Systemic and Intestinal Viral Reservoirs in CD4+ T Cell Subsets in Primary SIV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Virus
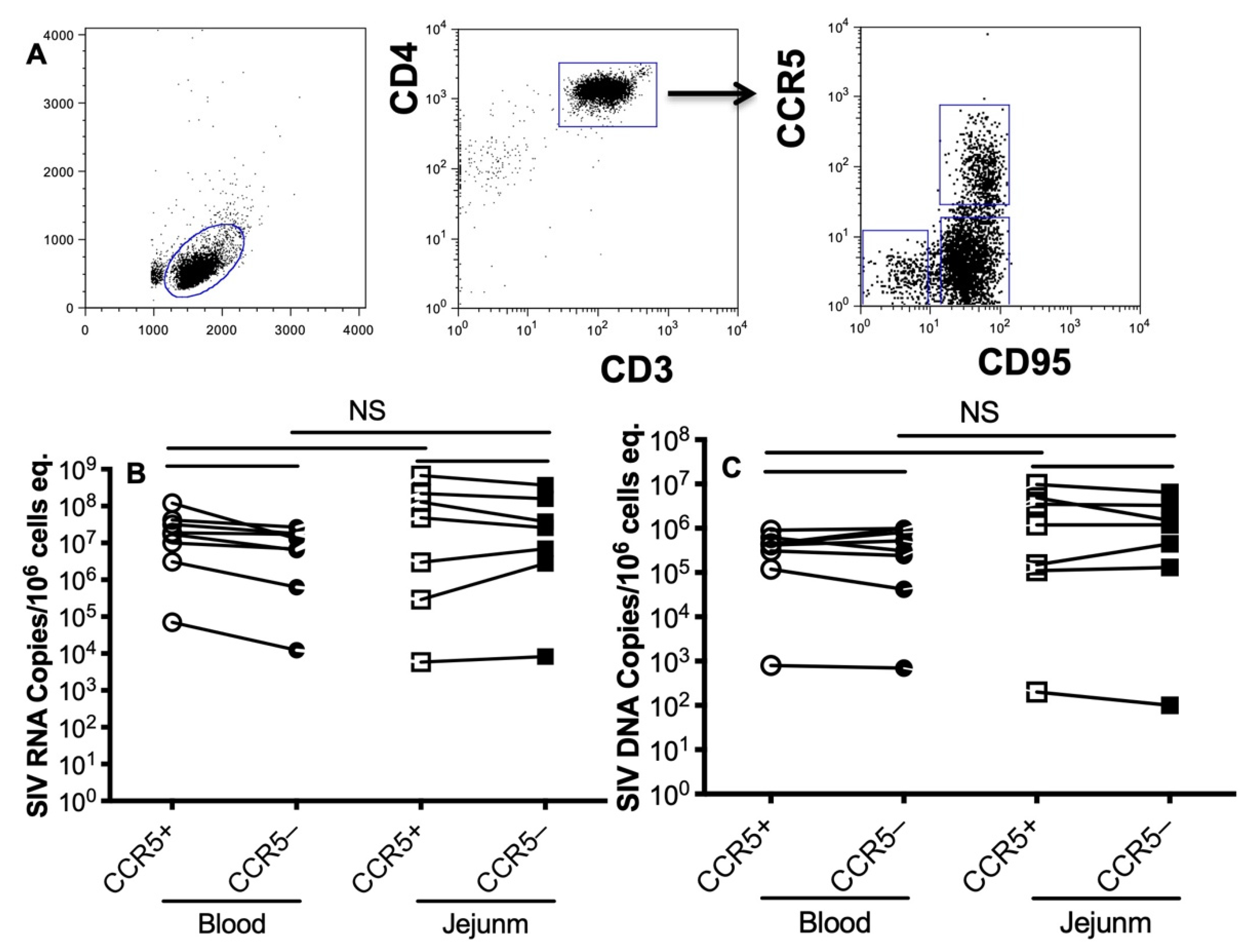
2.3. Sorting CD4+ T Cell Subsets
2.4. Genomic DNA and Total RNA Extraction
2.5. Quantification of Plasma Viral Load and Cell-Associated SIV RNA and DNA
2.6. Statistical Analysis
3. Results
3.1. Levels of SIV RNA/DNA in Peripheral and Intestinal CD4+ T Cells in Primary SIV Infection
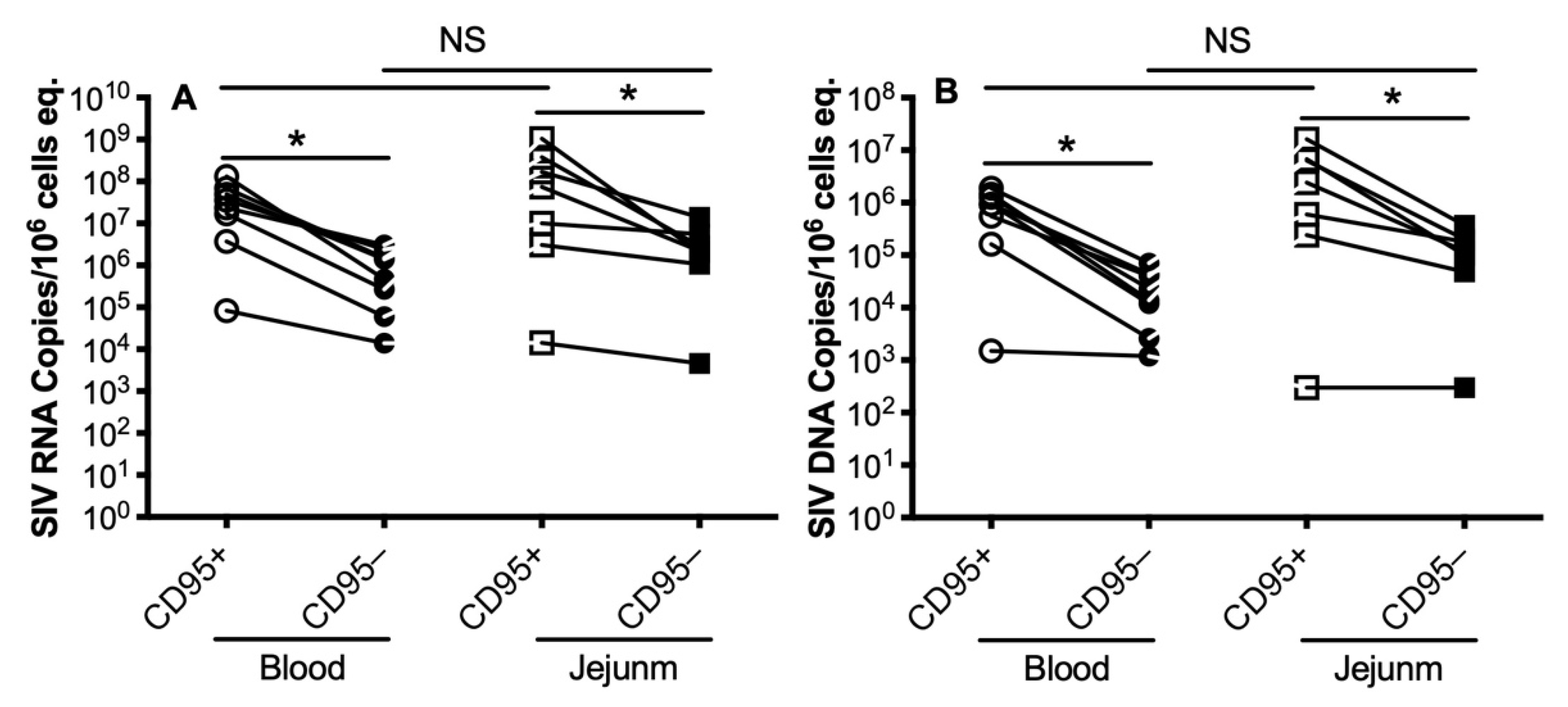
3.2. Levels of SIV RNA/DNA in Peripheral and Intestinal Memory CD4+ T Cells during Primary SIV Infection
3.3. Levels of SIV RNA/DNA in CCR5-Expressing Memory CD4+ T Cells during Primary SIV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, S.; Poles, M.A.; Tenner-Racz, K.; Horowitz, A.; Hurley, A.; Hogan, C.; Boden, D.; Racz, P.; Markowitz, M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004, 200, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Veazey, R.S. Mucosal immunology of HIV infection. Immunol. Rev. 2013, 254, 10–33. [Google Scholar] [CrossRef] [Green Version]
- Picker, L.J.; Hansen, S.G.; Lifson, J.D. New paradigms for HIV/AIDS vaccine development. Annu. Rev. Med. 2012, 63, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Veazey, R.S.; Wang, X.; Lackner, A.A.; Xu, H.; Pahar, B. Simian immunodeficiency virus infection in rhesus macaques induces selective tissue specific B cell defects in double positive CD21+CD27+ memory B cells. Clin. Immunol. 2011, 140, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, X.; Pahar, B.; Alvarez, X.; Rasmussen, K.K.; Lackner, A.A.; Veazey, R.S. Rapid down-regulation of gammac on T cells in early SIV infection correlates with impairment of T-cell function. FASEB J. 2012, 26, 2294–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, H.; Pahar, B.; Lackner, A.A.; Veazey, R.S. Divergent kinetics of proliferating T cell subsets in simian immunodeficiency virus (SIV) infection: SIV eliminates the “first responder” CD4+ T cells in primary infection. J. Virol. 2013, 87, 7032–7038. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, X.; Lackner, A.A.; Veazey, R.S. Type 3 innate lymphoid cell depletion is mediated by TLRs in lymphoid tissues of simian immunodeficiency virus-infected macaques. FASEB J. 2015, 29, 5072–5080. [Google Scholar] [CrossRef] [Green Version]
- Ziani, W.; Shao, J.; Wang, X.; Russell-Lodrigue, K.; Liu, Y.Z.; Montaner, L.J.; Veazey, R.S.; Xu, H. Increased proviral DNA in circulating cells correlates with plasma viral rebound in SIV-infected rhesus macaques after antiretroviral therapy interruption. J. Virol. 2021, 95, 6. [Google Scholar] [CrossRef] [PubMed]
- Okoye, A.A.; Hansen, S.G.; Vaidya, M.; Fukazawa, Y.; Park, H.; Duell, D.M.; Lum, R.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 2018, 24, 1430–1440. [Google Scholar] [CrossRef]
- Sloan, R.D.; Wainberg, M.A. The role of unintegrated DNA in HIV infection. Retrovirology 2011, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Koelsch, K.K.; Liu, L.; Haubrich, R.; May, S.; Havlir, D.; Gunthard, H.F.; Ignacio, C.C.; Campos-Soto, P.; Little, S.J.; Shafer, R.; et al. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J. Infect. Dis. 2008, 197, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, F.B.; Kim, J.; Shin, C.G. Distribution and fate of HIV-1 unintegrated DNA species: A comprehensive update. AIDS Res. Ther. 2017, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Craigie, R.; Bushman, F.D. HIV DNA integration. Cold Spring Harb. Perspect. Med. 2012, 2, a006890. [Google Scholar] [CrossRef] [Green Version]
- Okoye, A.A.; Picker, L.J. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantero-Perez, J.; Grau-Exposito, J.; Serra-Peinado, C.; Rosero, D.A.; Luque-Ballesteros, L.; Astorga-Gamaza, A.; Castellvi, J.; Sanhueza, T.; Tapia, G.; Lloveras, B.; et al. Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat. Commun. 2019, 10, 4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.J.; Li, Q.; Abel, K.; Kim, E.Y.; Ma, Z.M.; Wietgrefe, S.; La Franco-Scheuch, L.; Compton, L.; Duan, L.; Shore, M.D.; et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 2005, 79, 9217–9227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Schuler, T.; Zupancic, M.; Wietgrefe, S.; Staskus, K.A.; Reimann, K.A.; Reinhart, T.A.; Rogan, M.; Cavert, W.; Miller, C.J.; et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 1999, 286, 1353–1357. [Google Scholar] [CrossRef]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.M.; Maldarelli, F. The role of integration and clonal expansion in HIV infection: Live long and prosper. Retrovirology 2018, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Katusiime, M.G.; Halvas, E.K.; Wright, I.; Joseph, K.; Bale, M.J.; Kirby-McCullough, B.; Engelbrecht, S.; Shao, W.; Hu, W.S.; Cotton, M.F.; et al. Intact HIV Proviruses Persist in Children Seven to Nine Years after Initiation of Antiretroviral Therapy in the First Year of Life. J. Virol. 2020, 94, e01519-19. [Google Scholar] [CrossRef] [PubMed]
- Hodel, F.; Patxot, M.; Snaka, T.; Ciuffi, A. HIV-1 latent reservoir: Size matters. Future Virol. 2016, 11, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Avettand-Fenoel, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.P.; Rouzioux, C. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mexas, A.M.; Graf, E.H.; Pace, M.J.; Yu, J.J.; Papasavvas, E.; Azzoni, L.; Busch, M.P.; Di Mascio, M.; Foulkes, A.S.; Migueles, S.A.; et al. Concurrent measures of total and integrated HIV DNA monitor reservoirs and ongoing replication in eradication trials. AIDS 2012, 26, 2295–2306. [Google Scholar] [CrossRef]
- Yukl, S.A.; Kaiser, P.; Kim, P.; Telwatte, S.; Joshi, S.K.; Vu, M.; Lampiris, H.; Wong, J.K. HIV latency in isolated patient CD4(+) T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci. Transl. Med. 2018, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Rutsaert, S.; Bosman, K.; Trypsteen, W.; Nijhuis, M.; Vandekerckhove, L. Digital PCR as a tool to measure HIV persistence. Retrovirology 2018, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S.; Lane, H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar] [CrossRef] [Green Version]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Siliciano, J.D.; Siliciano, R.F. Assays to Measure Latency, Reservoirs, and Reactivation. Curr. Top. Microbiol. Immunol. 2018, 417, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H. Residual Proviral Reservoirs: A High Risk for HIV Persistence and Driving Forces for Viral Rebound after Analytical Treatment Interruption. Viruses 2021, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Minang, J.T.; Trivett, M.T.; Coren, L.V.; Barsov, E.V.; Piatak, M., Jr.; Ott, D.E.; Ohlen, C. Nef-mediated MHC class I down-regulation unmasks clonal differences in virus suppression by SIV-specific CD8(+) T cells independent of IFN-gamma and CD107a responses. Virology 2009, 391, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, X.; Lackner, A.A.; Veazey, R.S. CD8 down-regulation and functional impairment of SIV-specific cytotoxic T lymphocytes in lymphoid and mucosal tissues during SIV infection. J. Leukoc. Biol. 2013, 93, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Rusconi, S.; Mavilio, D.; Fogli, M.; Murdaca, G.; Pende, D.; Mingari, M.C.; Galli, M.; Moretta, L.; De Maria, A. Differential disappearance of inhibitory natural killer cell receptors during HAART and possible impairment of HIV-1-specific CD8 cytotoxic T lymphocytes. AIDS 2001, 15, 965–974. [Google Scholar] [CrossRef]
- Bronnimann, M.P.; Skinner, P.J.; Connick, E. The B-Cell Follicle in HIV Infection: Barrier to a Cure. Front. Immunol. 2018, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Huot, N.; Jacquelin, B.; Garcia-Tellez, T.; Rascle, P.; Ploquin, M.J.; Madec, Y.; Reeves, R.K.; Derreudre-Bosquet, N.; Muller-Trutwin, M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 2017, 23, 1277–1286. [Google Scholar] [CrossRef]
- Ram, D.R.; Manickam, C.; Lucar, O.; Shah, S.V.; Reeves, R.K. Adaptive NK cell responses in HIV/SIV infections: A roadmap to cell-based therapeutics? J. Leukoc. Biol. 2019, 105, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Liu, D.X.; Moroney-Rasmussen, T.; Lackner, A.A.; Veazey, R.S. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal. Immunol. 2012, 5, 658–669. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Morici, L.A.; Pahar, B.; Veazey, R.S. Early divergent host responses in SHIVsf162P3 and SIVmac251 infected macaques correlate with control of viremia. PLoS ONE 2011, 6, e17965. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H.; Alvarez, X.; Pahar, B.; Moroney-Rasmussen, T.; Lackner, A.A.; Veazey, R.S. Distinct expression patterns of CD69 in mucosal and systemic lymphoid tissues in primary SIV infection of rhesus macaques. PLoS ONE 2011, 6, e27207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keele, B.F.; Li, H.; Learn, G.H.; Hraber, P.; Giorgi, E.E.; Grayson, T.; Sun, C.; Chen, Y.; Yeh, W.W.; Letvin, N.L.; et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 2009, 206, 1117–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Animal | Category | Dose of SIVmac251 | Plasma Viral Load (Copies/mL) | CD4+ T Cell Count |
|---|---|---|---|---|
| T108 | 8 day p.i. | 100 TCID50 | 57,000 | 653 |
| BA57 | 8 day p.i. | 100 TCID50 | 14,000,000 | 416 |
| HI52 | 8 day p.i. | 100 TCID50 | 3,700,000 | 664 |
| HI53 | 8 day p.i. | 100 TCID50 | 3,600,000 | 325 |
| AV91 | 10 day p.i. | 100 TCID50 | 160,000,000 | 472 |
| M992 | 13 day p.i. | 100 TCID50 | 35,000,000 | 227 |
| HI58 | 13 day p.i. | 100 TCID50 | 8,000,000 | 651 |
| HI63 | 13 day p.i. | 100 TCID50 | 24,310,900 | 438 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ziani, W.; Veazey, R.S.; Xu, H. Systemic and Intestinal Viral Reservoirs in CD4+ T Cell Subsets in Primary SIV Infection. Viruses 2021, 13, 2398. https://doi.org/10.3390/v13122398
Wang X, Ziani W, Veazey RS, Xu H. Systemic and Intestinal Viral Reservoirs in CD4+ T Cell Subsets in Primary SIV Infection. Viruses. 2021; 13(12):2398. https://doi.org/10.3390/v13122398
Chicago/Turabian StyleWang, Xiaolei, Widade Ziani, Ronald S. Veazey, and Huanbin Xu. 2021. "Systemic and Intestinal Viral Reservoirs in CD4+ T Cell Subsets in Primary SIV Infection" Viruses 13, no. 12: 2398. https://doi.org/10.3390/v13122398
APA StyleWang, X., Ziani, W., Veazey, R. S., & Xu, H. (2021). Systemic and Intestinal Viral Reservoirs in CD4+ T Cell Subsets in Primary SIV Infection. Viruses, 13(12), 2398. https://doi.org/10.3390/v13122398







