Enteroviruses in Respiratory Samples from Paediatric Patients of a Tertiary Care Hospital in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Viral Detection and Typing
2.3. Statistics
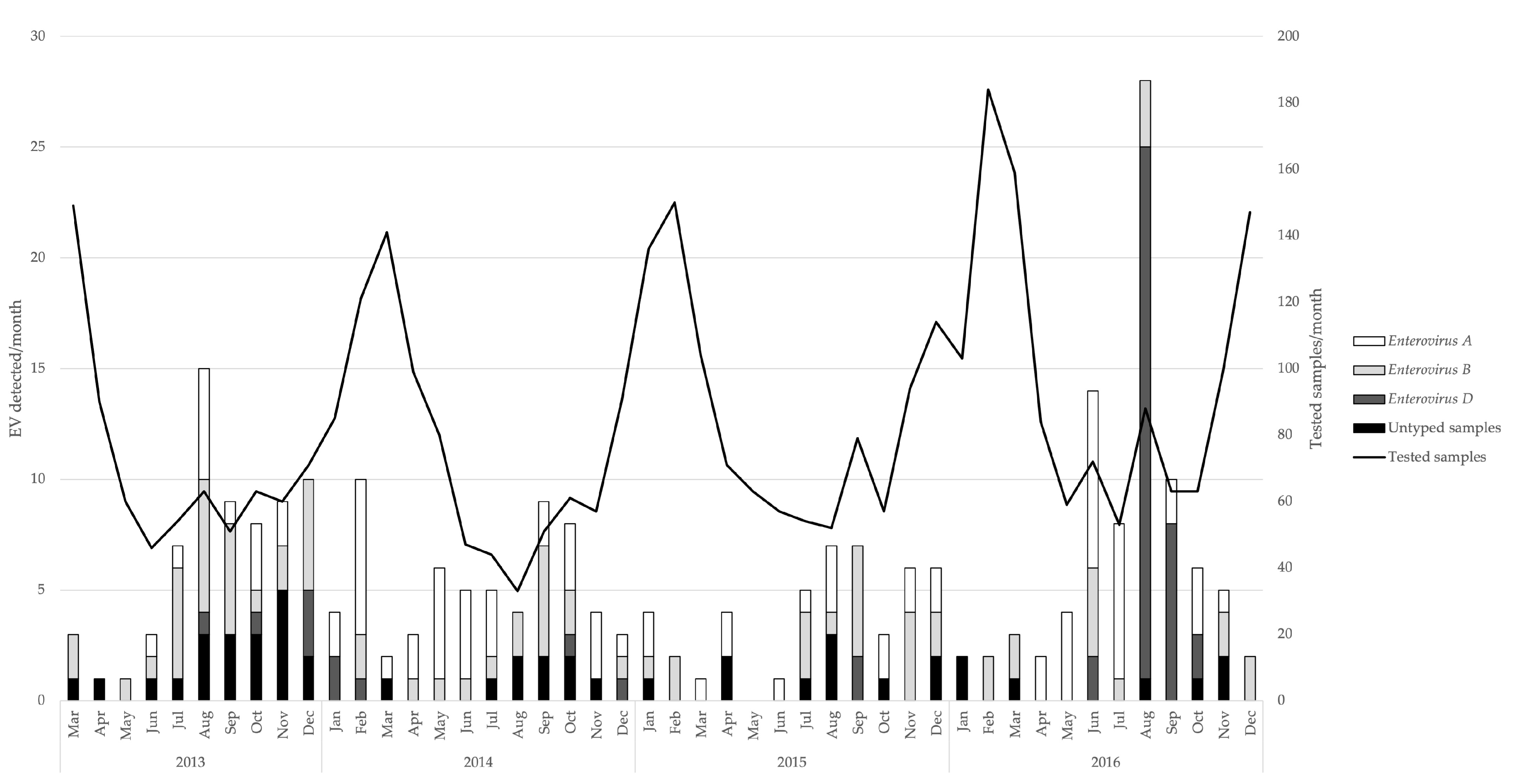
3. Results
3.1. Detection of Enteroviruses and Molecular Characterisation
3.2. Patients’ Characteristics and Clinical Symptoms
3.3. Pre-Existing Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oberste, M.S.; Gerber, S.I. Enteroviruses and Parechoviruses: Echoviruses, Coxsackieviruses, and Others. In Viral Infections of Humans; Kaslow, R.A., Stanberry, L.R., Le Duc, J.W., Eds.; Springer US: Boston, MA, USA, 2014; pp. 225–252. ISBN 978-1-4899-7447-1. [Google Scholar]
- Picornavirus Home. Available online: https://www.picornaviridae.com (accessed on 19 December 2019).
- Pallansch, M.; Roos, R. Enteroviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Volume 1, pp. 839–888. [Google Scholar]
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, Seminested PCR Amplification of VP1 Sequences for Direct Identification of All Enterovirus Serotypes from Original Clinical Specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvala, H.; Broberg, E.; Benschop, K.; Berginc, N.; Ladhani, S.; Susi, P.; Christiansen, C.; McKenna, J.; Allen, D.; Makiello, P.; et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J. Clin. Virol. 2018, 101, 11–17. [Google Scholar] [CrossRef]
- Royston, L.; Tapparel, C. Rhinoviruses and Respiratory Enteroviruses: Not as Simple as ABC. Viruses 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- De Crom, S.C.M.; Rossen, J.W.A.; Van Furth, A.M.; Obihara, C.C. Enterovirus and parechovirus infection in children: A brief overview. Eur. J. Nucl. Med. Mol. Imaging 2016, 175, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013, 14, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Tapparel, C.; Junier, T.; Gerlach, D.; Van Belle, S.; Turin, L.; Cordey, S.; Mühlemann, K.; Regamey, N.; Aubert, J.-D.; Soccal, P.M.; et al. New Respiratory Enterovirus and Recombinant Rhinoviruses among Circulating Picornaviruses. Emerg. Infect. Dis. 2009, 15, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.; Vila, J.; Gimferrer, L.; Piñana, M.; Esperalba, J.; Codina, M.G.; Barnés, M.; Martín, M.C.; Fuentes, F.; Rubio, S.; et al. Surveillance of enteroviruses from paediatric patients attended at a tertiary hospital in Catalonia from 2014 to 2017. J. Clin. Virol. 2019, 110, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Bray-Aschenbrenner, A.; Williams, H.; Buchanan, P.; Werner, J. The Resource Burden of Infections with Rhinovirus/Enterovirus, Influenza, and Respiratory Syncytial Virus in Children. Clin. Pediatr. 2018, 58, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Holm-Hansen, C.C.; Midgley, S.E.; Fischer, T.K. Global emergence of enterovirus D68: A systematic review. Lancet Infect. Dis. 2016, 16, e64–e75. [Google Scholar] [CrossRef]
- Holm-Hansen, C.C.; Midgley, S.E.; Schjørring, S.; Fischer, T.K. The importance of enterovirus surveillance in a Post-polio world. Clin. Microbiol. Infect. 2017, 23, 352–354. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A.; Benschop, K.S.; Donker, G.; Van Der Avoort, H.G. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011. Eurosurveillance 2014, 19, 20935. [Google Scholar] [CrossRef]
- Rapid Risk Assessment—Enterovirus Detections Associated with Severe Neurological Symptoms in Children and Adults in European Countries, 8 August 2016. Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/01-08-2016-RRA-Enterovirus%2071-Spain,%20France,%20Netherlands.pdf (accessed on 23 April 2018).
- Montes, M.; Oñate, E.; Muguruza, A.; Tamayo, E.; Carrera, I.M.; Iturzaeta, A.; Cilla, G. Enterovirus D68 Causing Acute Respiratory Infection: Clinical Characteristics and Differences with Acute Respiratory Infections Associated With Enterovirus Non-D. Pediatr. Infect. Dis. J. 2019, 38, 687–691. [Google Scholar] [CrossRef]
- Gelaw, A.; Pietsch, C.; Tigabu, Z.; Liebert, U.G. Genotyping of enteroviruses and human parechoviruses highlights their diversity in Northwest Ethiopia. J. Med. Virol. 2020, 92, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Kroneman, A.; Vennema, H.; Deforche, K.; Avoort, H.; Peñaranda, S.; Oberste, M.; Vinjé, J.; Koopmans, M. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 2011, 51, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, M.; Díaz-Cerio, M.; Muñoz-Almagro, C.; Rabella, N.; Tarragó, D.; Romero, M.P.; Pena, M.J.; Calvo, C.; Rey-Cao, S.; Moreno-Docón, A.; et al. Molecular epidemiology of enterovirus and parechovirus infections according to patient age over a 4-year period in Spain. J. Med. Virol. 2017, 89, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Poelman, R.; Schölvinck, E.H.; Borger, R.; Niesters, H.G.; Van Leer-Buter, C. The emergence of enterovirus D68 in a Dutch University Medical Center and the necessity for routinely screening for respiratory viruses. J. Clin. Virol. 2015, 62, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poelman, R.; Schuffenecker, I.; Van Leer-Buter, C.; Josset, L.; Niesters, H.G.; Lina, B. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J. Clin. Virol. 2015, 71, 1–9. [Google Scholar] [CrossRef] [Green Version]
- González-Sanz, R.; Taravillo, I.; Reina, J.; Navascués, A.; Moreno-Docón, A.; Aranzamendi, M.; Romero, M.P.; Del Cuerpo, M.; Pérez-González, C.; Pérez-Castro, S.; et al. Enterovirus D68-associated respiratory and neurological illness in Spain, 2014. Emerg. Microbes Infect. 2019, 8, 1438–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piralla, A.; Principi, N.; Ruggiero, L.; Girello, A.; Giardina, F.; De Sando, E.; Caimmi, S.; Bianchini, S.; Marseglia, G.L.; Lunghi, G.; et al. Enterovirus-D68 (EV-D68) in pediatric patients with respiratory infection: The circulation of a new B3 clade in Italy. J. Clin. Virol. 2018, 99–100, 91–96. [Google Scholar] [CrossRef]
- Knoester, M.; Schölvinck, E.H.; Poelman, R.; Smit, S.; Vermont, C.L.; Niesters, H.G.M.; Van Leer-Buter, C.C. Upsurge of Enterovirus D68, the Netherlands. Emerg. Infect. Dis. 2017, 23, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Dyrdak, R.; Grabbe, M.; Hammas, B.; Ekwall, J.; Hansson, K.; Luthander, J.; Naucler, P.; Reinius, H.; Rotzén-Östlund, M.; Albert, J. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September. Eurosurveillance 2016, 21, 30403. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Sabatier, M.; Wirth, T.; Pichon, M.; Lina, B.; Schuffenecker, I.; Josset, L. Molecular diversity and biennial circulation of enterovirus D68: A systematic screening study in Lyon, France, 2010 to 2016. Eurosurveillance 2018, 23, 1700711. [Google Scholar] [CrossRef] [Green Version]
- Piralla, A.; Girello, A.; Grignani, M.; Gozalo-Margüello, M.; Marchi, A.; Marseglia, G.; Baldanti, F. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J. Med. Virol. 2013, 86, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Benschop, K.S.M.; Rahamat-Langendoen, J.C.; Van Der Avoort, H.G.A.M.; Claas, E.C.J.; Pas, S.D.; Schuurman, R.; Verweij, J.J.; Wolthers, K.C.; Niesters, H.G.M.; Koopmans, M.P.G.; et al. VIRO-TypeNed, systematic molecular surveillance of enteroviruses in the Netherlands between 2010 and 2014. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- de Crom, S.; Rossen, J.; de Moor, R.; Veldkamp, E.; van Furth, A.; Obihara, C. Prospective assessment of clinical symptoms associated with enterovirus and parechovirus genotypes in a multicenter study in Dutch children. J. Clin. Virol. 2016, 77, 15–20. [Google Scholar] [CrossRef]
- Antona, D.; Kossorotoff, M.; Schuffenecker, I.; Mirand, A.; Leruez-Ville, M.; Bassi, C.; Aubart, M.; Moulin, F.; Lévy-Bruhl, D.; Henquell, C.; et al. Severe paediatric conditions linked with EV-A71 and EV-D68, France, May to October. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef]
- Böttcher, S.; Prifert, C.; Weißbrich, B.; Adams, O.; Aldabbagh, S.; Eis-Hübinger, A.M.; Diedrich, S. Detection of enterovirus D68 in patients hospitalised in three tertiary university hospitals in Germany, 2013 to 2014. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- Khetsuriani, N.; LaMonte, A.; Oberste, M.S.; Pallansch, M. Neonatal Enterovirus Infections Reported to the National Enterovirus Surveillance System in the United States, 1983–2003. Pediatr. Infect. Dis. J. 2006, 25, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; Murti, M.; Gustafson, R.; Pollock, S.; Hoyano, D.; Rempel, S.; Allison, S.; De Serres, G.; et al. Systematic community- and hospital-based surveillance for enterovirus-D68 in three Canadian provinces, August to December. Eurosurveillance 2015, 20, 30047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiche, J.; Böttcher, S.; Diedrich, S.; Buchholz, U.; Buda, S.; Haas, W.; Schweiger, B.; Wolff, T. Low-level Circulation of Enterovirus D68–Associated Acute Respiratory Infections, Germany. Emerg. Infect. Dis. 2015, 21, 837–841. [Google Scholar] [CrossRef]
- Hixon, A.M.; Yu, G.; Leser, J.S.; Yagi, S.; Clarke, P.; Chiu, C.Y.; Tyler, K.L. A mouse model of paralytic myelitis caused by enterovirus D. PLoS Pathog. 2017, 13, e1006199. [Google Scholar] [CrossRef] [Green Version]
- Dyda, A.; Stelzer-Braid, S.; Adam, D.; Chughtai, A.; MacIntyre, C.R. The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68)—What is the evidence for causation? Eurosurveillance 2018, 23, 17-00310. [Google Scholar] [CrossRef] [Green Version]
- Messacar, K.; Schreiner, T.L.; Maloney, J.; Wallace, A.; Ludke, J.; Oberste, M.S.; Nix, W.A.; Robinson, C.C.; Glodé, M.P.; Abzug, M.J.; et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015, 385, 1662–1671. [Google Scholar] [CrossRef]
- Bragstad, K.; Jakobsen, K.; Rojahn, A.E.; Skram, M.K.; Vainio, K.; Holberg-Petersen, M.; Hungnes, O.; Dudman, S.G.; Kran, A.-M.B. High frequency of enterovirus D68 in children hospitalised with respiratory illness in Norway, autumn 2014. Influ. Other Respir. Viruses 2014, 9, 59–63. [Google Scholar] [CrossRef]
- Sabanathan, S.; Van Tan, L.; Thwaites, L.; Wills, B.; Qui, P.T.; Van Doorn, H.R. Enterovirus 71 related severe hand, foot and mouth disease outbreaks in South-East Asia: Current situation and ongoing challenges. J. Epidemiol. Community Heal. 2014, 68, 500–502. [Google Scholar] [CrossRef] [Green Version]
- Van Der Sanden, S.; Koopmans, M.; Uslu, G.; Van Der Avoort, H.; on behalf of the Dutch Working Group for Clinical Virology. Epidemiology of Enterovirus 71 in The Netherlands, 1963 to 2008. J. Clin. Microbiol. 2009, 47, 2826–2833. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, Safety, and Immunogenicity of an Enterovirus 71 Vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; Poelman, R.; Knoester, M.; Van Leer-Buter, C.C.; Niesters, H.G.M. Enterovirus D68—The New Polio? Front. Microbiol. 2018, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Jasir, A.; Penttinen, P.; Celentano, L.P.; Greco, D.; Broberg, E. Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European Economic Area. Eurosurveillance 2017, 22, 16-00807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Species | Genotypes | 2013 | 2014 | 2015 | 2016 | Total |
|---|---|---|---|---|---|---|
| Enterovirus A | CVA2 | 4 | 2 | - | 2 | 8 |
| CVA4 | - | 3 | 3 | 2 | 8 | |
| CVA5 | 1 | 2 | 2 | - | 5 | |
| CVA6 | 3 | 18 | 9 | 7 | 37 | |
| CVA10 | - | 4 | - | 5 | 9 | |
| CVA16 | 2 | 4 | - | 1 | 7 | |
| EV-A71, Subgenogroup C1 | 1 | - | 2 | 10 | 13 | |
| EV-A71, Subgenogroup C2 | 2 | - | 1 | 1 | 4 | |
| Enterovirus B | CVA9 | 1 | 2 | - | 1 | 4 |
| CVB1 | 2 | - | 1 | - | 3 | |
| CVB2 | 2 | 2 | 3 | 1 | 8 | |
| CVB3 | 5 | 2 | - | 2 | 9 | |
| CVB4 | 5 | - | 2 | 1 | 8 | |
| CVB5 | 1 | 2 | 2 | 4 | 9 | |
| E3 | 2 | 2 | - | - | 4 | |
| E5 | - | - | - | 1 | 1 | |
| E6 | - | 1 | - | - | 1 | |
| E7 | - | - | 5 | 2 | 7 | |
| E9 | 1 | 2 | - | - | 3 | |
| E11 | 1 | - | - | - | 1 | |
| E18 | - | 2 | 3 | 2 | 7 | |
| E20 | - | - | 1 | - | 1 | |
| E25 | 1 | - | - | 1 | 2 | |
| E27 | - | - | 1 | - | 1 | |
| E30 | 9 | - | - | 2 | 11 | |
| Enterovirus D | EV-D68, Clade A2 | - | 1 | - | - | 1 |
| EV-D68, Clade B1 | 1 | 3 | - | - | 4 | |
| EV-D68, Clade B2 | 15 | 4 | - | 1 | 20 | |
| EV-D68, Clade B3 | - | - | 2 | 35 | 37 | |
| Untyped | 4 | 2 | 7 | 9 | 22 | |
| Total | 63 | 58 | 44 | 90 | 255 |
| Enterovirus A | Enterovirus B | Enterovirus D | Total | p * | |
|---|---|---|---|---|---|
| Number | 90 | 78 | 62 | 230 | |
| Characteristics | |||||
| Median age, months | 19 b,d | 24 | 32 | 22 | <0.001 |
| Median duration of hospital stay, days | 3 | 3 | 3.5 | 3 | 0.155 |
| Median duration of ICU stay, days | 5.5 | 4 | 7 | 6 | 0.757 |
| Oxygen demand, n (%) | 8 (17.8%) | 11 (24.4%) | 26 (57.8%) a,b | 45 | <0.001 |
| Clinical Symptoms | |||||
| Upper respiratory tract, n (%) | 51 (48.6%) d | 43 (41.0%) d | 11 (10.5%) | 105 | <0.001 |
| Obstructive bronchitis, n (%) | 8 (13.1%) | 11 (18.0%) | 42 (68.9%) a,b | 61 | <0.001 |
| Pneumonia, n (%) | 2 (10.5%) | 8 (42.1%) a | 9 (47.4%) a | 19 | 0.013 |
| Other respiratory symptoms, n (%) | 3 (42.9%) | 1 (14.3%) | 3 (42.9%) | 7 | 0.439 |
| Gastrointestinal symptoms, n (%) | 29 (59.2%) b,d | 13 (26.5%) | 7 (14.3%) | 49 | 0.004 |
| HFMD/exanthema, n (%) | 23 (76.7%) b,d | 7 (23.3%) d | 0 | 30 | <0.001 |
| Meningitis/encephalitis, n (%) | 5 (50.0%) | 4 (40.0%) | 1 (10.0%) | 10 | 0.510 |
| Other neurological symptoms, n (%) | 6 (60.0%) | 3 (30.0%) | 1 (10.0%) | 10 | 0.327 |
| Seizures, n (%) | 22 (53.7%) d | 15 (36.6%) d | 4 (9.8%) | 41 | 0.011 |
| Fever, n (%) | 33 (51.6%) d | 28 (43.8%) d | 3 (4.7%) | 64 | <0.001 |
| Others, n (%) | 16 (55.2%) d | 13 (44.8%) d | 0 | 29 | 0.002 |
| Characteristics | ICU Admission | Oxygen Demand | Duration of Hospital Stay 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | OR (95% CI) | Yes | No | p | OR (95% CI) | >3 Days | ≤3 Days | p | OR (95% CI) | |
| Sex | ||||||||||||
| Male | 15 | 121 | 0.681 | 1.21 (0.49–2.99) | 23 | 113 | 0.120 | 0.59 (0.31–1.15) | 57 | 79 | 0.601 | 1.16 (0.67–2.01) |
| Female | 8 | 78 | 22 | 64 | 33 | 53 | ||||||
| Age 2 | ||||||||||||
| ≤22 months | 14 | 100 | 0.337 | 1.54 (0.64–3.72) | 27 | 87 | 0.195 | 1.55 (0.80–3.02) | 50 | 64 | 0.301 | 1.33 (0.78–2.27) |
| >23 months | 9 | 99 | 18 | 90 | 40 | 68 | ||||||
| Prematurity | ||||||||||||
| Yes | 3 | 17 | 0.479 | 1.61 (0.43–5.96) | 9 | 11 | 0.006 * | 3.77 (1.46–9.77) | 12 | 8 | 0.069 | 2.39 (0.93–6.10) |
| No | 20 | 182 | 36 | 166 | 78 | 124 | ||||||
| Asthma | ||||||||||||
| Yes | 2 | 10 | 0.467 | 1.80 (0.37–8.77) | 4 | 8 | 0.256 | 2.06 (0.59–7.18) | 5 | 7 | 0.935 | 1.05 (0.32–3.42) |
| No | 21 | 189 | 41 | 169 | 85 | 125 | ||||||
| Epilepsy | ||||||||||||
| Yes | 2 | 9 | 0.391 | 2.01 (0.41–9.93) | 3 | 8 | 0.556 | 1.51 (0.38–5.93) | 7 | 4 | 0.122 | 2.70 (0.77–9.51) |
| No | 21 | 190 | 42 | 169 | 83 | 128 | ||||||
| Immunocompromised status | ||||||||||||
| Yes | 1 | 5 | 0.612 | 1.76 (0.20–15.7) | 0 | 6 | NA | NA | 4 | 2 | 0.207 | 3.02 (0.54–16.87) |
| No | 22 | 194 | 45 | 171 | 86 | 130 | ||||||
| EV–D68 infection | ||||||||||||
| Yes | 9 | 45 | 0.086 | 2.20 (0.89–5.42) | 26 | 28 | <0.001 | 7.28 (3.56–14.90) | 27 | 27 | 0.105 | 1.67 (0.90–3.09) |
| No | 14 | 154 | 19 | 149 | 63 | 105 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baertl, S.; Pietsch, C.; Maier, M.; Hönemann, M.; Bergs, S.; Liebert, U.G. Enteroviruses in Respiratory Samples from Paediatric Patients of a Tertiary Care Hospital in Germany. Viruses 2021, 13, 882. https://doi.org/10.3390/v13050882
Baertl S, Pietsch C, Maier M, Hönemann M, Bergs S, Liebert UG. Enteroviruses in Respiratory Samples from Paediatric Patients of a Tertiary Care Hospital in Germany. Viruses. 2021; 13(5):882. https://doi.org/10.3390/v13050882
Chicago/Turabian StyleBaertl, Susanne, Corinna Pietsch, Melanie Maier, Mario Hönemann, Sandra Bergs, and Uwe G. Liebert. 2021. "Enteroviruses in Respiratory Samples from Paediatric Patients of a Tertiary Care Hospital in Germany" Viruses 13, no. 5: 882. https://doi.org/10.3390/v13050882
APA StyleBaertl, S., Pietsch, C., Maier, M., Hönemann, M., Bergs, S., & Liebert, U. G. (2021). Enteroviruses in Respiratory Samples from Paediatric Patients of a Tertiary Care Hospital in Germany. Viruses, 13(5), 882. https://doi.org/10.3390/v13050882






