Three-Year Clinical Follow-Up of Children Intrauterine Exposed to Zika Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Diagnosis
2.2. Statistical Analysis
2.3. Ethical Procedures
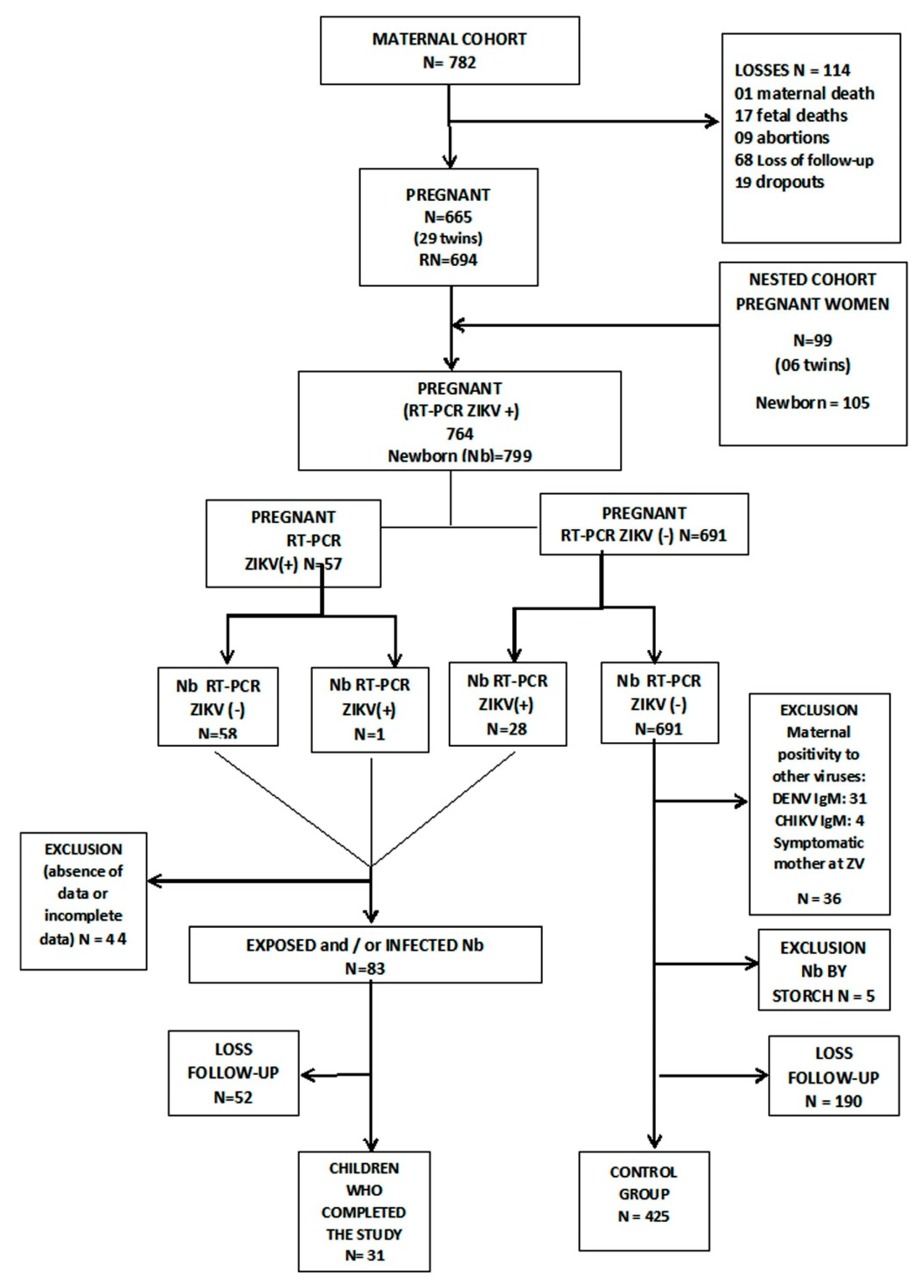
3. Results
Cohort General Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregg, N.M. Congenital cataract following German measles in the mother. Epidemiol. Infect. 1991, 107, iii–xiv. [Google Scholar] [CrossRef] [PubMed]
- Madrid, L.; Varo, R.; Sitoe, A.; Bassat, Q. Congenital and perinatally-acquired infections in resource-constrained settings. Expert Rev. Anti Infect. Ther. 2016, 14, 845–861. [Google Scholar] [CrossRef]
- Costello, A.; Dua, T.; Duran, P.; Gülmezoglu, M.; Oladapo, O.T.; Perea, W.; Pires, J.; Ramon-Pardo, P.; Rollins, N.; Saxena, S. Defining the syndrome associated with congenital Zika virus infection. Bull. World Health Organ. 2016, 94, 406. [Google Scholar] [CrossRef]
- Gulland, A. Zika virus may be linked to several birth defects, expert warns. BMJ 2016, 352, i1322. [Google Scholar] [CrossRef]
- Aragao, M.F.V.V.; Holanda, A.C.; Brainer-Lima, A.M.; Petribu, N.C.L.; Castillo, M.; van der Linden, V.; Serpa, S.C.; Tenório, A.G.; Travassos, P.T.C.; Cordeiro, M.T.; et al. Nonmicrocephalic Infants with Congenital Zika Syndrome Suspected Only after Neuroimaging Evaluation Compared with Those with Microcephaly at Birth and Postnatally: How Large Is the Zika Virus “Iceberg”? AJNR Am. J. Neuroradiol 2017, 38, 1427–1434. [Google Scholar] [CrossRef]
- Pan American Health Organization. Zika Suspected and Confirmed CASES Reported by Countries and Territories in the Americas Cumulative Cases, 2015-2017; Pan American Health Organization: Washington, DC, USA, 2018. [Google Scholar]
- Ministério da Saúde (BR). Protocolo de Vigilância e Resposta à Ocorrência de Microcefalia Relacionada à Infecção Pelo Vírus Zika Brasília DF; Ministério da Saúde: Brasilia, Brazil, 2015. [Google Scholar]
- Teixeira, M.G.; Costa, M.A.C.; de Oliveira, W.K.; Nunes, M.L.; Rodrigues, L.C. The Epidemic of Zika Virus-Related Microcephaly in Brazil: Detection, Control, Etiology, and Future Scenarios. Am. J. Public Health 2016, 106, 601–605. [Google Scholar] [CrossRef]
- Teixeira, G.A.; Dantas, D.N.A.; Carvalho, G.A.F.L.; Silva, A.N.D.; Lira, A.L.B.C.; Enders, B.C. Analysis of the concept of the Zika Virus congenital syndrome. Cien Saude Colet 2020, 25, 567–574. [Google Scholar] [CrossRef]
- Kuper, H.; Lyra, T.M.; Moreira, M.E.L.; de Albuquerque, M.D.S.V.; de Araújo, T.V.B.; Fernandes, S.; Jofre-Bonet, M.; Larson, H.; Lopes de Melo, A.P.; Mendes, C.H.F.; et al. Social and economic impacts of congenital Zika syndrome in Brazil: Study protocol and rationale for a mixed-methods study. Wellcome Open Res. 2018, 3, 127. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.F.P.M.; Souza, W.V.; Araújo, T.V.B.; Braga, M.C.; Miranda Filho, D.B.; Ximenes, R.A.A.; de Melo Filho, D.A.; Brito, C.A.A.; Valongueiro, S.; Melo, A.P.L.; et al. The microcephaly epidemic and Zika virus: Building knowledge in epidemiology. Cad. Saude Publica 2018, 34, e00069018. [Google Scholar] [PubMed]
- Ministerio de Saúde Saúde(BR), Secretaria de Vigilância em Saúde. Síndrome congênita associada à infecção pelo virus Zika: Situação epidemiológica, ações desenvolvidas e desafios, 2015 a 2019. Bol. Epidemiol. Internet 2019, 50, 1–31. (In Spanish) [Google Scholar]
- Sanchez Clemente, N.; Rodrigues, M.; Pascalicchio, A.P.; Gazeta, R.E.; Vedovello, D.; Brickley, E.B.; De Almeida, M.F.; Passos, S.D. Cohort profile: The Jundiaí Zika cohort (JZC), a pregnancy and birth cohort in São Paulo state, Brazil. BMJ Open 2019, 9, e027947. [Google Scholar] [CrossRef] [PubMed]
- Tambalo, D. Avaliação da Qualidade da Assistência do Pré-Natal em Gestantes da Coorte Zika Vírus Jundiaí. Master’s Thesis, Faculdade de Medicina de Jundiaí, São Paulo, Brazil, 2019. [Google Scholar]
- World Health Organization. Screening, Assessment and Management of Neonates and Infants with Complications Associated with Zika Virus Exposure in Utero: Rapid Advice Guideline; World Health Organization: Geneva, Switzerland, 2016; pp. 1–15. [Google Scholar]
- Capurro, H.; Konichezky, S.; Fonseca, D.; Caldeyro-Barcia, R. A simplified method for diagnosis of gestational age in the newborn infant. J. Pediatr. 1978, 93, 120–122. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Kennedy, S.H.; Salomon, L.J.; Altman, D.G.; Ohuma, E.O.; Stones, W.; Gravett, M.G.; Barros, F.C.; Victora, C.; Purwar, M.; et al. The INTERGROWTH-21st fetal growth standards: Toward the global integration of pregnancy and pediatric care. Am. J. Obs. Gynecol. 2018, 218, S630–S640. [Google Scholar] [CrossRef]
- Ministério da Saúde (BR), S. d. V. e. S., Secretaria de Atenção à Saúde Orientações Integradas de Vigilância e Atenção à Saúde no Âmbito da Emergência de Saúde Pública de Importância Nacional: Procedimentos para o Monitoramento das Alterações no Crescimento e Desenvolvimento a Partir da Gestação até a Primeira Infância, Relacionadas à Infecção pelo Vírus Zika e Outras Etiologias Infeciosas Dentro da Capacidade Operacional do SUS. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/orientacoes_integradas_vigilancia_atencao_emergencia_saude_publica.pdf (accessed on 6 June 2020).
- Teller, D.Y.; McDonald, M.A.; Preston, K.; Sebris, S.L.; Dobson, V. Assessment of visual acuity in infants and children: The acuity card procedure. Dev. Med. Child. Neurol 1986, 28, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Nellis, L.; Gridley, B.E. Review of the Bayley Scales of i nfant development-Second Edition. J. Sch. Pychol. 1994, 32, 201–209. [Google Scholar] [CrossRef]
- Babson, S.G. Growth of low-birth-weight infants. J. Pediatr. 1970, 77, 11–18. [Google Scholar] [CrossRef]
- Ramsay, M.; Martel, C.; Porporino, M.; Zygmuntowicz, C. The Montreal Children’s Hospital Feeding Scale: A brief bilingual screening tool for identifying feeding problems. Paediatr. Child. Health 2011, 16, 147–e17. [Google Scholar] [CrossRef]
- Almeida, F.C.F. Clinical Evaluation Protocol of Pediatric Dysphagia (PAD-PED). In Base de Dados Nacionais; ID: sms-10505; Secretaria Municipal da Saúde: São Paulo, Brazil, 2014. [Google Scholar]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Leal, M.C.; Muniz, L.F.; Ferreira, T.S.; Santos, C.M.; Almeida, L.C.; Van Der Linden, V.; Ramos, R.C.; Rodrigues, L.C.; Neto, S.S. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection—Brazil, November 2015-May 2016. MMWR Morb. Mortal Wkly Rep. 2016, 65, 917–919. [Google Scholar] [CrossRef]
- Coelho, A.V.C.; Crovella, S. Microcephaly Prevalence in Infants Born to Zika Virus-Infected Women: A Systematic Review and Meta-Analysis. Int J. Mol. Sci 2017, 18, 1714. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Osgood-Zimmerman, A.; Kassebaum, N.J.; Ray, S.E.; de Araújo, V.E.M.; da Nóbrega, A.A.; Frutuoso, L.C.V.; Lecca, R.C.R.; Stevens, A.; Zoca de Oliveira, B.; et al. The association between Zika virus infection and microcephaly in Brazil 2015-2017: An observational analysis of over 4 million births. PLoS Med. 2019, 16, e1002755. [Google Scholar] [CrossRef]
- Kikuti, M.; Cardoso, C.W.; Prates, A.P.B.; Paploski, I.A.D.; Kitron, U.; Reis, M.G.; Mochida, G.H.; Ribeiro, G.S. Congenital brain abnormalities during a Zika virus epidemic in Salvador, Brazil, April 2015 to July 2016. Euro Surveill 2018, 23, 1700757. [Google Scholar] [CrossRef] [PubMed]
- Prata-Barbosa, A.; Martins, M.M.; Guastavino, A.B.; Cunha, A.J.L.A. , Effects of Zika infection on growth. J. Pediatr 2019, 95 (Suppl. S1), 30–41. [Google Scholar] [CrossRef]
- Barbosa, M.H.M.; Garcia, C.F.D.; Magalhães Barbosa, M.C.; Robaina, J.R.; Prata-Barbosa, A.; Lima, M.A.M.T.; Cunha, A.J.L.A. Normal Hearing Function in Children Prenatally Exposed to Zika Virus. Int. Arch. Otorhinolaryngol. 2020, 24, e299–e307. [Google Scholar] [CrossRef] [PubMed]
- Gerzson, L.R.; de Almeida, C.S.; Silva, J.H.D.; Feitosa, M.M.A.; de Oliveira, L.N.; Schuler-Faccini, L. Neurodevelopment of Nonmicrocephalic Children, After 18 Months of Life, Exposed Prenatally to Zika Virus. J. Child. Neurol 2020, 35, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Vasconcelos, Z.; Gabaglia, C.R.; Damasceno, L.; Pone, M.; Abreu de Carvalho, L.M.; Pone, S.M.; Zin, A.A.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabié, A.; Callier, C.; Carles, G.; Cassadou, S.; Césaire, R.; et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N. Eng. J. Med. 2018, 378, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.C.S.; Hernandez Alves Ribeiro César, C.P.; Paranhos, L.R.; Guedes-Granzotti, R.B.; Lewis, D.R. Speech-language disorders in children with congenital Zika virus syndrome: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110309. [Google Scholar] [CrossRef]
- Valdes, V.; Zorrilla, C.D.; Gabard-Durnam, L.; Muler-Mendez, N.; Rahman, Z.I.; Rivera, D.; Nelson, C.A. Cognitive Development of Infants Exposed to the Zika Virus in Puerto Rico. JAMA Netw. Open 2019, 2, e1914061. [Google Scholar] [CrossRef]
- O’Hare, A.; Bremner, L. Management of developmental speech and language disorders: Part 1. Arch. Dis. Child. 2016, 101, 272–277. [Google Scholar] [CrossRef]
- Azevedo, R.S.S.; Araujo, M.T.; Oliveira, C.S.; Filho, A.J.M.; Nunes, B.T.D.; Henriques, D.F.; Silva, E.V.P.; Carvalho, V.L.; Chiang, J.O.; Martins, L.C.; et al. Zika Virus Epidemic in Brazil. II. Post-Mortem Analyses of Neonates with Microcephaly, Stillbirths, and Miscarriage. J. Clin. Med. 2018, 7, 496. [Google Scholar] [CrossRef]
- van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Júnior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragão, M.F.; et al. Description of 13 Infants Born During October 2015-January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth—Brazil. MMWR Morb Mortal Wkly Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; van der Linden, V.; Bezerra, T.P.; de Valois, L.; Borges, A.C.G.; Antunes, M.M.C.; Brandt, K.G.; Moura, C.X.; Rodrigues, L.C.; Ximenes, C.R. Characteristics of Dysphagia in Infants with Microcephaly Caused by Congenital Zika Virus Infection, Brazil, 2015. Emerg. Infect. Dis. 2017, 23, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Mendoza, C.K.; Rice, M.E.; Galang, R.R.; Fulton, A.C.; VanMaldeghem, K.; Prado, M.V.; Ellis, E.; Anesi, M.S.; Simeone, R.M.; Petersen, E.E.; et al. Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy—U.S. Territories, January 1, 2016–April 25, 2017. Mmwr Morb Mortal Wkly. Rep. 2017, 66, 615–621. [Google Scholar]
- Soriano-Arandes, A.; Frick, M.A.; García López-Hortelano, M.; Sulleiro, E.; Rodó, C.; Sánchez-Seco, M.P.; Cabrera-Lafuente, M.; Suy, A.; De la Calle, M.; Santos, M.; et al. Clinical Outcomes of a Zika Virus Mother-Child Pair Cohort in Spain. Pathogens 2020, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.L.; Nery Júnior, N.R.R.; Estofolete, C.F.; Bernardes Terzian, A.C.; Guimarães, G.F.; Zini, N.; Alves da Silva, R.; Dutra Silva, G.C.; Junqueira Franco, L.C.; Rahal, P.; et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin. Microbiol. Infect. 2018, 24, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.V.; Albuquerque, M.F.P.M.; Vazquez, E.; Bezerra, L.C.A.; Mendes, A.D.C.G.; Lyra, T.M.; Araujo, T.V.B.; Oliveira, A.L.S.; Braga, M.C.; Ximenes, R.A.A.; et al. Microcephaly epidemic related to the Zika virus and living conditions in Recife, Northeast Brazil. Bmc Public Health 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.F.; Rückert, C.; Cavany, S.M.; Perkins, T.A.; Ebel, G.D.; Grubaugh, N.D. Arbovirus coinfection and co-transmission: A neglected public health concern? PLoS Biol. 2019, 17, e3000130. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Olsen, P.C.; Costa, F.; Wang, Q.; Oliveira, T.Y.; Nery, N.; Aromolaran, A.; do Rosário, M.S.; Sacramento, G.A.; Cruz, J.S.; et al. Risk of Zika microcephaly correlates with features of maternal antibodies. J. Exp. Med. 2019, 216, 2302–2315. [Google Scholar] [CrossRef]
- Langerak, T.; Mumtaz, N.; Tolk, V.I.; van Gorp, E.C.M.; Martina, B.E.; Rockx, B.; Koopmans, M.P.G. The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLS Pathog 2019, 15, e1007640. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Pezzuto, P.; Higa, L.M.; Dias, A.A.; Vasconcelos, J.M.; Santos, T.M.P.; Ferreira, J.C.C.G.; Ferreira, R.O.; Dutra, F.F.; Rossi, A.D.; et al. Congenital Zika syndrome is associated with maternal protein malnutrition. Sci. Adv. 2020, 6, eaaw6284. [Google Scholar] [CrossRef]
- Kigawa, M.; Tsuchida, A.; Matsumura, K.; Takamori, A.; Ito, M.; Tanaka, T.; Hamazaki, K.; Adachi, Y.; Saito, S.; Origasa, H.; et al. Factors of non-responsive or lost-to-follow-up Japanese mothers during the first year post partum following the Japan Environment and Children’s Study: A longitudinal cohort study. BMJ Open 2019, 9, e031222. [Google Scholar] [CrossRef]
- Cameron, C.M.; Osborne, J.M.; Spinks, A.B.; Davey, T.M.; Sipe, N.; McClure, R.J. Impact of participant attrition on child injury outcome estimates: A longitudinal birth cohort study in Australia. BMJ Open 2017, 7, e015584. [Google Scholar] [CrossRef] [PubMed]
- Gustavson, K.; von Soest, T.; Karevold, E.; Røysamb, E. Attrition and generalizability in longitudinal studies: Findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health 2012, 2012, 12918. [Google Scholar] [CrossRef] [PubMed]
| Sociodemographic Characteristics | Pregnant Women (n = 764) |
|---|---|
| Age (years) (n = 763) | |
| Mean (±standard deviation) | 27.4 (±7.3) |
| Median (IIQ 25–75%) | 27 (21–33) |
| Minimum–maximum | 13–46 |
| Age (years) | n (%) |
| 13 to 19 | 123 (16.1) |
| 20 to 34 | 497 (65.1) |
| 35 to 46 | 143 (18.7) |
| Not informed | 1 (0.1) |
| Ethnicity | |
| White | 398 (52.1) |
| Brown | 267 (34.9) |
| Black | 79 (10.3) |
| Indigenous | 2 (0.3) |
| Not informed | 18 (2.4) |
| Paid work | |
| Yes | 346 (45.3) |
| No | 401 (52.5) |
| Not informed | 17 (2.2) |
| Risk factors | |
| Gestational or type I diabetes mellitus | 199 (26) |
| Chronic or gestational arterial hypertension | 78 (10.2) |
| Early Pregnancy | 71 (9.3) |
| Microcephaly (suspected or confirmed) | 50 (6.5) |
| Twinning | 25 (3.3) |
| Suggestive symptoms Zika1 | 11 (1.4) |
| Others | 274 (35.8) |
| No information | 14 (2.6) |
| Changes in obstetric ultrasound compatible with Zika | |
| Yes | 41 (5.4) |
| No | 713 (93.3) |
| Not informed | 10 (1.3) |
| Type of delivery | |
| Vaginal | 358 (46.9) |
| Cesarean | 371 (48.6) |
| Forceps | 18 (2.4) |
| No information | 16 (2.1) |
| Zika symptoms | |
| Yes | 40 (5.2) |
| No | 723 (94.7) |
| Not informed | 1 (0.1) |
| Zika Virus PCR | |
| Detectable | 57 (7.5) |
| Not detectable | 691 (90.4) |
| No information | 16 (2.1) |
| Dengue (IgM) | |
| Detectable | 31 (4.0) |
| Not detectable | 624 (8.,7) |
| No information | 109 (14.3) |
| Chikungunya (IgM) | |
| Detectable | 4 (0.5) |
| Not detectable | 731 (95.7) |
| No information | 29 (3.8) |
| Viral co-infection | |
| Zika and dengue | 4 (0.5) |
| Clinical Characteristics | Total | Children with Zika or Exposed (Mothers Confirmed with Zika) | Control (Non-Reactive) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| Gestational age (weeks) [n (%)] | n = 797 | n = 83 | n = 672 | ||
| ≤31 | 21 (2.6) | 1 (1.2) | 20 (3.0) | 0.496 1 | 0.38 (0.05–2.88) |
| 32 to 36 | 114 (14.3) | 10 (12.1) | 104 (15.5) | 0.376 2 | 0.73 (0.37–1.47) |
| ≥37 | 620 (77.8) | 72 (86.7) | 548 (81.5) | - | 1 |
| Weight (grams) | n = 777 | n = 82 | n = 695 | ||
| Mean (±standard deviation) | 2970.9 (±636.3) | 3147.4 (±549.3) | 2950.1 (±643.0) | - | - |
| Median (IIQ 25–75%) | 3015 (2618–3398) | 3222 (2790–3526) | 2980 (2595–3375) | 0.006 3 | - |
| Minimum–maximum | 560–4525 | 1270–4285 | 560–4525 | - | - |
| Low weight at birth | n = 83 | n = 672 | |||
| Yes (<2.500 g) | 151 (19.4) | 7 (8.5) | 144 (20.7) | 0.008 2 | 0.35 (0.16–0.79) |
| No (≥2.500 g) | 626 (80.6) | 75 (91.5) | 551 (79.3) | - | 1 |
| Length (cm) | n = 82 | n = 692 | |||
| Mean (± standard deviation) | 47.4 (±3.3) | 48.3 (±2.7) | 47.3 (±3.3) | - | - |
| Median (IIQ 25–75%) | 48 (46–50) | 48 (47–50) | 48 (46–50) | 0.004 3 | - |
| Minimum–maximum | 28.5–54.5 | 38.0–53.0 | 28.5–54.5 | - | - |
| APGAR 5 min | n = 695 | n = 78 | n = 617 | ||
| Mean (±standard deviation) | 9.3 (±0.8) | 9.3 (±0.8) | 9.3 (±0.8) | 0.692 3 | - |
| Median (IIQ 25–75%) | 9 (9–10) | 9 (9–10) | 9 (9–10) | - | - |
| Minimum–maximum | 4–10 | 5–10 | 4–10 | - | - |
| APGAR 5 min | n = 695 | n = 83 | n = 672 | ||
| 1 a 6 | 8 (1.2) | 1 (1.3) | 7 (1.1) | 1.000 1 | 1.13 (0.14–9.32) |
| 7 a 10 (normal) | 687 (98.8) | 77 (98.7) | 610 (98.9) | - | 1 |
| Head circumference (cm) | n = 768 | n = 82 | n = 686 | ||
| Mean (±standard deviation) | 33.6 (±2.3) | 34.1 (±1.9) | 33.6 (±2.3) | - | - |
| Median (IIQ 25–75%) | 34 (33–35) | 34 (33–35) | 34 (32–35) | 0.163 3 | - |
| Minimum–maximum | 21–38.5 | 27–38 | 21–38.5 | - | - |
| Microcephaly [n (%)] | n = 797 | n = 83 | n = 694 | ||
| Yes | 48 (6.0) | 3 (3.6) | 45 (6.5) | 0.295 2 | 0.53 (0.16–1.76) |
| No | 730 (91.6) | 81 (96.4) | 649 (93.5) | 1 |
| Clinical Characteristics | Total | Children with Zika or Exposed (Mothers Confirmed with Zika) | Control (Non-Reactive) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| Pregnant women with diabetes mellitus 1 | |||||
| Weight (grams) | n = 206 | n = 28 | n = 178 | ||
| Mean (±standard deviation) | 3206.1 (±532.1) | 3280.0 (±403.7) | 3194.5 (±549.6) | - | - |
| Median (IIQ 25–75%) | 3250 (2890–3555) | 3250 (2950–3599) | 3250 (2960–3598) | 0.582 2 | - |
| Minimum–maximum | 720–4330 | 2560–4090 | 720–4330 | - | - |
| Low weight at birth | |||||
| Yes (<2.500 g) | 14 (6.8) | - | 14 (100.0) | 0.224 3 | 0.20 (0.11–3.43) |
| No (≥2.500 g) | 192 (93.2) | 28 (14.6) | 164 (85.4) | - | 1 |
| Pregnant women without diabetes mellitus | |||||
| Weight (grams) | n = 553 | n = 53 | n = 500 | ||
| Mean (±standard deviation) | 2877.9 (±642.7) | 3074.1 (±608.2) | 2857.1 (±654.4) | - | - |
| Median (IIQ 25–75%) | 2930 (2515–3330) | 3115 (2680–3495) | 2920 (2486–3281) | 0.020 2 | - |
| Minimum–maximum | 560–4525 | 1270–4285 | 560–4525 | - | - |
| Low weight at birth | |||||
| Yes (<2.500 g) | 135 (24.4) | 7 (5.2) | 128 (94.8) | 0.046 4 | 0.44 (0.18–0.97) |
| No (≥2.500 g) | 418 (75.6) | 46 (11.0) | 372 (89.0) | - | 1 |
| Clinical Characteristics | Total | Children with Zika or Exposed (Mothers Confirmed with Zika) | Control (Non-Reactive) | p-Value | OR (IC95%) |
|---|---|---|---|---|---|
| Preterm birth 1 | |||||
| Weight (grams) | n = 11 | n = 120 | |||
| Mean (±standard deviation) | 2293.4 (±712.1) | 2337.3 (±551.4) | 2289.4 (±726.8) | - | - |
| Median (IIQ 25–75%) | 2320 (1840–2865) | 2370 (2200–2695) | 2305 (1825–2876) | 0.845 3 | - |
| Minimum–maximum | 560–3573 | 1270–3055 | 560–3573 | - | - |
| Low weight at birth | |||||
| Yes (<2.500 g) | 76 (58.0) | 6 (7.9) | 70 (92.1) | >0.999 4 | 0.86 (0.25–2.97) |
| No (≥2.500 g) | 55 (42.0) | 5 (9.1) | 50 (90.9) | - | 1 |
| Term birth 2 | |||||
| Weight (grams) | n = 615 | n = 70 | n = 545 | ||
| Mean (±standard deviation) | 3119.0 (±513.7) | 3269.2 (±434.0) | 3099.7 (±520.2) | - | - |
| Median (IIQ 25−75%) | 3120 (2780–3475) | 3272 (2924–3555) | 3090 (2760–3455) | 0.009 3 | - |
| Minimum–maximum | 1320–4525 | 2340–4285 | 1320–4525 | - | - |
| Low weight at birth | |||||
| Yes (<2.500 g) | 69 (11.2) | 1 (1.4) | 68 (98.6) | 0.006 5 | 0.10 (0.01–0.74) |
| No (≥2.500 g) | 546 (88.8) | 69 (12.6) | 477 (87.4) | - | 1 |
| Variables | Total | Children with Zika or Exposed (Mothers Confirmed with Zika) | Control (Non-Reactive) | p-Value | OR (IC95%) | |
|---|---|---|---|---|---|---|
| Result of dengue in pregnant women (exposure) | ||||||
| Positive | 34 (4.3) | 8 (10.5) | 26 (4.3) | 0.043 1 | 2.62 (1.14–6.03) | |
| Negative | 648 (81.3) | 68 (89.5) | 580 (95.7) | - | 1 | |
| No information | 115 (14.4) | - | - | - | - | |
| Result of chikungunya in pregnant women (exposure) | ||||||
| Positive (IgM) | 4 (0.5) | 1 (1.2) | 3 (0.4) | 0.369 1 | 2.76 (0.28–26.84) | |
| Negative | 761 (95.5) | 82 (98.8) | 679 (99.6) | - | 1 | |
| No information | 32 (4.0) | - | - | - | - | |
| Vision impairment | ||||||
| Yes | 8(1) | 1 | 7 (3.0) | 0.353 1 | 0.27 (0.01–4835.00) 2 | |
| No | 281 (35.3) | 55 (100.0) | 226 (97.0) | - | 1 | |
| No information | 509 (63.8) | - | - | - | - | |
| Arthrogryposis | ||||||
| Yes | - | - | - | - | - | |
| No | 572 (71.8) | 65 (100.0) | 507 (100.0) | - | 1 | |
| No information | 225 (28.2) | - | - | - | - | |
| Hearing Loss | ||||||
| Yes | 8 (1.0) | 3 (4.9) | 4 (1.5) | 0.123 1 | 3.40 (0.74–15.61) | |
| No | 312 (39.1) | 58 (95.1) | 263 (98.5) | - | 1 | |
| No information | 479 (59.9) | - | - | - | - | |
| Abnormalities in post-natal imaging | ||||||
| Yes | 17 (2.1) | 5 (11.1) | 12 (13.0) | 0.747 2 | 0.83 (0.28–2.53) | |
| No | 120 (15.1) | 40 (88.9) | 80 (87.0) | - | 1 | |
| No information | 660 (82.8) | - | - | - | - | |
| Abnormalities of neuropsychomotor development | ||||||
| Yes | 27 (3.4) | 9 (13.2) | 18 (3.8) | 0.003 1 | 3.85 (1.65–8.96) | |
| No | 513 (64.4) | 59 (86.8) | 454 (96.2) | - | 1 | |
| No information | 257 (32.2) | - | - | - | - | |
| Dysphagia | ||||||
| Yes | 15 (1.9) | 6 (10.0) | 9 (2.9) | 0.022 1 | 3.68 (1.26–10.76) | |
| No | 352 (44.2) | 54 (90.0) | 298 (97.1) | - | 1 | |
| No information | 429 (53.9) | - | - | - | - | |
| Variables | Univariate Analysis OR (95% CI) | Multivariate Analysis OR (95% CI) |
|---|---|---|
| Positive dengue result in pregnant women | 2.62 (1.14–6.03) | 4.13 (1.04–16.42) |
| Hearing Loss | 3.40 (0.74–15.61) | 1.29 (0.12–13.57) |
| Abnormalities of neuropsychomotor development | 3.85 (1.65–8.96) | 10.33 (1.96–54.50) |
| Dysphagia | 3.68 (1.26–10.76) | 1.99 (0.38–10.45) |
| Low weight at birth | 0.35 (0.16–0.79) | 0.54 (0.15–2.01) |
| Microcephaly | 0.46 (0.18–1.17) | 0.28 (0.06–1.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazeta, R.E.; Bertozzi, A.P.A.P.; Dezena, R.d.C.d.A.B.; Silva, A.C.B.; Fajardo, T.C.G.; Catalan, D.T.; Rizzo, M.d.F.V.; Moron, A.F.; Soriano-Arandes, A.; Clemente, N.S.; et al. Three-Year Clinical Follow-Up of Children Intrauterine Exposed to Zika Virus. Viruses 2021, 13, 523. https://doi.org/10.3390/v13030523
Gazeta RE, Bertozzi APAP, Dezena RdCdAB, Silva ACB, Fajardo TCG, Catalan DT, Rizzo MdFV, Moron AF, Soriano-Arandes A, Clemente NS, et al. Three-Year Clinical Follow-Up of Children Intrauterine Exposed to Zika Virus. Viruses. 2021; 13(3):523. https://doi.org/10.3390/v13030523
Chicago/Turabian StyleGazeta, Rosa Estela, Ana Paula Antunes Pascalicchio Bertozzi, Rita de Cássia de Aguirre Bernardes Dezena, Andrea Cristina Botelho Silva, Thamirys Cosmo Gillo Fajardo, Daniel T. Catalan, Maria de Fátima Valente Rizzo, Antonio Fernandes Moron, Antoni Soriano-Arandes, Nuria Sanchez Clemente, and et al. 2021. "Three-Year Clinical Follow-Up of Children Intrauterine Exposed to Zika Virus" Viruses 13, no. 3: 523. https://doi.org/10.3390/v13030523
APA StyleGazeta, R. E., Bertozzi, A. P. A. P., Dezena, R. d. C. d. A. B., Silva, A. C. B., Fajardo, T. C. G., Catalan, D. T., Rizzo, M. d. F. V., Moron, A. F., Soriano-Arandes, A., Clemente, N. S., Quintella, T., Ventura, D. F., Damico, F. M., França, V. d. C. R. d. M., Almeida, J. P. G. d., Zara, A. L. d. S. A., Pires, L. C., Jundiaí, C. Z. v., & Passos, S. D. (2021). Three-Year Clinical Follow-Up of Children Intrauterine Exposed to Zika Virus. Viruses, 13(3), 523. https://doi.org/10.3390/v13030523








