Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling in Switzerland
2.1.1. Goat Flock A
2.1.2. Goat Flocks B, C and D
2.1.3. Diagnostic Clarifications
2.2. RNA Extraction and RT-qPCR
2.3. Virus Isolation in Cell Culture
2.4. Serological Analyses
2.4.1. Production of a Polyclonal Antiserum
2.4.2. ELISA
2.4.3. Virus Neutralization Test
2.5. Experimental Infection of Goats
2.6. Sequence Analysis
3. Results
3.1. Sampling in Switzerland
3.1.1. Clinical Findings
3.1.2. Pathological Findings
3.2. BTV RT-qPCR
3.3. Virus Isolation In Vitro
3.4. Serological Investigations
3.4.1. Polyclonal Antiserum
3.4.2. ELISA Results of Field Sera
3.4.3. Virus Neutralization
3.5. Experimental Inoculation of Goats
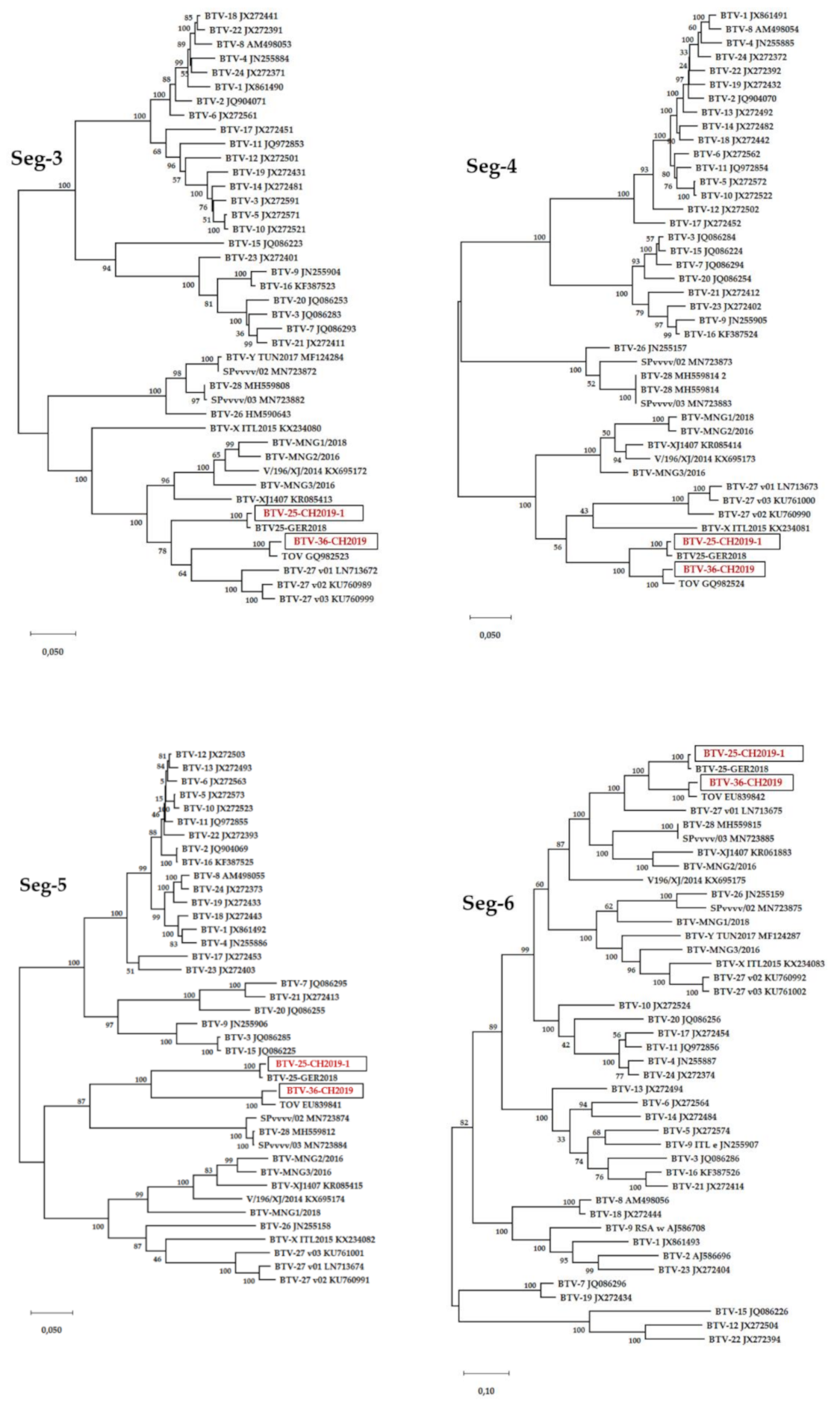
3.6. Sequence Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, P. Bluetongue virus structure and assembly. Curr. Opin. Virol. 2017, 24, 115–123. [Google Scholar] [CrossRef]
- Mertens, P.P.; Diprose, J.; Maan, S.; Singh, K.P.; Attoui, H.; Samuel, A.R. Bluetongue virus replication, molecular and structural biology. Vet. Ital. 2004, 40, 426–437. [Google Scholar] [PubMed]
- Ries, C.; Sharav, T.; Tseren-Ochir, E.O.; Beer, M.; Hoffmann, B. Putative Novel Serotypes ‘33’ and ‘35’ in Clinically Healthy Small Ruminants in Mongolia Expand the Group of Atypical BTV. Viruses 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- OIE. Terrestial Manual. Chapter 3.1.3. Bluetongue (Infection with Bluetongue Virus). 2018. Available online: https://www.oie.int/international-standard-setting/terrestrial-manual (accessed on 20 January 2021).
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Darpel, K.E.; Barber, J.; Hope, A.; Wilson, A.J.; Gubbins, S.; Henstock, M.; Frost, L.; Batten, C.; Veronesi, E.; Moffat, K.; et al. Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Darpel, K.E.; Monaghan, P.; Simpson, J.; Anthony, S.J.; Veronesi, E.; Brooks, H.W.; Elliott, H.; Brownlie, J.; Takamatsu, H.H.; Mellor, P.S.; et al. Involvement of the skin during bluetongue virus infection and replication in the ruminant host. Vet. Res. 2012, 43, 40. [Google Scholar] [CrossRef]
- Drew, C.P.; Heller, M.C.; Mayo, C.; Watson, J.L.; Maclachlan, N.J. Bluetongue virus infection activates bovine monocyte-derived macrophages and pulmonary artery endothelial cells. Vet. Immunol. Immunopathol. 2010, 136, 292–296. [Google Scholar] [CrossRef]
- Barratt-Boyes, S.M.; MacLachlan, N.J. Dynamics of viral spread in bluetongue virus infected calves. Vet. Microbiol. 1994, 40, 361–371. [Google Scholar] [CrossRef]
- Caporale, M.; Di Gialleonorado, L.; Janowicz, A.; Wilkie, G.; Shaw, A.; Savini, G.; Van Rijn, P.A.; Mertens, P.; Di Ventura, M.; Palmarini, M. Virus and host factors affecting the clinical outcome of bluetongue virus infection. J. Virol. 2014, 88, 10399–10411. [Google Scholar] [CrossRef]
- Gibbs, E.P.; Greiner, E.C. The epidemiology of bluetongue. Comp. Immunol. Microbiol. Infect. Dis. 1994, 17, 207–220. [Google Scholar] [CrossRef]
- Wilson, A.; Mellor, P. Bluetongue in Europe: Vectors, epidemiology and climate change. Parasitol. Res. 2008, 103 (Suppl. 1), S69–S77. [Google Scholar] [CrossRef]
- Pinior, B.; Firth, C.L.; Loitsch, A.; Stockreiter, S.; Hutter, S.; Richter, V.; Lebl, K.; Schwermer, H.; Kasbohrer, A. Cost distribution of bluetongue surveillance and vaccination programmes in Austria and Switzerland (2007–2016). Vet. Rec. 2018, 182, 257. [Google Scholar] [CrossRef]
- Racloz, V.; Straver, R.; Kuhn, M.; Thur, B.; Vanzetti, T.; Stark, K.D.; Griot, C.; Cagienard, A. Establishment of an early warning system against bluetongue virus in Switzerland. Schweiz. Arch. Tierheilkd. 2006, 148, 593–598. [Google Scholar] [CrossRef]
- Hofmann, M.; Griot, C.; Chaignat, V.; Perler, L.; Thur, B. Bluetongue disease reaches Switzerland. Schweiz. Arch. Tierheilkd. 2008, 150, 49–56. [Google Scholar] [CrossRef]
- Worwa, G.; Hilbe, M.; Chaignat, V.; Hofmann, M.A.; Griot, C.; Ehrensperger, F.; Doherr, M.G.; Thur, B. Virological and pathological findings in Bluetongue virus serotype 8 infected sheep. Vet. Microbiol. 2010, 144, 264–273. [Google Scholar] [CrossRef]
- European Comission. Animal Disease Notification System. 2017. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ad_adns_overview_2017.pdf (accessed on 14 December 2020).
- European Comission. Animal Disease Notifciation System. 2018. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ad_adns_overview_2018.pdf (accessed on 14 December 2020).
- European Comission. Animal Disease Notifcation System. 2019. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ad_adns_overview_2019.pdf (accessed on 14 December 2020).
- European Comission. Animal Disease Notification System. 2020. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ad_adns_overview_2020.pdf (accessed on 14 December 2020).
- Hofmann, M.A.; Renzullo, S.; Mader, M.; Chaignat, V.; Worwa, G.; Thuer, B. Genetic characterization of toggenburg orbivirus, a new bluetongue virus, from goats, Switzerland. Emerg. Infect. Dis. 2008, 14, 1855–1861. [Google Scholar] [CrossRef]
- Chaignat, V.; Worwa, G.; Scherrer, N.; Hilbe, M.; Ehrensperger, F.; Batten, C.; Cortyen, M.; Hofmann, M.; Thuer, B. Toggenburg Orbivirus, a new bluetongue virus: Initial detection, first observations in field and experimental infection of goats and sheep. Vet. Microbiol. 2009, 138, 11–19. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Nomikou, K.; Batten, C.; Antony, F.; Belaganahalli, M.N.; Samy, A.M.; Reda, A.A.; Al-Rashid, S.A.; El Batel, M.; et al. Novel bluetongue virus serotype from Kuwait. Emerg. Infect. Dis. 2011, 17, 886–889. [Google Scholar] [CrossRef]
- Zientara, S.; Sailleau, C.; Viarouge, C.; Hoper, D.; Beer, M.; Jenckel, M.; Hoffmann, B.; Romey, A.; Bakkali-Kassimi, L.; Fablet, A.; et al. Novel bluetongue virus in goats, Corsica, France, 2014. Emerg. Infect. Dis. 2014, 20, 2123–2125. [Google Scholar] [CrossRef]
- Sun, E.C.; Huang, L.P.; Xu, Q.Y.; Wang, H.X.; Xue, X.M.; Lu, P.; Li, W.J.; Liu, W.; Bu, Z.G.; Wu, D.L. Emergence of a Novel Bluetongue Virus Serotype, China 2014. Transbound. Emerg. Dis. 2016, 63, 585–589. [Google Scholar] [CrossRef]
- Savini, G.; Puggioni, G.; Meloni, G.; Marcacci, M.; Di Domenico, M.; Rocchigiani, A.M.; Spedicato, M.; Oggiano, A.; Manunta, D.; Teodori, L.; et al. Novel putative Bluetongue virus in healthy goats from Sardinia, Italy. Infect. Genet. Evol. 2017, 51, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Planzer, J.; Kaufmann, C.; Worwa, G.; Gavier-Widen, D.; Hofmann, M.A.; Chaignat, V.; Thur, B. In vivo and in vitro propagation and transmission of Toggenburg orbivirus. Res. Vet. Sci. 2011, 91, e163–e168. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Sant, S.; Mangone, I.; Goria, M.; Dondo, A.; Zoppi, S.; van Gennip, R.G.P.; Radaelli, M.C.; Camma, C.; van Rijn, P.A.; et al. One after the other: A novel Bluetongue virus strain related to Toggenburg virus detected in the Piedmont region (North-western Italy), extends the panel of novel atypical BTV strains. Transbound. Emerg. Dis. 2018, 65, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Domes, U.; Janowetz, B.; Bottcher, J.; Burkhardt, K.; Miller, T.; Beer, M.; Hoffmann, B. Isolation and Cultivation of a New Isolate of BTV-25 and Presumptive Evidence for a Potential Persistent Infection in Healthy Goats. Viruses 2020, 12, 983. [Google Scholar] [CrossRef]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Methods 2006, 136, 200–209. [Google Scholar] [CrossRef]
- Bahnemann, H.G. Inactivation of viral antigens for vaccine preparation with particular reference to the application of binary ethylenimine. Vaccine 1990, 8, 299–303. [Google Scholar] [CrossRef]
- Chrzastek, K.; Lee, D.H.; Smith, D.; Sharma, P.; Suarez, D.L.; Pantin-Jackwood, M.; Kapczynski, D.R. Use of Sequence-Independent, Single-Primer-Amplification (SISPA) for rapid detection, identification, and characterization of avian RNA viruses. Virology 2017, 509, 159–166. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Rajko-Nenow, P.; Golender, N.; Bumbarov, V.; Brown, H.; Frost, L.; Darpel, K.; Tennakoon, C.; Flannery, J.; Batten, C. Complete Coding Sequence of a Novel Bluetongue Virus Isolated from a Commercial Sheeppox Vaccine. Microbiol. Resour. Announc. 2020, 9. [Google Scholar] [CrossRef]
- Schulz, C.; Breard, E.; Sailleau, C.; Jenckel, M.; Viarouge, C.; Vitour, D.; Palmarini, M.; Gallois, M.; Hoper, D.; Hoffmann, B.; et al. Bluetongue virus serotype 27: Detection and characterization of two novel variants in Corsica, France. J. Gen. Virol. 2016, 97, 2073–2083. [Google Scholar] [CrossRef]
- Batten, C.A.; Henstock, M.R.; Steedman, H.M.; Waddington, S.; Edwards, L.; Oura, C.A. Bluetongue virus serotype 26: Infection kinetics, pathogenesis and possible contact transmission in goats. Vet. Microbiol. 2013, 162, 62–67. [Google Scholar] [CrossRef]
- Breard, E.; Schulz, C.; Sailleau, C.; Bernelin-Cottet, C.; Viarouge, C.; Vitour, D.; Guillaume, B.; Caignard, G.; Gorlier, A.; Attoui, H.; et al. Bluetongue virus serotype 27: Experimental infection of goats, sheep and cattle with three BTV-27 variants reveal atypical characteristics and likely direct contact transmission BTV-27 between goats. Transbound. Emerg. Dis. 2018, 65, e251–e263. [Google Scholar] [CrossRef]
- Santos, D.S.; Silva, C.C.; Araujo, V.O.; de Fatima Souza, M.; Lacerda-Lucena, P.B.; Simoes, S.V.; Riet-Correa, F.; Lucena, R.B. Primary photosensitization caused by ingestion of Froelichia humboldtiana by dairy goats. Toxicon 2017, 125, 65–69. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Colegate, S.M.; Knoppel, E.L.; Rood, K.A.; Collett, M.G. Wild parsnip (Pastinaca sativa)-induced photosensitization. Toxicon 2019, 167, 60–66. [Google Scholar] [CrossRef]
- Garcia, J.A.; Romero, A.; Uzal, F.A.; Tarigo, L.; Affolter, V.K.; Dutra, F. Solar-induced dorsal skin necrosis in sheep. Vet. Derm. 2019, 30, 442-e137. [Google Scholar] [CrossRef]
- Batten, C.; Darpel, K.; Henstock, M.; Fay, P.; Veronesi, E.; Gubbins, S.; Graves, S.; Frost, L.; Oura, C. Evidence for transmission of bluetongue virus serotype 26 through direct contact. PLoS ONE 2014, 9, e96049. [Google Scholar] [CrossRef]
- Bonneau, K.R.; Mullens, B.A.; MacLachlan, N.J. Occurrence of genetic drift and founder effect during quasispecies evolution of the VP2 and NS3/NS3A genes of bluetongue virus upon passage between sheep, cattle, and Culicoides sonorensis. J. Virol. 2001, 75, 8298–8305. [Google Scholar] [CrossRef]
- Samy, A.M.; Peterson, A.T. Climate Change Influences on the Global Potential Distribution of Bluetongue Virus. PLoS ONE 2016, 11, e0150489. [Google Scholar] [CrossRef]
- Maan, N.S.; Maan, S.; Nomikou, K.; Guimera, M.; Pullinger, G.; Singh, K.P.; Belaganahalli, M.N.; Mertens, P.P.C. The Genome Sequence of Bluetongue Virus Type 2 from India: Evidence for Reassortment between Eastern and Western Topotype Field Strains. J. Virol. 2012, 86, 5967–5968. [Google Scholar] [CrossRef]



| Organ Material | |||||||
|---|---|---|---|---|---|---|---|
| Goat ID | EDTA Blood | Skin 1 | Skin 2 | Lung | Liver | Spleen | Mesenteric Lymph Node |
| #12 | 29.9 | - | - | 32.3 | 32.1 | 29.2 | - |
| #13 | 30.23 | - | - | 34.4 | 32.4 | 30.7 | - |
| #14 | 29.47 | - | - | 33.2 | 31.1 | 33.1 | - |
| #15 | 30.05 | - | - | 32.3 | 31.7 | 31.4 | - |
| #17 | - | - | - | - | - | - | - |
| #18 | - | - | - | - | - | - | - |
| #19 | - | - | - | - | - | - | - |
| Segment/Protein (Accesion No.) | Serotype (nt/aa) | Strain (nt/aa) | Accession No. (nt/aa) | Identity Level % (nt/aa) | Query Cover % |
|---|---|---|---|---|---|
| 25/25 | TOV/TOV | GQ982522.1/ACY02806.1 | 96.52/98.23 | 100/99 | |
| 1/VP1 (LR993239) | 27/unknown | BTV-27-FRA2014-v02/BTV-MNG3-2016 | KU760987.1/CAD2286107.1 | 85.21/93.55 | 100/99 |
| 27/unknown | BTV-27-FRA2014-v01/BTV-MNG1-2018 | LN713671.1/CAD2286086.1 | 85.01/93.24 | 100/99 | |
| Unknown/unknown | BTV-XJ1407/BTV-MNG3-2016 | KR061882.1/CAD2286108.1 | 66.83/63.39 | 86/99 | |
| 2/VP2 (LR993240) | Unknown/unknown | BTV-MNG3-2016/BTV-Y-TUN2017 | LR877359.1/AVQ09328.1 | 66.34/63.19 | 95/96 |
| Unknown/unknown | BTV-MNG2-2016/V196-XJ-2014 | LR877348.1/ASW41947.1 | 66.14/62.41 | 99/99 | |
| 25/25 | TOV/TOV | GQ982523.1/ACY02807.1 | 97.93/99.11 | 100/99 | |
| 3/VP3 (LR993241) | 27/unknown | BTV-27-FRA2014-v03/BTV-MNG3-2016 | KU760999.1/CAD2286109.1 | 84.85/95.34 | 100/99 |
| 27/unknown | BTV-27-FRA2014-v02/BTV-MNG2-2016 | KU760989.1/CAD2286091.1 | 84.66/95.34 | 100/99 | |
| 25/25 | TOV/TOV | GQ982524.1/ACY02808.1 | 97.99/98.60 | 100/99 | |
| 4/VP4 (LR993242) | Unknown/unknown | BTV-Z ITL2017/BTV-Z ITL2017 | MF673723.2/AVA16291.2 | 90.63/97.27 | 91/91 |
| Unknown/unknown | BTV-25-GER2018/BTV-MNG3-2016 | LR798444.1/CAD2286110.1 | 90.59/92.24 | 100/99 | |
| 25/25 | TOV/TOV | EU839841.1/ACJ06703.1 | 97.33/96.72 | 99/99 | |
| 5/NS1 (LR993243) | Unknown/unknown | BTV-25-GER2018/BTV-25-GER2018 | LR798445.1/CAB5237905.1 | 78.34/83.24 | 99/99 |
| 4/11 | MOR2004-02/USA2013-WA 13-031503 | KP821423.1/AKM21154.1 | 75.17/79.60 | 99/99 | |
| 25/25 | TOV/TOV | EU839842.1/ACJ06704.1 | 96.58/97.15 | 100/99 | |
| 6/VP5 (LR993244) | Unknown/27 | BTV-25-GER2018/BTV-27-FRA2014-v01 | LR798446.1/CEK41875.1 | 82.86/86.69 | 100/99 |
| 27/unknown | BTV-27-FRA2014-v01/BTV-28-1537-14 | LN713675.1/QDH76491.1 | 77.74/84.79 | 100/99 | |
| 25/25 | TOV/TOV | EU839843.1/ACJ06705.1 | 97.52/99.71 | 100/99 | |
| 7/VP7 (LR993245) | 27/26 | BTV-27-FRA2014-v03/KUW2010-02 | KU760993.1/AED99449.1 | 84.48/97.99 | 100/99 |
| 27/unknown | BTV-27-FRA2014-v02/Spvvvv-03 | KU761003.1/QGW56811.1 | 84.19/97.71 | 100/99 | |
| 25/25 | TOV/TOV | EU839844.1/ACJ06706.1 | 97.46/97.45 | 100/99 | |
| 8/NS2 (LR993246) | 25/27 | BTV-25-GER2018/BTV-27-FRA2014-v03 | LR798448.1/AMQ36834.1 | 96.61/85.84 | 100/99 |
| 27/27 | BTV-27-FRA2014-v03/BTV-27-FRA2014-v01 | KU761004.1/CEK41877.1 | 82.67/85.55 | 100/99 | |
| 9/VP6 (LR993247) | 25/25 | TOV/TOV | EU839845.1/ACJ06707.1 | 97.17/96.35 | 99/99 |
| Unknown/unknown | BTV-25-GER2018/BTV-25-GER2018 | LR798449.1/CAB5237909.1 | 85.96/83.59 | 99/99 | |
| Unknown/unknown | BTV-MNG1-2018/BTV-MNG1-2018 | LR877345.1/CAD2286102.1 | 81.89/79.03 | 99/99 | |
| 10/NS3 (LR993248) | 25/27 | TOV/BTV-27-FRA2014-v02 | EU839846.1/AMQ36826.1 | 94.64/95.20 | 100/99 |
| Unknown/25 | BTV-25-GER2018/TOV | LR798450.1/ACJ06708.1 | 87.25/93.89 | 100/99 | |
| 27/unknown | BTV-27-FRA2014-v02/BTV-X ITL2015 | KU760996.1/APC23697.2 | 86.96/93.01 | 100/99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ries, C.; Vögtlin, A.; Hüssy, D.; Jandt, T.; Gobet, H.; Hilbe, M.; Burgener, C.; Schweizer, L.; Häfliger-Speiser, S.; Beer, M.; et al. Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland. Viruses 2021, 13, 721. https://doi.org/10.3390/v13050721
Ries C, Vögtlin A, Hüssy D, Jandt T, Gobet H, Hilbe M, Burgener C, Schweizer L, Häfliger-Speiser S, Beer M, et al. Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland. Viruses. 2021; 13(5):721. https://doi.org/10.3390/v13050721
Chicago/Turabian StyleRies, Christina, Andrea Vögtlin, Daniela Hüssy, Tabea Jandt, Hansjörg Gobet, Monika Hilbe, Carole Burgener, Luzia Schweizer, Stephanie Häfliger-Speiser, Martin Beer, and et al. 2021. "Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland" Viruses 13, no. 5: 721. https://doi.org/10.3390/v13050721
APA StyleRies, C., Vögtlin, A., Hüssy, D., Jandt, T., Gobet, H., Hilbe, M., Burgener, C., Schweizer, L., Häfliger-Speiser, S., Beer, M., & Hoffmann, B. (2021). Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland. Viruses, 13(5), 721. https://doi.org/10.3390/v13050721






