1 | IRF8 | NCF4 | PRKCD | SBDS | TET2 | ZNF341 |
| BLOC1S6 | CD79A | DEF6 | HPS4 | IRF9 | NCKAP1L | PRKDC | SEC61A1 | TFRC | CR2 |
| BTK | CD79B | DKC1 | HPS6 | ISG15 | NCSTN | PSENEN | SERPING1 | TGFB1 | |
| C1QA | CD81 | DNAJC21 | HTRA2 | ITCH | NFE2L2 | PSMA3 | SGPL1 | TICAM1 | |
| C1QB | CD8A | DNASE1L3 | ICOS | ITGB2 | NFKB1 | PSMB10 | SH2D1A | TINF2 | |
| C1QC | CDC42 | DNASE2 | IFIH1 | ITK | NFKB2 | PSMB4 | SKIV2L | TLR3 | |
| C1R | CDCA7 | DNMT3B | IFNAR1 | ITPKB | NFKBIA | PSMB8 | SLC29A3 | TLR7 | |
| C1S | CEBPE | DOCK2 | IFNGR1 | IVNS1ABP | NHEJ1 | PSMB9 | SLC35C1 | TMC6 | |
| C2 | CFB | DOCK8 | IFNGR2 | JAGN1 | NHP2 | PSTPIP1 | SLC37A4 | TMC8 | |
| C3 | CFD | EFL1 | IGHM | JAK3 | NLRC4 | PTEN | SLC39A7 | TNFAIP3 | |
| C4A | CFH | ELANE | IGKC | KRAS | NLRP1 | PTPN2 | SLC46A1 | TNFRSF11A | |
| C4B | CFHR1 | EPG5 | IGLL1 | LAMTOR2 | NLRP12 | PTPRC | SLC7A7 | TNFRSF13C | |
| C5 | CFHR3 | ERBIN | IKBKB | LAT | NLRP3 | RAB27A | SMARCAL1 | TNFRSF1A | |
| C6 | CFHR4 | ERCC6L2 | IKBKG | LCK | NOD2 | RAC2 | SMARCD2 | TNFRSF9 | |
| C7 | CFHR5 | EXTL3 | IKZF1 | LIG1 | NOP10 | RAG1 | SOCS1 | TOP2B | |
| C8A | CFI | F12 | IL10 | LIG4 | NRAS | RAG2 | SP110 | TPP2 |
| Case | Age | Vaccine | Interval-1 | Interval-2 |
|---|---|---|---|---|
| 1 | 1.2 year | 1 | 11 weeks | – |
| 2 | 3.5 years | 1 | 20 months | – |
| 3 | 4 years | 1 | 18 months | – |
| 4 | 4 years | 1 | 32 months | – |
| 5 | 7 years | 1 | 6 years | – |
| 6 | 8 years | 1 | 7 years | – |
| 7 | 9 years | 1 | 8 years | – |
| 8 | 12 years | 1 | 11 years | – |
| 9 | 14 years | 2 | 13 years | 2 years |
| 10 | 14 years | 2 | 13 years | 10 years |
| 11 | 14 years | 2 | 13 years | 4 years |
| 12 | 14 years | 2 | 13 years | 9 years |
| 13 | 7 years | 2 | 6 years | 2 years |
| 14 | 12 years | 2 | 11 years | 9 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, P.S.; Wilson, M.R.; Catho, G.; Blanchard-Rohner, G.; Schiess, N.; Cohrs, R.J.; Boutolleau, D.; Burrel, S.; Yoshikawa, T.; Wapniarski, A.; et al. Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling. Viruses 2021, 13, 2286. https://doi.org/10.3390/v13112286
Ramachandran PS, Wilson MR, Catho G, Blanchard-Rohner G, Schiess N, Cohrs RJ, Boutolleau D, Burrel S, Yoshikawa T, Wapniarski A, et al. Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling. Viruses. 2021; 13(11):2286. https://doi.org/10.3390/v13112286
Chicago/Turabian StyleRamachandran, Prashanth S., Michael R. Wilson, Gaud Catho, Geraldine Blanchard-Rohner, Nicoline Schiess, Randall J. Cohrs, David Boutolleau, Sonia Burrel, Tetsushi Yoshikawa, Anne Wapniarski, and et al. 2021. "Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling" Viruses 13, no. 11: 2286. https://doi.org/10.3390/v13112286
APA StyleRamachandran, P. S., Wilson, M. R., Catho, G., Blanchard-Rohner, G., Schiess, N., Cohrs, R. J., Boutolleau, D., Burrel, S., Yoshikawa, T., Wapniarski, A., Heusel, E. H., Carpenter, J. E., Jackson, W., Ford, B. A., & Grose, C. (2021). Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling. Viruses, 13(11), 2286. https://doi.org/10.3390/v13112286









