Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Search Strategy
2.2. Trend Analysis of Mosquito-Associated Viruses
3. Discovery of Mosquito-Associated Viruses
3.1. Flaviridae
3.2. Togaviridae
3.3. Peribunyaviridae
3.4. Other Virus Families
4. Vectors of Mosquito-Associated Viruses
4.1. Vectors of the Genera Aedes
4.2. Vectors of the Genera Culex
4.3. Vectors of the Genera Anopheles
5. Abiotic Factors Affecting Arboviruses
5.1. Effect of Urbanisation on Mosquito Species Diversity and Arboviruses
5.2. Human Population Growth Migration and Arboviruses
5.3. Effect of Land Use on Mosquito Diversity and Arboviruses
5.4. Effect of Climate Variability on Mosquito Diversity and Arbovirus Transmission
6. Discussion
6.1. Summary
6.2. Major Knowledge Gaps, Challenges, and Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-Borne Arboviruses of African Origin: Review of Key Viruses and Vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.; Shearer, F.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef] [Green Version]
- Buchwald, A.G.; Hayden, M.H.; Dadzie, S.K.; Paull, S.H.; Carlton, E.J. Aedes-Borne Disease Outbreaks in West Africa: A Call for Enhanced Surveillance. Acta Trop. 2020, 209, 105468. [Google Scholar] [CrossRef] [PubMed]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-Specific Viruses—Transmission and Interaction. Viruses 2019, 11, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junglen, S.; Marklewitz, M.; Zirkel, F.; Wollny, R.; Meyer, B.; Heidemann, H.; Metzger, S.; Annan, A.; Dei, D.; Leendertz, F.H.; et al. No Evidence of Gouléako and Herbert Virus Infections in Pigs, Côte d’Ivoire and Ghana. Emerg. Infect. Dis. 2015, 21, 2190–2193. [Google Scholar] [CrossRef] [Green Version]
- Kanouté, Y.B.; Gragnon, B.G.; Schindler, C.; Bonfoh, B.; Schelling, E. Epidemiology of Brucellosis, Q Fever and Rift Valley Fever at the Human and Livestock Interface in Northern Côte d’Ivoire. Acta Trop. 2017, 165, 66–75. [Google Scholar] [CrossRef]
- Durand, B.; Lo Modou, M.; Tran, A.; Ba, A.; Sow, F.; Belkhiria, J.; Fall, A.G.; Biteye, B.; Grosbois, V.; Chevalier, V. Rift Valley Fever in Northern Senegal: A Modelling Approach to Analyse the Processes Underlying Virus Circulation Recurrence. PLoS Negl. Trop. Dis. 2020, 14, e0008009. [Google Scholar] [CrossRef]
- Sule, W.F.; Oluwayelu, D.O.; Hernández-Triana, L.M.; Fooks, A.R.; Venter, M.; Johnson, N. Epidemiology and Ecology of West Nile Virus in Sub-Saharan Africa. Parasites Vectors 2018, 11, 414. [Google Scholar] [CrossRef] [Green Version]
- Vasilakis, N.; Guzman, H.; Firth, C.; Forrester, N.L.; Widen, S.G.; Wood, T.G.; Rossi, S.L.; Ghedin, E.; Popov, V.; Blasdell, K.R.; et al. Mesoniviruses Are Mosquito-Specific Viruses with Extensive Geographic Distribution and Host Range. Virol. J. 2014, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Vasilakis, N.; Tesh, R.B. Insect-Specific Viruses and Their Potential Impact on Arbovirus Transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Bolling, B.G.; Vasilakis, N.; Guzman, H.; Popov, V.L.; Wood, T.G.; Widen, S.G.; Thangamani, S.; Tesh, R.B. Insect-Specific Viruses Detected in Laboratory Mosquito Colonies and Their Potential Implications for Experiments Evaluating Arbovirus Vector Competence. Am. J. Trop. Med. Hyg. 2015, 92, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Hegde, S.; Rasgon, J.L.; Hughes, G.L. The Microbiome Modulates Arbovirus Transmission in Mosquitoes. Curr. Opin. Virol. 2015, 15, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauver, J.R.; Grubaugh, N.D.; Krajacich, B.J.; Weger-Lucarelli, J.; Lakin, S.M.; Fakoli, L.S.; Bolay, F.K.; Diclaro, J.W.; Dabiré, K.R.; Foy, B.D.; et al. West African Anopheles Gambiae Mosquitoes Harbor a Taxonomically Diverse Virome Including New Insect-Specific Flaviviruses, Mononegaviruses, and Totiviruses. Virology 2016, 498, 288–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanfack Minkeu, F.; Vernick, K. A Systematic Review of the Natural Virome of Anopheles Mosquitoes. Viruses 2018, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, J.H.; Needham, J.; Raychaudhuri, S.; Diamond, M.S.; Beasley, D.W.C.; Morkowski, S.; Salje, H.; Fernandez Salas, I.; Kim, D.Y.; Frolov, I.; et al. Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection. PLoS Negl. Trop. Dis. 2015, 9, e0004119. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.L.; Long, M.T. Perspectives on New Vaccines against Arboviruses Using Insect-Specific Viruses as Platforms. Vaccines 2021, 9, 263. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Weaver, S.C. Biotechnological Applications of an Insect-Specific Alphavirus. DNA Cell Biol. 2017, 36, 1045–1049. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Auguste, A.J.; Kaelber, J.T.; Luo, H.; Rossi, S.L.; Fenton, K.; Leal, G.; Kim, D.Y.; Chiu, W.; Wang, T.; et al. A Chikungunya Fever Vaccine Utilizing an Insect-Specific Virus Platform. Nat. Med. 2017, 23, 192–199. [Google Scholar] [CrossRef]
- Gressel, J. Microbiome Facilitated Pest Resistance: Potential Problems and Uses. Pest Manag. Sci. 2018, 74, 511–515. [Google Scholar] [CrossRef]
- Barnard, K.; Jeanrenaud, A.C.S.N.; Brooke, B.D.; Oliver, S.V. The Contribution of Gut Bacteria to Insecticide Resistance and the Life Histories of the Major Malaria Vector Anopheles Arabiensis (Diptera: Culicidae). Sci. Rep. 2019, 9, 9117. [Google Scholar] [CrossRef]
- Kwaśnik, M.; Rożek, W.; Rola, J. Rift Valley Fever—A Growing Threat to Humans and Animals. J. Vet. Res. 2021, 65, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Baudin, M.; Jumaa, A.M.; Jomma, H.J.E.; Karsany, M.S.; Bucht, G.; Näslund, J.; Ahlm, C.; Evander, M.; Mohamed, N. Association of Rift Valley Fever Virus Infection with Miscarriage in Sudanese Women: A Cross-Sectional Study. Lancet Glob. Health 2016, 4, e864–e871. [Google Scholar] [CrossRef] [Green Version]
- Stoler, J.; al Dashti, R.; Anto, F.; Fobil, J.N.; Awandare, G.A. Deconstructing “Malaria”: West Africa as the next Front for Dengue Fever Surveillance and Control. Acta Trop. 2014, 134, 58–65. [Google Scholar] [CrossRef]
- Zahouli, J.B.Z.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Effect of Land-Use Changes on the Abundance, Distribution, and Host-Seeking Behavior of Aedes Arbovirus Vectors in Oil Palm-Dominated Landscapes, Southeastern Côte d’Ivoire. PLoS ONE 2017, 12, e0189082. [Google Scholar] [CrossRef]
- Zahouli, J.B.Z.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Urbanization Is a Main Driver for the Larval Ecology of Aedes Mosquitoes in Arbovirus-Endemic Settings in South-Eastern Côte d’Ivoire. PLoS Negl. Trop. Dis. 2017, 11, e0005751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.-P.; et al. Climate and Urbanization Drive Mosquito Preference for Humans. Curr. Biol. 2020, 30, 3570–3579.e6. [Google Scholar] [CrossRef]
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Hemming, D.; Agusto, F.; Evans, K.J.; Fefferman, N.; Gaff, H.; Gumel, A.; LaDeau, S.; et al. Climate, Environmental and Socio-Economic Change: Weighing up the Balance in Vector-Borne Disease Transmission. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehorn, J.; Yacoub, S. Global Warming and Arboviral Infections. Clin. Med. 2019, 19, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global Expansion and Redistribution of Aedes-Borne Virus Transmission Risk with Climate Change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaythorpe, K.A.; Hamlet, A.; Cibrelus, L.; Garske, T.; Ferguson, N.M. The Effect of Climate Change on Yellow Fever Disease Burden in Africa. eLife 2020, 9, e55619. [Google Scholar] [CrossRef] [PubMed]
- Giesen, C.; Roche, J.; Redondo-Bravo, L.; Ruiz-Huerta, C.; Gomez-Barroso, D.; Benito, A.; Herrador, Z. The Impact of Climate Change on Mosquito-Borne Diseases in Africa. Pathog. Glob. Health 2020, 114, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate Change Could Shift Disease Burden from Malaria to Arboviruses in Africa. Lancet Planet. Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- WHO. Dengue Fever in Cape Verde—Update 1; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Guedes, D.R.D.; Gomes, E.T.B.; Paiva, M.H.S.; de Melo-Santos, M.A.V.; Alves, J.; Gómez, L.F.; Ayres, C.F.J. Circulation of DENV2 and DENV4 in Aedes aegypti (Diptera: Culicidae) Mosquitoes from Praia, Santiago Island, Cabo Verde. J. Insect Sci. 2017, 17, 86. [Google Scholar] [CrossRef] [Green Version]
- Manu, S.K.; Bonney, J.H.K.; Pratt, D.; Abdulai, F.N.; Agbosu, E.E.; Frimpong, P.O.; Adiku, T.K. Arbovirus Circulation among Febrile Patients at the Greater Accra Regional Hospital, Ghana. BMC Res. Notes 2019, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Amoako, N.; Duodu, S.; Dennis, F.E.; Bonney, J.H.K.; Asante, K.P.; Ameh, J.; Mosi, L.; Hayashi, T.; Agbosu, E.E.; Pratt, D.; et al. Detection of Dengue Virus among Children with Suspected Malaria, Accra, Ghana. Emerg. Infect. Dis. 2018, 24, 1544–1547. [Google Scholar] [CrossRef] [Green Version]
- Bonney, J.H.K.; Hayashi, T.; Dadzie, S.; Agbosu, E.; Pratt, D.; Nyarko, S.; Asiedu-Bekoe, F.; Ido, E.; Sarkodie, B.; Ohta, N.; et al. Molecular Detection of Dengue Virus in Patients Suspected of Ebola Virus Disease in Ghana. PLoS ONE 2018, 13, e0208907. [Google Scholar] [CrossRef]
- Robin, Y.; Cornet, M.; Heme, G.; Le Gonidec, G. Isolement Du Virus de La Dengue Au Sénégal [Dengue Virus Isolation in Senegal]. Ann. l’Institut Pasteur Virol. 1980, 131, 149–154. [Google Scholar] [CrossRef]
- Saluzzo, J.F.; Cornet, M.; Castagnet, P.; Rey, C.; Digoutte, J.P. Isolation of Dengue 2 and Dengue 4 Viruses from Patients in Senegal. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 5. [Google Scholar] [CrossRef]
- Traore-Lamizana, M.; Zeller, H.; Monlun, E.; Mondo, M.; Hervy, J.-P.; Adam, F.; Digoutte, J.-P. Dengue 2 Outbreak in Southeastern Senegal During 1990: Virus Isolations from Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 1994, 31, 623–627. [Google Scholar] [CrossRef]
- Dieng, I.; dos Passos Cunha, M.; Diagne, M.M.; Sembène, P.M.; de Andrade Zanotto, P.M.; Faye, O.; Faye, O.; Sall, A.A. Origin and Spread of the Dengue Virus Type 1, Genotype V in Senegal, 2015–2019. Viruses 2021, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Ba, Y.; Sall, A.A.; Diop, O.M.; Ndione, J.A.; Mondo, M.; Girault, L.; Mathiot, C. Amplification of the Sylvatic Cycle of Dengue Virus Type 2, Senegal, 1999–2000: Entomologic Findings and Epidemiologic Considerations. Emerg. Infect. Dis. 2003, 9, 362–367. [Google Scholar] [CrossRef]
- Faye, O.; Ba, Y.; Faye, O.; Talla, C.; Diallo, D.; Chen, R.; Mondo, M.; Ba, R.; Macondo, E.; Siby, T.; et al. Urban Epidemic of Dengue Virus Serotype 3 Infection, Senegal, 2009. Emerg. Infect. Dis. 2014, 20, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.; Causey, O.; Reddy, S.; Cooke, A. Dengue Viruses from Febrile Patients in Nigeria, 1964–1968. Lancet 1971, 297, 105–106. [Google Scholar] [CrossRef]
- Fagbami, A.H.; Fabiyi, A. Arbovirus Studies in Two Towns in Western State of Nigeria. Trop. Geogr. Med. 1975, 27, 59–62. [Google Scholar]
- Baba, M.M.; Talle, M. The Effect of Climate on Dengue Virus Infections in Nigeria. N. Y. Sci. J. 2011, 4, 28–33. [Google Scholar]
- Dawurung, J.; Baba, M.; Stephen, G.; Jonas, S.; Bukbuk, D.; Dawurung, C. Serological Evidence of Acute Dengue Virus Infection among Febrile Patients Attending Plateau State Specialist Hospital Jos, Nigeria. Rep. Opin. 2010, 2, 71–76. [Google Scholar]
- Idris, A.; Baba, M.; Thairu, Y.; Bamidele, O. Sero-Prevalence of Dengue Type-3 Virus among Patients with Febrile Illnesses Attending a Tertiary Hospital in Maiduguri, Nigeria. Int. J. Med. Med. Sci. 2013, 5, 560–563. [Google Scholar]
- Adedayo, F.; Nioma, I.; Olanrewaju, M.; Adeyinka, A.; Ebele, A. Serological Evidence of Recent Dengue Virus Infection Among Febrile Children in a Semi Arid Zone. Am. J. Infect. Dis. 2013, 9, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Oladipo, E.; Amanetu, C.; Gbadero, T.; Oloke, J. Detectable Anti-Dengue Virus IgM Antibodies among Healthy Individuals in Ogbomoso, Oyo State, Nigeria. Am. J. Infect. Dis. 2014, 10, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Eisenhut, M.; Schwarz, T.F.; Hegenscheid, B. Seroprevalence of Dengue, Chikungunya and Sindbis Virus Infections in German Aid Workers. Infection 1999, 27, 82–85. [Google Scholar] [CrossRef]
- Gautret, P.; Botelho-Nevers, E.; Charrel, R.N.; Parola, P. Dengue Virus Infections in Travellers Returning from Benin to France, July–August 2010. Euro Surveill. 2010, 15, 19657. [Google Scholar] [PubMed]
- Fourié, T.; Luciani, L.; Amrane, S.; Zandotti, C.; Leparc-Goffart, I.; Ninove, L.; Nougairède, A. Dengue Virus Type 1 Infection in Traveler Returning from Benin to France, 2019. Emerg. Infect. Dis. 2020, 26, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Bob, N.S.; Bâ, H.; Fall, G.; Ishagh, E.; Diallo, M.Y.; Sow, A.; Sembene, P.M.; Faye, O.; El Kouri, B.; Sidi, M.L.; et al. Detection of the Northeastern African Rift Valley Fever Virus Lineage During the 2015 Outbreak in Mauritania. Open Forum Infect. Dis. 2017, 4, ofx087. [Google Scholar] [CrossRef] [Green Version]
- Tougma, S.A.; Zoungrana Yaméogo, W.N.; Dahourou, D.L.; Salou Kagoné, I.A.; Compaoré, T.R.; Kaboré, A.; Kagoné, T.; Drabo, M.K.; Meda, N. Dengue Virus Infection and Pregnancy Outcomes during the 2017 Outbreak in Ouagadougou, Burkina Faso: A Retrospective Cohort Study. PLoS ONE 2020, 15, e0238431. [Google Scholar] [CrossRef] [PubMed]
- Tarnagda, Z.; Cissé, A.; Bicaba, B.W.; Diagbouga, S.; Sagna, T.; Ilboudo, A.K.; Tialla, D.; Lingani, M.; Sondo, K.A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018, 24, 170–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sondo, K.A.; Ouattara, A.; Diendéré, E.A.; Diallo, I.; Zoungrana, J.; Zémané, G.; Da, L.; Gnamou, A.; Meda, B.; Poda, A.; et al. Dengue Infection during Pregnancy in Burkina Faso: A Cross-Sectional Study. BMC Infect. Dis. 2019, 19, 997. [Google Scholar] [CrossRef] [PubMed]
- Hervy, J.-P.; Legros, F.; Roche, J.-C.; Monteny, N.; Diaco, B. Circulation Du Virus Dengue 2 Dans Plusieurs Milieux Boisés Des Savanes Soudaniennes de La Région de Bobo-Dioulasso (Burkina Faso). Cah. ORSTOM Entomol. Méd. Parasitol. 1984, 22, 135–143. [Google Scholar]
- Robert, V.; Lhuillier, M.; Meunier, D.; Sarthou, J.L.; Monteny, N.; Digoutte, J.P.; Cornet, M.; Germain, M.; Cordellier, R. Yellow Fever Virus, Dengue 2 and Other Arboviruses Isolated from Mosquitos, in Burkina Faso, from 1983 to 1986. Entomological and Epidemiological Considerations. Bull. Soc. Pathol. Exot. 1993, 86, 90–100. [Google Scholar]
- Ridde, V.; Agier, I.; Bonnet, E.; Carabali, M.; Dabiré, K.R.; Fournet, F.; Ly, A.; Meda, I.B.; Parra, B. Presence of Three Dengue Serotypes in Ouagadougou (Burkina Faso): Research and Public Health Implications. Infect. Dis. Poverty 2016, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.K.; Carabali, M.; Edwards, T.; Barro, A.; Lee, J.-S.; Dahourou, D.; Lee, K.S.; Nikiema, T.; Shin, M.Y.; Bonnet, E.; et al. Estimating the Force of Infection for Dengue Virus Using Repeated Serosurveys, Ouagadougou, Burkina Faso. Emerg. Infect. Dis. 2021, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Carabali, M.; Lee, J.-S.; Lee, K.-S.; Namkung, S.; Lim, S.-K.; Ridde, V.; Fernandes, J.; Lell, B.; Matendechero, S.H.; et al. Evaluating Dengue Burden in Africa in Passive Fever Surveillance and Seroprevalence Studies: Protocol of Field Studies of the Dengue Vaccine Initiative. BMJ Open 2018, 8, e017673. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Balasubramanian, R.; Ouedraogo, M.; Wandji Nana, L.R.; Mogeni, O.D.; Jeon, H.J.; van Pomeren, T.; Haselbeck, A.; Lim, J.K.; Prifti, K.; et al. The Epidemiology of Dengue Outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6, e04389. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kutsuna, S.; Maeki, T.; Tajima, S.; Takaya, S.; Katanami, Y.; Yamamoto, K.; Takeshita, N.; Hayakawa, K.; Kato, Y.; et al. A Case of Dengue Fever Imported from Burkina Faso to Japan in October 2016. Jpn. J. Infect. Dis. 2017, 70, 675–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, J.P.; Du Saussay, C.; Gautun, J.C.; McCormick, J.B.; Mouchet, J. Dengue in Burkina Faso (Ex-Upper Volta): Seasonal Epidemics in the Urban Area of Ouagadougou. Bull. Soc. Pathol. Exot. Fil. 1985, 78, 7–14. [Google Scholar]
- Fournet, F.; Rican, S.; Vaillant, Z.; Roudot, A.; Meunier-Nikiema, A.; Kassié, D.; Dabiré, R.; Salem, G. The Influence of Urbanization Modes on the Spatial Circulation of Flaviviruses within Ouagadougou (Burkina Faso). Int. J. Environ. Res. Public Health 2016, 13, 1226. [Google Scholar] [CrossRef] [Green Version]
- Eldin, C.; Gautret, P.; Nougairede, A.; Sentis, M.; Ninove, L.; Saidani, N.; Million, M.; Brouqui, P.; Charrel, R.; Parola, P. Identification of Dengue Type 2 Virus in Febrile Travellers Returning from Burkina Faso to France, Related to an Ongoing Outbreak, October to November 2016. Eurosurveillance 2016, 21, 30425. [Google Scholar] [CrossRef]
- Diallo, I.; Sondo, K.A.; Tieno, H.; Tamelokpo, E.Y.; Zoungrana, J.; Sagna, Y.; Savadogo, M.; Poda, A.; Guira, O.; Diendéré, E.A.; et al. À Propos de 98 Cas de Dengue Hospitalisés Dans Une Clinique Privée de Ouagadougou: Aspects Épidémiologiques, Diagnostiques et Évolutifs. Bull. Soc. Pathol. Exot. 2017, 110, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Collenberg, E.; Ouedraogo, T.; Ganamé, J.; Fickenscher, H.; Kynast-Wolf, G.; Becher, H.; Kouyaté, B.; Kräusslich, H.-G.; Sangaré, L.; Tebit, D.M. Seroprevalence of Six Different Viruses among Pregnant Women and Blood Donors in Rural and Urban Burkina Faso: A Comparative Analysis. J. Med. Virol. 2006, 78, 683–692. [Google Scholar] [CrossRef]
- Baronti, C.; Piorkowski, G.; Touret, F.; Charrel, R.; de Lamballerie, X.; Nougairede, A. Complete Coding Sequences of Two Dengue Virus Type 2 Strains Isolated from an Outbreak in Burkina Faso in 2016. Genome Announc. 2017, 5, e00209-17. [Google Scholar] [CrossRef] [Green Version]
- Safronetz, D.; Sacko, M.; Sogoba, N.; Rosenke, K.; Martellaro, C.; Traoré, S.; Cissé, I.; Maiga, O.; Boisen, M.; Nelson, D.; et al. Vectorborne Infections, Mali. Emerg. Infect. Dis. 2016, 22, 340–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoutrides, E.K.; Coulibaly, M.B.; George, C.M.; Sacko, A.; Traore, S.; Bessoff, K.; Wiley, M.R.; Kolivras, K.N.; Adelman, Z.; Traore, M.; et al. Dengue Virus Seroprevalence Among Febrile Patients in Bamako, Mali: Results of a 2006 Surveillance Study. Vector Borne Zoonotic Dis. 2011, 11, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Akoua-Koffi, C.; Diarrassouba, S.; Benie, V.; Ngbichi, J.; Bozoua, T.; Bosson, A.; Akran, V.; Carnevale, P.; Ehouman, A. Investigation Autour d’un Cas Mortel de Fièvre Jaune En Côte d’Ivoire En 1999. Bull. Soc. Pathol. Exot. 2001, 94, 227–230. [Google Scholar] [PubMed]
- Aoussi, E.B.F.; Ehui, E.; Kassi, N.A.; Kouakou, G.; Nouhou, Y.; Adjogoua, E.V.; Eholié, S.; Bissagnéné, E. Seven Native Cases of Dengue in Abidjan, Ivory Coast. Méd. Mal. Infect. 2014, 44, 433–436. [Google Scholar] [CrossRef] [PubMed]
- L’Azou, M.; Jean-Marie, J.; Bessaud, M.; Cabié, A.; Césaire, R.; de Lamballerie, X.; Courbil, R.; Richard, P. Dengue Seroprevalence in the French West Indies: A Prospective Study in Adult Blood Donors. Am. J. Trop. Med. Hyg. 2015, 92, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- WHO. Dengue Fever—Côte d’Ivoire—Disease Outbreak News 4 August 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Weekly Bulletin on Outbreaks and Other Emergencies—Week 42 2019, 14–20 October 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Butenko, A.M. Arbovirus Circulation in the Republic of Guinea. Med. Parazitol. 1996, 2, 40–45. [Google Scholar]
- Schoepp, R.J.; Rossi, C.A.; Khan, S.H.; Goba, A.; Fair, J.N. Undiagnosed Acute Viral Febrile Illnesses, Sierra Leone. Emerg. Infect. Dis. 2014, 20, 1176–1182. [Google Scholar] [CrossRef]
- de Araújo Lobo, J.M.; Mores, C.N.; Bausch, D.G.; Christofferson, R.C. Short Report: Serological Evidence of Under-Reported Dengue Circulation in Sierra Leone. PLoS Negl. Trop. Dis. 2016, 10, e0004613. [Google Scholar] [CrossRef] [Green Version]
- Dariano, D.F.; Taitt, C.R.; Jacobsen, K.H.; Bangura, U.; Bockarie, A.S.; Bockarie, M.J.; Lahai, J.; Lamin, J.M.; Leski, T.A.; Yasuda, C.; et al. Surveillance of Vector-Borne Infections (Chikungunya, Dengue, and Malaria) in Bo, Sierra Leone, 2012–2013. Am. J. Trop. Med. Hyg. 2017, 97, 1151–1154. [Google Scholar] [CrossRef]
- Ndiaye, E.H.; Diallo, D.; Fall, G.; Ba, Y.; Faye, O.; Dia, I.; Diallo, M. Arboviruses Isolated from the Barkedji Mosquito-Based Surveillance System, 2012–2013. BMC Infect. Dis. 2018, 18, 642. [Google Scholar] [CrossRef]
- Renaudet, J.; Jan, C.; Ridet, J.; Adam, C.; Robin, Y. A Serological Survey of Arboviruses in the Human Population of Senegal. Bull. Soc. Pathol. Exot. Fil. 1978, 71, 131–140. [Google Scholar]
- Diallo, M.; Nabeth, P.; Ba, K.; Sall, A.A.; Ba, Y.; Mondo, M.; Girault, L.; Abdalahi, M.O.; Mathiot, C. Mosquito Vectors of the 1998-1999 Outbreak of Rift Valley Fever and Other Arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med. Vet. Entomol. 2005, 19, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Macnamara, F.N.; Horn, D.W.; Porterfield, J.S. Yellow Fever and Other Arthropod-Borne Viruses; a Consideration of Two Serological Surveys Made in South Western Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1959, 53, 202–212. [Google Scholar] [CrossRef]
- Guyer, B. Serological Survey for Arboviruses in Igbo-Ora, Western Nigeria. Ann. Trop. Med. Parasitol. 1972, 66, 243–250. [Google Scholar] [CrossRef]
- Tomori, O.; Fagbami, A.; Fabiyi, A. Isolations of West Nile Virus from Man in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 103–104. [Google Scholar] [CrossRef]
- Fagbami, A.H. Zika Virus Infections in Nigeria: Virological and Seroepidemiological Investigations in Oyo State. J. Hyg. 1979, 83, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Olaleye, O.D.; Oladosu, L.A.; Omilabu, S.A.; Baba, S.S.; Fagbami, A.H. Complement Fixing Antibodies against Arboviruses in Horses at Lagos, Nigeria. Rev. Elev. Med. Vet. Pays Trop. 1989, 42, 321–325. [Google Scholar]
- Sule, W.F.; Oluwayelu, D.O.; Adedokun, R.A.M.; Rufai, N.; McCracken, F.; Mansfield, K.L.; Johnson, N. High Seroprevelance of West Nile Virus Antibodies Observed in Horses from Southwestern Nigeria. Vector Borne Zoonotic Dis. 2015, 15, 218–220. [Google Scholar] [CrossRef]
- Oderinde, B.S.; Mora-Cárdenas, E.; Carletti, T.; Baba, M.M.; Marcello, A. Prevalence of Locally Undetected Acute Infections of Flaviviruses in North-Eastern Nigeria. Virus Res. 2020, 286, 198060. [Google Scholar] [CrossRef]
- Campos, M.; Ward, D.; Morales, R.F.; Gomes, A.R.; Silva, K.; Sepúlveda, N.; Gomez, L.F.; Clark, T.G.; Campino, S. Surveillance of Aedes aegypti Populations in the City of Praia, Cape Verde: Zika Virus Infection, Insecticide Resistance and Genetic Diversity. Parasites Vectors 2020, 13, 481. [Google Scholar] [CrossRef]
- Diarra, I.; Nurtop, E.; Sangaré, A.K.; Sagara, I.; Pastorino, B.; Sacko, S.; Zeguimé, A.; Coulibaly, D.; Fofana, B.; Gallian, P.; et al. Zika Virus Circulation in Mali. Emerg. Infect. Dis. 2020, 26, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.R.; Bailey, A.L.; Weiler, A.M.; Barry, G.L.; Breitbach, M.E.; Stewart, L.M.; Jasinska, A.J.; Freimer, N.B.; Apetrei, C.; Phillips-Conroy, J.E.; et al. Seroprevalence of Zika Virus in Wild African Green Monkeys and Baboons. mSphere 2017, 2, e00393-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaun, J.J.; Germain, M.; Robert, V.; Robin, Y.; Monath, T.P.; Camicas, J.L.; Digoutte, J.P. Yellow Fever in Senegal from 1976 to 1980 (Author’s Transl). Med. Trop. 1981, 41, 45–51. [Google Scholar]
- Yaro, S.; Zango, A.; Rouamba, J.; Diabaté, A.; Dabiré, R.; Kambiré, C.; Tiendrebeogo, S.M.R.; Yonli, T.; Ouango, J.G.; Diagbouga, S.P. Situation Épidémiologique de La Fièvre Jaune Au Burkina Faso de 2003 à 2008. Bull. Soc. Pathol. Exot. 2010, 103, 44–47. [Google Scholar] [CrossRef]
- Baudon, D.; Robert, V.; Roux, J.; Lhuillier, M.; Saluzzo, J.F.; Sarthou, J.L.; Cornet, M.; Stanghellini, A.; Gazin, P.; Molez, J.F. The 1983 Yellow Fever Epidemic in Burkina Faso. Bull. World Health Organ. 1986, 64, 873–882. [Google Scholar]
- Barennes, H.; Baldet, T.; Cassel, A.-M.; Kabiré, C.; Kambou, C. An Epidemic Risk of Yellow Fever in Burkina Faso despite a Rapid Immunisation Riposte: Role of a Multidisciplinary Investigation Team. Sante 2002, 12, 323–329. [Google Scholar]
- Cordellier, R. The Epidemiology of Yellow Fever in Western Africa. Bull. World Health Organ. 1991, 69, 73–84. [Google Scholar]
- Agadzi, V.K.; Boatin, B.A.; Appawu, M.A.; Mingle, J.A.; Addy, P.A. Yellow Fever in Ghana, 1977–1980. Bull. World Health Organ. 1984, 62, 577–583. [Google Scholar]
- WHO. Yellow Fever in Ghana (Emergencies Preparedness, Response); WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Cornet, M.; Robin, Y.; Chateau, R.; Heme, G.; Adam, C.; Valade, M.; Gonidec, G.; Jan, C.; Renaudet, J.; Dieng, P.L.; et al. Isolements d’arbovirus Au Sénégal Oriental à Partir de Moustiques (1972–1977) et Notes Sur l’épidémiologie Des Virus Transmis Par Les Aedes, En Particulier Du Virus Amaril. Cah. ORSTOM. Sér. Entomol. Méd. Parasitol. 1979, 17, 149–163. [Google Scholar]
- Nikolay, B.; Diallo, M.; Boye, C.S.B.; Sall, A.A. Usutu Virus in Africa. Vector Borne Zoonotic Dis. 2011, 11, 1417–1423. [Google Scholar] [CrossRef]
- Kemp, G.; Humburg, J.; Alhaji, I. Isolation and Identification of African Horse-Sickness Virus in Nigeria. Vet. Rec. 1971, 89, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.; Calisher, C.; Mcguire, K. Spondweni Virus Infection in a Foreign Resident of Upper Volta. Lancet 1982, 320, 1306–1308. [Google Scholar] [CrossRef]
- Robin, Y.; Cornet, M.; Le Gonidec, G.; Chateau, R.; Heme, G. [Kedougou Virus (Ar D14701): A New Arbovirus (“Flavivirus”) Isolated in Senegal (Author’s Transl)]. Ann. Microbiol. 1978, 129, 239–244. [Google Scholar]
- Woodruff, A.W.; Bowen, E.T.; Platt, G.S. Viral Infections in Travellers from Tropical Africa. BMJ 1978, 1, 956–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boorman, J.P.T.; Draper, C.C. Isolations of Arboviruses in the Lagos Area of Nigeria, and a Survey of Antibodies to Them in Man and Animals. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 269–277. [Google Scholar] [CrossRef]
- Moore, D.L.; Reddy, S.; Akinkugbe, F.M.; Lee, V.H.; David-West, T.S.; Causey, O.R.; Carey, D.E. An Epidemic of Chikungunya Fever at Ibadan, Nigeria, 1969. Ann. Trop. Med. Parasitol. 1974, 68, 59–68. [Google Scholar] [CrossRef]
- Tomori, O.; Fagbami, A.; Fabiyi, A. The 1974 Epidemic of Chikungunya Fever in Children in Ibadan. Trop. Geogr. Med. 1975, 27, 413–417. [Google Scholar] [PubMed]
- Sow, A.; Faye, O.; Diallo, M.; Diallo, D.; Chen, R.; Faye, O.; Diagne, C.T.; Guerbois, M.; Weidmann, M.; Ndiaye, Y.; et al. Chikungunya Outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum Infect. Dis. 2018, 5, ofx259. [Google Scholar] [CrossRef]
- Bacci, A.; Marchi, S.; Massougbodji, A.; Perrin, R.X.; Chippaux, J.-P.; Sambri, V.; Landini, M.P.; Fievet, N.; Varani, S.; Rossini, G. High Seroprevalence of Chikungunya Virus Antibodies Among Pregnant Women Living in an Urban Area in Benin, West Africa. Am. J. Trop. Med. Hyg. 2015, 92, 1133–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansumana, R.; Jacobsen, K.H.; Gbakima, A.A.; Hodges, M.H.; Lamin, J.M.; Leski, T.A.; Malanoski, A.P.; Lin, B.; Bockarie, M.J.; Stenger, D.A. Presumptive Self-Diagnosis of Malaria and Other Febrile Illnesses in Sierra Leone. Pan Afr. Med. J. 2013, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, F.; Xia, L.X.; Zhu, L.W.; Kamara, I.L.; Huang, K.Q.; Zhang, Y.; Liu, J.; Kargbo, B.; Wang, J.; et al. Next-Generation Sequencing Study of Pathogens in Serum from Patients with Febrile Jaundice in Sierra Leone. Biomed. Environ. Sci. 2019, 32, 363–370. [Google Scholar] [PubMed]
- Odend’hal, S. Semliki Forest Virus. In The Geographical Distribution of Animal Viral Diseases; Elsevier: Amsterdam, The Netherlands, 1983; pp. 373–375. [Google Scholar]
- Hubálek, Z.; Rudolf, I.; Nowotny, N. Arboviruses Pathogenic for Domestic and Wild Animals. Adv. Virus. Res. 2014, 89, 201–275. [Google Scholar] [PubMed]
- Gonzalez, J.P.; Le Guenno, B.; Some, M.J.R.; Akakpo, J.A. Serological Evidence in Sheep Suggesting Phlebovirus Circulation in a Rift Valley Fever Enzootic Area in Burkina Faso. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 680–682. [Google Scholar] [CrossRef]
- Boussini, H.; Lamien, C.E.; Nacoulma, O.G.; Kabore, A.; Poda, G.; Viljoen, G.J. Prevalence of Rift Valley Fever in Domestic Ruminants in the Central and Northern Regions of Burkina Faso. Rev. Sci. Tech. OIE 2014, 33, 893–901. [Google Scholar] [CrossRef]
- Akakpo, A.J.; Some, M.J.; Bornarel, P.; Jouan, A.; Gonzalez, J.P. Epidemiology of Rift Valley Fever in Western Africa. I. Serologic Survey in Domestic Ruminants of Burkina Faso. Bull. Soc. Pathol. Exot. Fil. 1989, 82, 321–331. [Google Scholar]
- Boiro, I.; Konstaninov, O.K.; Numerov, A.D. Isolation of Rift Valley Fever Virus from Bats in the Republic of Guinea. Bull. Soc. Pathol. Exot. Fil. 1987, 80, 62–67. [Google Scholar]
- Tong, C.; Javelle, E.; Grard, G.; Dia, A.; Lacrosse, C.; Fourié, T.; Gravier, P.; Watier-Grillot, S.; Lancelot, R.; Letourneur, F.; et al. Tracking Rift Valley Fever: From Mali to Europe and Other Countries, 2016. Eurosurveillance 2019, 24, 1800213. [Google Scholar] [CrossRef]
- Haneche, F.; Leparc-Goffart, I.; Simon, F.; Hentzien, M.; Martinez-Pourcher, V.; Caumes, E.; Maquart, M. Rift Valley Fever in Kidney Transplant Recipient Returning from Mali with Viral RNA Detected in Semen up to Four Months from Symptom Onset, France, Autumn 2015. Eurosurveillance 2016, 21, 30222. [Google Scholar] [CrossRef]
- Subudhi, S.; Dakouo, M.; Sloan, A.; Stein, D.R.; Grolla, A.; Jones, S.; Dibernardo, A.; Rosenke, K.; Sas, M.; Traore, A.; et al. Seroprevalence of Rift Valley Fever Virus Antibodies in Cattle in Mali, 2005–2014. Am. J. Trop. Med. Hyg. 2018, 98, 872–874. [Google Scholar] [CrossRef] [Green Version]
- Lagare, A.; Fall, G.; Ibrahim, A.; Ousmane, S.; Sadio, B.; Abdoulaye, M.; Alhassane, A.; Mahaman, A.E.; Issaka, B.; Sidikou, F.; et al. First Occurrence of Rift Valley Fever Outbreak in Niger, 2016. Vet. Med. Sci. 2019, 5, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, W. Identification of Rift Valley Fever in Nigeria. Bull. Epizoot Dis. Afr. 1959, 7, 317–318. [Google Scholar]
- Musa, A.A.; Yila, S.A.; Allam, L.; Sackey, A.; Alhaji, N.B.; Garba, B.S.; Mambula-Machunga, S.; Nafarnda, W.D.; Owolodun, O.A.; Dzikwi, A.A. Serological Evidence of Rift Valley Fever Infection and Risk Factors among One-Humped Camels (Camelus Dromedarius) in Northern Nigeria. bioRxiv 2020. [Google Scholar] [CrossRef]
- Diallo, M.; Lochouarn, L.; Sall, A.A.; Mondo, M.; Ba, K.; Girault, L.; Mathiot, C. First Isolation of the Rift Valley Fever Virus from Culex Poicilipes (Diptera: Culicidae) in Nature. Am. J. Trop. Med. Hyg. 2000, 62, 702–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomor, O.; Monath, T.P.; Lee, V.; Fagbami, A.; Fabiyi, A. Bwamba Virus Infection: A Sero-Survey of Veterbrates in Five Ecological Zones in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1974, 68, 461–465. [Google Scholar] [CrossRef]
- Eiden, M.; Vina-Rodriguez, A.; El Mamy, B.O.; Isselmou, K.; Ziegler, U.; Höper, D.; Jäckel, S.; Balkema-Buschmann, A.; Unger, H.; Doumbia, B.; et al. Ngari Virus in Goats during Rift Valley Fever Outbreak, Mauritania, 2010. Emerg. Infect. Dis. 2014, 20, 2174–2176. [Google Scholar] [CrossRef] [Green Version]
- Digoutte, J.P.; Gagnard, V.J.; Bres, P.; Pajot, F.X. Nyando Virus Infection in Man. Bull. Soc. Pathol. Exot. Filiales 1972, 65, 751–758. [Google Scholar] [PubMed]
- Oluwayelu, D.O.; Aiki-Raji, C.O.; Umeh, E.C.; Mustapha, S.O.; Adebiyi, A.I. Serological Investigation of Akabane Virus Infection in Cattle and Sheep in Nigeria. Adv. Virol. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.S.; Olaleye, O.D.; Ayanbadejo, O.A. Haemagglutination-Inhibiting Antibodies against African Horse Sickness Virus in Domestic Animals in Nigeria. Vet. Res. 1993, 24, 483–487. [Google Scholar]
- Oladosu, L.A.; Olayeye, O.D.; Baba, S.S.; Omilabu, S.A. Isolation and Identification of African Horse Sickness Virus during an Outbreak in Lagos, Nigeria. Rev. Sci. Tech. OIE 1993, 12, 873–877. [Google Scholar] [CrossRef]
- Adeyefa, C.A.; Hamblin, C. Continuing Prevalence of African Horse Sickness in Nigeria. Rev. Elev. Med. Vet. Pays Trop. 1995, 48, 31–33. [Google Scholar] [CrossRef]
- Amoa-Bosompem, M.; Kobayashi, D.; Murota, K.; Faizah, A.N.; Itokawa, K.; Fujita, R.; Osei, J.H.N.; Agbosu, E.; Pratt, D.; Kimura, S.; et al. Entomological Assessment of the Status and Risk of Mosquito-Borne Arboviral Transmission in Ghana. Viruses 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junglen, S.; Korries, M.; Grasse, W.; Wieseler, J.; Kopp, A.; Hermanns, K.; León-Juárez, M.; Drosten, C.; Kümmerer, B.M. Host Range Restriction of Insect-Specific Flaviviruses Occurs at Several Levels of the Viral Life Cycle. mSphere 2017, 2, e00375-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junglen, S.; Kopp, A.; Kurth, A.; Pauli, G.; Ellerbrok, H.; Leendertz, F.H. A New Flavivirus and a New Vector: Characterization of a Novel Flavivirus Isolated from Uranotaenia Mosquitoes from a Tropical Rain Forest. J. Virol. 2009, 83, 4462–4468. [Google Scholar] [CrossRef] [Green Version]
- Hermanns, K.; Zirkel, F.; Kopp, A.; Marklewitz, M.; Rwego, I.B.; Estrada, A.; Gillespie, T.R.; Drosten, C.; Junglen, S. Discovery of a Novel Alphavirus Related to Eilat Virus. J. Gen. Virol. 2017, 98, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Marklewitz, M.; Zirkel, F.; Kurth, A.; Drosten, C.; Junglen, S. Evolutionary and Phenotypic Analysis of Live Virus Isolates Suggests Arthropod Origin of a Pathogenic RNA Virus Family. Proc. Natl. Acad. Sci. USA 2015, 112, 7536–7541. [Google Scholar] [CrossRef] [Green Version]
- Marklewitz, M.; Zirkel, F.; Rwego, I.B.; Heidemann, H.; Trippner, P.; Kurth, A.; Kallies, R.; Briese, T.; Lipkin, W.I.; Drosten, C.; et al. Discovery of a Unique Novel Clade of Mosquito-Associated Bunyaviruses. J. Virol. 2013, 87, 12850–12865. [Google Scholar] [CrossRef] [Green Version]
- Quan, P.-L.; Junglen, S.; Tashmukhamedova, A.; Conlan, S.; Hutchison, S.K.; Kurth, A.; Ellerbrok, H.; Egholm, M.; Briese, T.; Leendertz, F.H.; et al. Moussa Virus: A New Member of the Rhabdoviridae Family Isolated from Culex Decens Mosquitoes in Côte d’Ivoire. Virus Res. 2010, 147, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Diagne, M.M.; Gaye, A.; Ndione, M.H.D.; Faye, M.; Fall, G.; Dieng, I.; Widen, S.G.; Wood, T.G.; Popov, V.; Guzman, H.; et al. Dianke Virus: A New Mesonivirus Species Isolated from Mosquitoes in Eastern Senegal. Virus Res. 2020, 275, 197802. [Google Scholar] [CrossRef]
- Zirkel, F.; Kurth, A.; Quan, P.-L.; Briese, T.; Ellerbrok, H.; Pauli, G.; Leendertz, F.H.; Lipkin, W.I.; Ziebuhr, J.; Drosten, C.; et al. An Insect Nidovirus Emerging from a Primary Tropical Rainforest. MBio 2011, 2, e00077-11. [Google Scholar] [CrossRef] [Green Version]
- Zirkel, F.; Roth, H.; Kurth, A.; Drosten, C.; Ziebuhr, J.; Junglen, S. Identification and Characterization of Genetically Divergent Members of the Newly Established Family Mesoniviridae. J. Virol. 2013, 87, 6346–6358. [Google Scholar] [CrossRef] [Green Version]
- Hermanns, K.; Zirkel, F.; Kurth, A.; Drosten, C.; Junglen, S. Cimodo Virus Belongs to a Novel Lineage of Reoviruses Isolated from African Mosquitoes. J. Gen. Virol. 2014, 95, 905–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marklewitz, M.; Handrick, S.; Grasse, W.; Kurth, A.; Lukashev, A.; Drosten, C.; Ellerbrok, H.; Leendertz, F.H.; Pauli, G.; Junglen, S. Gouleako Virus Isolated from West African Mosquitoes Constitutes a Proposed Novel Genus in the Family Bunyaviridae. J. Virol. 2011, 85, 9227–9234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilakis, N.; Forrester, N.L.; Palacios, G.; Nasar, F.; Savji, N.; Rossi, S.L.; Guzman, H.; Wood, T.G.; Popov, V.; Gorchakov, R.; et al. Negevirus: A Proposed New Taxon of Insect-Specific Viruses with Wide Geographic Distribution. J. Virol. 2013, 87, 2475–2488. [Google Scholar] [CrossRef] [Green Version]
- Parry, R.; Naccache, F.; Ndiaye, E.H.; Fall, G.; Castelli, I.; Lühken, R.; Medlock, J.; Cull, B.; Hesson, J.C.; Montarsi, F.; et al. Identification and RNAi Profile of a Novel Iflavirus Infecting Senegalese Aedes vexans arabiensis Mosquitoes. Viruses 2020, 12, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, S.; Zirkel, F.; Kurth, A.; van Cleef, K.W.R.; Drosten, C.; van Rij, R.P.; Junglen, S. A Unique Nodavirus with Novel Features: Mosinovirus Expresses Two Subgenomic RNAs, a Capsid Gene of Unknown Origin, and a Suppressor of the Antiviral RNA Interference Pathway. J. Virol. 2014, 88, 13447–13459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallies, R.; Kopp, A.; Zirkel, F.; Estrada, A.; Gillespie, T.; Drosten, C.; Junglen, S. Genetic Characterization of Goutanap Virus, a Novel Virus Related to Negeviruses, Cileviruses and Higreviruses. Viruses 2014, 6, 4346–4357. [Google Scholar] [CrossRef]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes: Identification, Ecology and Control; Fascinating Life Sciences; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Brown, J.E.; Evans, B.R.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human Impacts Have Shaped Historical and Recent Evolution in Aedes aegypti, the Dengue and Yellow Fever Mosquito. Evolution 2014, 68, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Mattingly, P.F. Genetical Aspects of the Aedes aegypti Problem. I. Taxonom: And Bionomics. Ann. Trop. Med. Parasitol. 1957, 51, 392–408. [Google Scholar] [CrossRef]
- Powell, J.R. Mosquitoes on the Move. Science 2016, 354, 971–972. [Google Scholar] [CrossRef]
- Kotsakiozi, P.; Evans, B.R.; Gloria-Soria, A.; Kamgang, B.; Mayanja, M.; Lutwama, J.; Le Goff, G.; Ayala, D.; Paupy, C.; Badolo, A.; et al. Population Structure of a Vector of Human Diseases: Aedes aegypti in Its Ancestral Range, Africa. Ecol. Evol. 2018, 8, 7835–7848. [Google Scholar] [CrossRef] [Green Version]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of Mosquito Preference for Humans Linked to an Odorant Receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Tabachnick, W.J. History of Domestication and Spread of Aedes aegypti—A Review. Mem. Inst. Oswaldo Cruz 2013, 108, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Serra, O.P.; Cardoso, B.F.; Ribeiro, A.L.M.; dos Santos, F.A.L.; Slhessarenko, R.D. Mayaro Virus and Dengue Virus 1 and 4 Natural Infection in Culicids from Cuiabá, State of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Ashley, R.F. Demonstration of Venezuelan Equine Encephalomyelitis Virus in Tissues of Aedes aegypti. Am. J. Trop. Med. Hyg. 1971, 20, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Dohm, D.J.; Sardelis, M.R.; O’guinn, M.L.; Andreadis, T.G.; Blow, J.A. An Update on the Potential of North American Mosquitoes (Diptera: Culicidae) to Transmit West Nile Virus. J. Med. Entomol. 2005, 42, 57–62. [Google Scholar] [CrossRef]
- Black, W.C.; Bennett, K.E.; Gorrochótegui-Escalante, N.; Barillas-Mury, C.V.; Fernández-Salas, I.; de Lourdes Muñoz, M.; Farfán-Alé, J.A.; Olson, K.E.; Beaty, B.J. Flavivirus Susceptibility in Aedes aegypti. Arch. Med. Res. 2002, 33, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Diallo, M.; Ba, Y.; Faye, O.; Soumare, M.L.; Dia, I.; Sall, A.A. Vector Competence of Aedes aegypti Populations from Senegal for Sylvatic and Epidemic Dengue 2 Virus Isolated in West Africa. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 493–498. [Google Scholar] [CrossRef]
- Diallo, M.; Sall, A.A.; Moncayo, A.C.; Ba, Y.; Fernandez, Z.; Ortiz, D.; Coffey, L.L.; Mathiot, C.; Tesh, R.B.; Weaver, S.C. Potential Role of Sylvatic and Domestic African Mosquito Species in Dengue Emergence. Am. J. Trop. Med. Hyg. 2005, 73, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Dickson, L.B.; Sanchez-Vargas, I.; Sylla, M.; Fleming, K.; Black, W.C. Vector Competence in West African Aedes aegypti Is Flavivirus Species and Genotype Dependent. PLoS Negl. Trop. Dis. 2014, 8, e3153. [Google Scholar] [CrossRef] [Green Version]
- Adhami, J.; Reiter, P. Introduction and Establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J. Am. Mosq. Control Assoc. 1998, 14, 340–343. [Google Scholar]
- Hawley, W.; Reiter, P.; Copeland, R.; Pumpuni, C.; Craig, G. Aedes albopictus in North America: Probable Introduction in Used Tires from Northern Asia. Science 1987, 236, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.J.; Hunt, R.H. Aedes albopictus in Africa? First Records of Live Specimens in Imported Tires in Cape Town. J. Am. Mosq. Control Assoc. 1991, 7, 107–108. [Google Scholar] [PubMed]
- Savage, H.M.; Ezike, V.I.; Nwankwo, A.C.; Spiegel, R.; Miller, B.R. First Record of Breeding Populations of Aedes albopictus in Continental Africa: Implications for Arboviral Transmission. J. Am. Mosq. Control Assoc. 1992, 8, 101–103. [Google Scholar]
- Fontenille, D. Aedes (Stegomyia) albopictus (Skuse), a Potential New Dengue Vector in Southern Cameroon. Emerg. Infect. Dis. 2001, 7, 1066–1067. [Google Scholar] [CrossRef] [Green Version]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an Arbovirus Vector: From the Darkness to the Light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A.; et al. Mosquito, Bird and Human Surveillance of West Nile and Usutu Viruses in Emilia-Romagna Region (Italy) in 2010. PLoS ONE 2012, 7, e38058. [Google Scholar] [CrossRef] [Green Version]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent Chikungunya and Dengue Virus Infections during Simultaneous Outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593. [Google Scholar] [CrossRef]
- Kamgang, B.; Yougang, A.P.; Tchoupo, M.; Riveron, J.M.; Wondji, C. Temporal Distribution and Insecticide Resistance Profile of Two Major Arbovirus Vectors Aedes aegypti and Aedes albopictus in Yaoundé, the Capital City of Cameroon. Parasites Vectors 2017, 10, 469. [Google Scholar] [CrossRef]
- Juliano, S.A.; O’Meara, G.F.; Morrill, J.R.; Cutwa, M.M. Desiccation and Thermal Tolerance of Eggs and the Coexistence of Competing Mosquitoes. Oecologia 2002, 130, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Ngoagouni, C.; Kamgang, B.; Nakouné, E.; Paupy, C.; Kazanji, M. Invasion of Aedes albopictus (Diptera: Culicidae) into Central Africa: What Consequences for Emerging Diseases? Parasites Vectors 2015, 8, 191. [Google Scholar] [CrossRef]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus Fever: The Role of Host and Vector Susceptibility and Interspecific Competition in Shaping the Current and Future Distributions of the Sylvatic Cycles of Dengue Virus and Yellow Fever Virus. Infect. Genet. Evol. 2013, 19, 292–311. [Google Scholar] [CrossRef] [Green Version]
- Nikolay, B.; Diallo, M.; Faye, O.; Boye, C.S.; Sall, A.A. Vector Competence of Culex Neavei (Diptera: Culicidae) for Usutu Virus. Am. J. Trop. Med. Hyg. 2012, 86, 993–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jöst, H.; Bialonski, A.; Günther, S.; Schmidt-Chanasit, J.; Schmetz, C.; Becker, N. Isolation and Phylogenetic Analysis of Batai Virus, Germany. Am. J. Trop. Med. Hyg. 2011, 84, 241–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.-L.; Yang, C.-F.; Teng, H.-J.; Lu, L.-C.; Lin, C.; Tsai, K.-H.; Chen, Y.-Y.; Chen, L.-Y.; Chang, S.-F.; Shu, P.-Y. Molecular Epidemiology of Japanese Encephalitis Virus in Mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 2014, 8, e3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugman, V.A.; Hernández-Triana, L.M.; Prosser, S.W.J.; Weland, C.; Westcott, D.G.; Fooks, A.R.; Johnson, N. Molecular Species Identification, Host Preference and Detection of Myxoma Virus in the Anopheles Maculipennis Complex (Diptera: Culicidae) in Southern England, UK. Parasites Vectors 2015, 8, 421. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.C.; Woodall, J.P.; Corbet, P.S. Nyando Virus: A Hitherto Undescribed Virus Isolated FromAnopheles Funestus Giles Collected in Kenya. Arch. Gesamte Virusforsch. 1965, 15, 422–427. [Google Scholar] [CrossRef]
- Maquart, M.; Boyer, S.; Rakotoharinome, V.M.; Ravaomanana, J.; Tantely, M.L.; Heraud, J.-M.; Cardinale, E. High Prevalence of West Nile Virus in Domestic Birds and Detection in 2 New Mosquito Species in Madagascar. PLoS ONE 2016, 11, e0147589. [Google Scholar] [CrossRef] [Green Version]
- Ratovonjato, J.; Olive, M.-M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reynes, J.-M.; Elissa, N. Detection, Isolation, and Genetic Characterization of Rift Valley Fever Virus from Anopheles (Anopheles) Coustani, Anopheles (Anopheles) Squamosus, and Culex (Culex) Antennatus of the Haute Matsiatra Region, Madagascar. Vector Borne Zoonotic Dis. 2011, 11, 753–759. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats. Antivir. Res 2010, 85, 328. [Google Scholar] [CrossRef] [Green Version]
- Saghir, J.; Santoro, J. Urbanization in Sub-Saharan Africa: Meeting Challenges by Bridging Stakeholders; Center for Strategic and International Studies (CSIS): Washington, DC, USA, 2018. [Google Scholar]
- The Economist. Africa’s Population Will Double by 2050. Available online: https://www.economist.com/special-report/2020/03/26/africas-population-will-double-by-2050 (accessed on 6 April 2021).
- Powell, J.R.; Gloria-Soria, A.; Kotsakiozi, P. Recent History of Aedes aegypti: Vector Genomics and Epidemiology Records. BioScience 2018, 68, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Chinery, W.A. Impact of Rapid Urbanization on Mosquitoes and Their Disease Transmission Potential in Accra and Tema, Ghana. Afr. J. Med. Med. Sci. 1995, 24, 179–188. [Google Scholar]
- Walsh, J.F.; Molyneux, D.H.; Birley, M.H. Deforestation Effects on Vector-Borne Disease. Parasitology 1993, 106, S55–S75. [Google Scholar] [CrossRef] [PubMed]
- Richman, R.; Diallo, D.; Diallo, M.; Sall, A.A.; Faye, O.; Diagne, C.T.; Dia, I.; Weaver, S.C.; Hanley, K.A.; Buenemann, M. Ecological Niche Modeling of Aedes Mosquito Vectors of Chikungunya Virus in Southeastern Senegal. Parasites Vectors 2018, 11, 1–17. [Google Scholar] [CrossRef]
- Anyamba, A.; Small, J.L.; Britch, S.C.; Tucker, C.J.; Pak, E.W.; Reynolds, C.A.; Crutchfield, J.; Linthicum, K.J. Recent Weather Extremes and Impacts on Agricultural Production and Vector-Borne Disease Outbreak Patterns. PLoS ONE 2014, 9, e92538. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.L.; Shija, F.; Linton, Y.-M.; Misinzo, G.; Kaddumukasa, M.; Djouaka, R.; Anyaele, O.; Harris, A.; Irish, S.; Hlaing, T.; et al. Historical Environmental Change in Africa Drives Divergence and Admixture of Aedes aegypti Mosquitoes: A Precursor to Successful Worldwide Colonization? Mol. Ecol. 2016, 25, 4337–4354. [Google Scholar] [CrossRef] [Green Version]
- Gloria-Soria, A.; Ayala, D.; Bheecarry, A.; Calderon-Arguedas, O.; Chadee, D.D.; Chiappero, M.; Coetzee, M.; Elahee, K.B.; Fernandez-Salas, I.; Kamal, H.A.; et al. Global Genetic Diversity of Aedes aegypti. Mol. Ecol. 2016, 25, 5377–5395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef] [Green Version]
- United Nations. Revision of World Population Prospects 2019. Available online: https://www.un.org/en/development/desa/population/events/pdf/other/21/21June_FINAL%20PRESS%20RELEASE_WPP17.pdf (accessed on 11 May 2021).
- Shenton, F.C.; Addissie, A.; Alabaster, G.; Baziwe, D.; Carrasco Tenezaca, M.; Chinula, D.; Jatta, E.; Jawara, M.; Jones, R.; Knudsen, J.; et al. Research Agenda for Preventing Mosquito-Transmitted Diseases through Improving the Built Environment in Sub-Saharan Africa. Cities Health 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mordecai, E.A.; Cohen, J.M.; Evans, M.V.; Gudapati, P.; Johnson, L.R.; Lippi, C.A.; Miazgowicz, K.; Murdock, C.C.; Rohr, J.R.; Ryan, S.J.; et al. Detecting the Impact of Temperature on Transmission of Zika, Dengue, and Chikungunya Using Mechanistic Models. PLoS Negl. Trop. Dis. 2017, 11, e0005568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.S.; Honorio, N.A.; Garcia, L.M.T.; de Sá Carvalho, L.C. Aedes aegypti Control in Urban Areas: A Systemic Approach to a Complex Dynamic. PLoS Negl. Trop. Dis. 2017, 11, e0005632. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Special Programme for Research and Training in Tropical Diseases (2017)—Global Vector Control Response 2017–2030; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Multisectoral Approach to ThenPrevention and Control of Vector-Borne Diseases; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Connor, M.E.; Monroe, W.M. Stegomyia Indices and Their Value in Yellow Fever Control 1. Am. J. Trop. Med. Hyg. 1923, 1–3, 9–19. [Google Scholar] [CrossRef]
- WHO. Technical Quide for a System of Yellow Fever Surveillance; WHO: Geneva, Switzerland, 1971. [Google Scholar]
- WHO. Yellow Fever: Rapid Field Entomological Assessment During Yellow Fever Outbreaks in Africa: Handbook: Methodo-Logical Field Approaches for Scientists with a Basic Background in Entomology; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Roddy, P.; Dalrymple, U.; Jensen, T.O.; Dittrich, S.; Rao, V.B.; Pfeffer, D.A.; Twohig, K.A.; Roberts, T.; Bernal, O.; Guillen, E. Quantifying the Incidence of Severe-Febrile-Illness Hospital Admissions in Sub-Saharan Africa. PLoS ONE 2019, 14, e0220371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elven, J.; Dahal, P.; Ashley, E.A.; Thomas, N.V.; Shrestha, P.; Stepniewska, K.; Crump, J.A.; Newton, P.N.; Bell, D.; Reyburn, H.; et al. Non-Malarial Febrile Illness: A Systematic Review of Published Aetiological Studies and Case Reports from Africa, 1980–2015. BMC Med. 2020, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, Y.; Abe, H.; Nguema Ondo, G.; Bikangui, R.; Massinga Loembé, M.; Zadeh, V.R.; Essimengane, J.G.E.; Mbouna, A.V.N.; Bache, E.B.; Agnandji, S.T.; et al. Surveillance of the Major Pathogenic Arboviruses of Public Health Concern in Gabon, Central Africa: Increased Risk of West Nile Virus and Dengue Virus Infections. BMC Infect. Dis. 2021, 21, 265. [Google Scholar] [CrossRef]
- Stoler, J.; Awandare, G.A. Febrile Illness Diagnostics and the Malaria-Industrial Complex: A Socio-Environmental Perspective. BMC Infect. Dis. 2016, 16, 683. [Google Scholar] [CrossRef] [Green Version]
- L’Azou, M.; Succo, T.; Kamagaté, M.; Ouattara, A.; Gilbernair, E.; Adjogoua, E.; Luxemburger, C. Dengue: Etiology of Acute Febrile Illness in Abidjan, Côte d’Ivoire, in 2011–2012. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 717–722. [Google Scholar] [CrossRef]
- Niang, M.; Loucoubar, C.; Sow, A.; Diagne, M.M.; Faye, O.; Faye, O.; Diallo, M.; Toure-Balde, A.; Sall, A.A. Genetic Diversity of Plasmodium Falciparum Isolates from Concurrent Malaria and Arbovirus Co-Infections in Kedougou, Southeastern Senegal. Malar. J. 2016, 15, 155. [Google Scholar] [CrossRef] [Green Version]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.S.; Dia, A.T.; Faye, O.; Weaver, S.C.; Diallo, M.; et al. Concurrent Malaria and Arbovirus Infections in Kedougou, Southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Herrera, B.B.; Chang, C.A.; Hamel, D.J.; Mboup, S.; Ndiaye, D.; Imade, G.; Okpokwu, J.; Agbaji, O.; Bei, A.K.; Kanki, P.J. Continued Transmission of Zika Virus in Humans in West Africa, 1992–2016. J. Infect. Dis. 2017, 215, 1546–1550. [Google Scholar] [CrossRef]
- Mathé, P.; Egah, D.Z.; Müller, J.A.; Shehu, N.Y.; Obishakin, E.T.; Shwe, D.D.; Pam, V.C.; Okolo, M.O.; Yilgwan, C.; Gomerep, S.S.; et al. Low Zika Virus Seroprevalence among Pregnant Women in North Central Nigeria, 2016. J. Clin. Virol. 2018, 105, 35–40. [Google Scholar] [CrossRef]

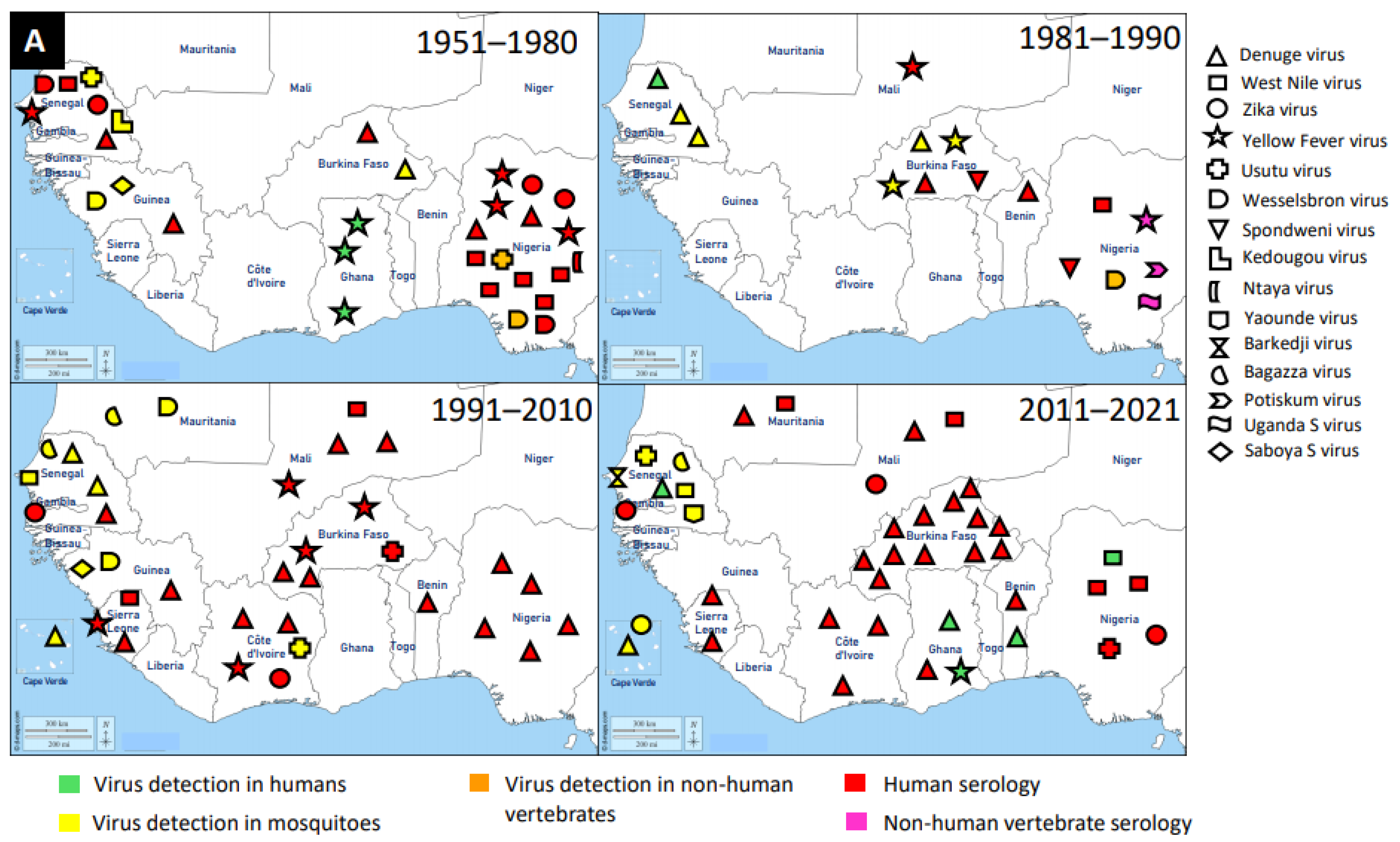
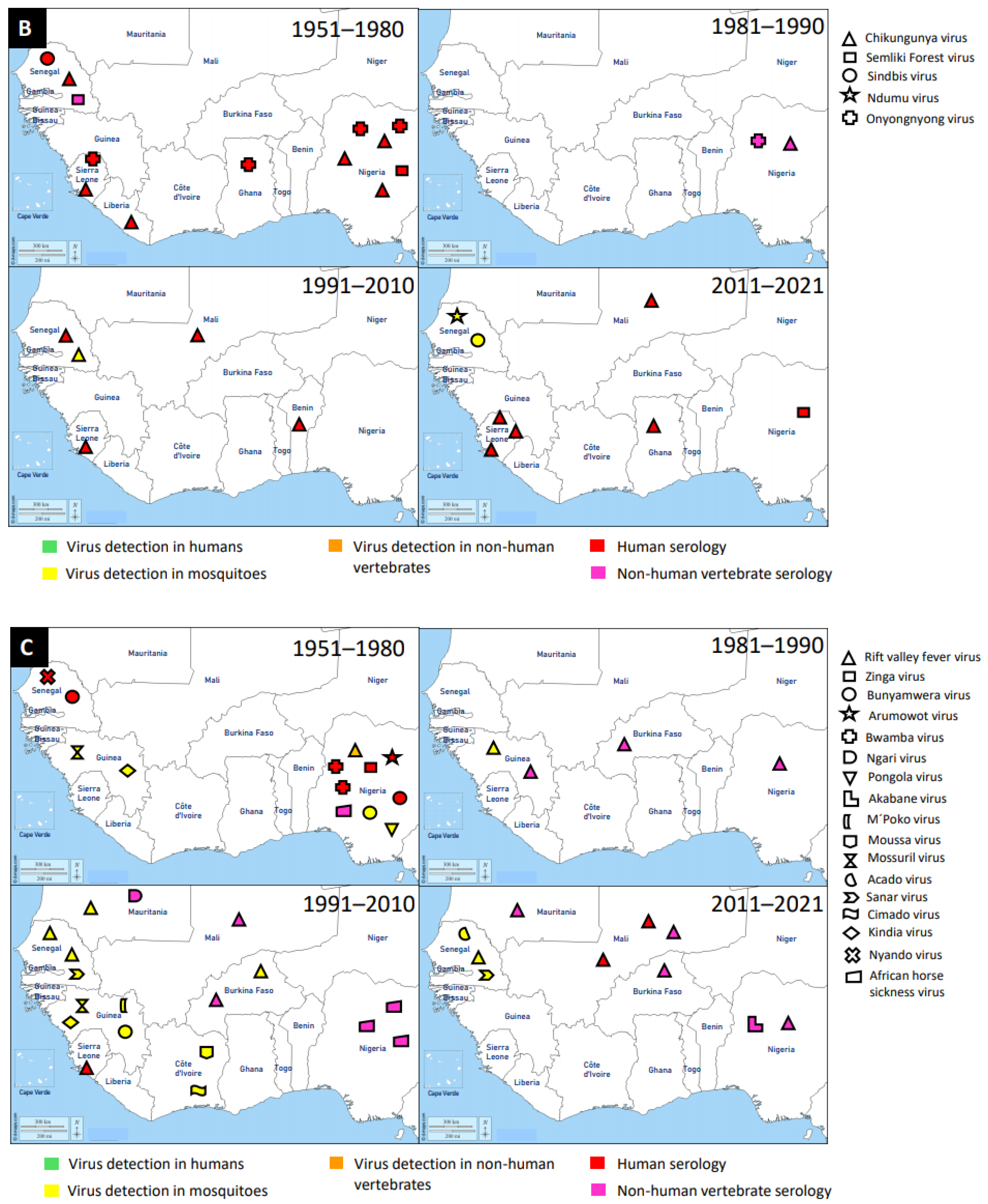

| Family | Name of Virus | Year of Virus Detection | Country | Source of Virus Detection | Reference |
|---|---|---|---|---|---|
| Flaviviridae | Mosquito-borne viruses | ||||
| Dengue | 2009 | Cape Verde | Aedes aegypti | [34] | |
| 2014/15 | Cape Verde | Ae. aegypti | [35] | ||
| 2016/17 | Ghana | Serology/Human | [36] | ||
| 2016/17 | Ghana | Human | [37] | ||
| 2014/2016 | Ghana | Serology/Human | [38] | ||
| 1974 | Senegal | Ae. luteocephalus, Serology/Human | [39] | ||
| 1983 | Senegal | Human | [40] | ||
| 1990 | Senegal | Aedes sp | [41] | ||
| 2015/19 | Senegal | Human | [42] | ||
| 1999 | Senegal | Aedes sp | [43] | ||
| 2009/10 | Senegal | Ae. aegypti, Serology/Human | [44] | ||
| 1964 | Nigeria | Serology/Human | [45] | ||
| 1975 | Nigeria | Serology/Human | [46] | ||
| 2001 | Nigeria | Serology/Human | [47] | ||
| 2010 * | Nigeria | Serology/Human | [48] | ||
| 2011 | Nigeria | Serology/Human | [49] | ||
| 2013 * | Nigeria | Serology/Human | [50] | ||
| 2014 * | Nigeria | Serology/Human | [51] | ||
| 1987/93 | Benin | Serology/Human | [52] | ||
| 2010 | Benin | Serology/Human | [53] | ||
| 2019 | Benin | Serology/Human; Human | [54] | ||
| 2015 | Mauritania | Serology/Human | [55] | ||
| 2017 | Burkina Faso | Serology/Human | [56] | ||
| 2016 | Burkina Faso | Serology/Human | [57] | ||
| 2019 | Burkina Faso | Serology/Human | [58] | ||
| 1980 | Burkina Faso | Aedes sp | [59] | ||
| 1983/86 | Burkina Faso | Aedes sp | [60] | ||
| 2013/14 | Burkina Faso | Serology/Human | [61] | ||
| 2015/17 | Burkina Faso | Serology/Human | [62] | ||
| 2014 | Burkina Faso | Serology/Human | [63] | ||
| 2016/17 | Burkina Faso | Serology/Human | [64] | ||
| 2016 | Burkina Faso | Serology/Human | [65] | ||
| 1982 | Burkina Faso | Serology/Human | [66] | ||
| 2004 | Burkina Faso | Serology/Human | [67] | ||
| 2016 | Burkina Faso | Serology/Human | [68] | ||
| 2013/14 | Burkina Faso | Serology/Human | [69] | ||
| 2017 | Burkina Faso | Serology/Human | [56] | ||
| 2003/4 | Burkina Faso | Serology/Human | [70] | ||
| 2016 | Burkina Faso | Serology/Human | [71] | ||
| 2009/13 | Mali | Serology/Human | [72] | ||
| 2006 | Mali | Serology/Human | [73] | ||
| 1999 | Côte d’Ivoire | Mosquitoes, Serology/Human | [74] | ||
| 2010 | Côte d’Ivoire | Serology/Human, Human | [75] | ||
| 2011/12 | Côte d’Ivoire | Serology/Human, Human | [76] | ||
| 2017 | Côte d’Ivoire | Serology/Human, Human | [77] | ||
| 2019 | Côte d’Ivoire | Serology/Human | [78] | ||
| Dengue | 1978/91 | Guinea | Mosquitoes | [79] | |
| 2006/8 | Sierra Leone | Serology/Human | [80] | ||
| 2016 * | Sierra Leone | Serology/Human | [81] | ||
| 2012/13 | Sierra Leone | Serology/Human | [82] | ||
| West Nile | 2012/13 | Senegal | Culex sp, Aedes sp | [83] | |
| 1972/75 | Senegal | Serology/Human | [84] | ||
| 1998/99 | Senegal | Masonia uniformis | [85] | ||
| 1951/55 | Nigeria | Serology/Human | [86] | ||
| 1968/69 | Nigeria | Serology/human | [87] | ||
| 1963 | Nigeria | Serology/Human | [88] | ||
| 1975 | Nigeria | Serology/Human | [89] | ||
| 1987 | Nigeria | Serology/Horses | [90] | ||
| 2011/12 | Nigeria | Serology/Horses | [91] | ||
| 2018 | Nigeria | Human | [92] | ||
| 2015 | Mauritania | Serology/Human | [55] | ||
| 2009/13 | Mali | Serology/Human | [72] | ||
| 2006/8 | Sierra Leone | Serology/Human | [80] | ||
| Zika | 2016 | Cape Verde | Ae. aegytpi | [93] | |
| 2016 | Mali | Serology/Human | [94] | ||
| 1972/75 | Senegal | Serology/Human | [84] | ||
| 1999 | Côte d’Ivoire | Mosquitoes, Serology/Human | [74] | ||
| 2010/14 | Gambia | Serology | [95] | ||
| 1975 * | Nigeria | Serology | [46] | ||
| 1951/55 | Nigeria | Serology/Human | [86] | ||
| 2018 | Nigeria | Serology | [92] | ||
| Yellow Fever | 1989 | Nigeria | Serology/horses | [90] | |
| 1951/55 | Nigeria | Serology/Human | [86] | ||
| 1968/69 | Nigeria | Serology/Human | [87] | ||
| 1975 | Nigeria | Serology/Human | [89] | ||
| 1972/75 | Senegal | Serology/Human | [84] | ||
| 1976 | Senegal | Aedes sp | [96] | ||
| 2003/8 | Burkina Faso | Serology/Human | [97] | ||
| 1983/86 | Burkina Faso | Aedes sp | [60] | ||
| 1983 | Burkina Faso | Aedes sp | [98] | ||
| 1999 | Burkina Faso | Serology/Human | [99] | ||
| 1987 | Mali | Serology/Human | [100] | ||
| 2006 | Mali | Serology/Human | [73] | ||
| 1999 | Côte d’Ivoire | Mosquitoes, Serology/Human | [74] | ||
| 2006/09 | Sierra Leone | Serology/Human | [80] | ||
| 1977/80 | Ghana | Human | [101] | ||
| 1963 | Ghana | Human | [101] | ||
| 1969/70 | Ghana | Human | [101] | ||
| 2011 | Ghana | Human | [102] | ||
| Usutu | 2012/13 | Senegal | Culex sp | [83] | |
| 1972/1977 | Senegal | Aedes sp | [103] | ||
| 1972 | Nigeria | Turdus libonyanus | [104] | ||
| 2018 | Nigeria | Serology/Human | [92] | ||
| 2004 | Burkina Faso | Serology/Human | [104] | ||
| 2004 | Côte d’Ivoire | Culex quinquefasciatus | [104] | ||
| Wesselsbron | 1972/75 | Senegal | Serology/Human | [84] | |
| 1971 * | Nigeria | Serology/Horse | [105] | ||
| 1975 | Nigeria | Serology/Human | [89] | ||
| 1989 * | Nigeria | Serology/Horse | [90] | ||
| 1998 | Mauritania | Aedes vexans | [85] | ||
| 1978/91 | Guinea | Mosquitoes | [79] | ||
| Spondweni | 1982 * | Burkina Faso | Serology/Human | [106] | |
| Kedougou | 1978 * | Senegal | Aedes sp | [107] | |
| Ntaya | 1977 | Nigeria | Serology/Human | [108] | |
| Yaounde | 2012/13 | Senegal | Culex sp | [83] | |
| Bagazza | 2012/13 | Senegal | Culex sp | [83] | |
| 1998/99 | Senegal | Aedes fowleri | [85] | ||
| 1998/99 | Mauritania | Culex neavei | [85] | ||
| Barkedji | 2012/13 | Senegal | Culex sp, Aedes sp | [83] | |
| Potiskum | 1989 * | Nigeria | Serology/Horses | [90] | |
| Uganda S | 1989 * | Nigeria | Serology/Horses | [90] | |
| Saboya | 1978/91 | Guinea | Mosquitoes | [79] | |
| Togaviridae | |||||
| Chikungunya | 1963 | Nigeria | Aedes sp | [109] | |
| 1969 | Nigeria | Serology/Human | [110] | ||
| 1968/69 | Nigeria | Serology/Human | [87] | ||
| 1989 * | Nigeria | Serology/Horse | [90] | ||
| 1974 | Nigeria | Serology/Human | [111] | ||
| 2009/10 | Senegal | Serology, Aedes sp | [112] | ||
| 1972/75 | Senegal | Serology/Human | [84] | ||
| 2016/17 | Ghana | Serology/Human | [36] | ||
| 2006 | Benin | Serology/Human | [113] | ||
| 2009/13 | Mali | Serology/Human | [72] | ||
| Togaviridae | |||||
| Chikungunya | 2012/13 | Sierra Leone | Serology/Human | [114] | |
| 2016/17 | Sierra Leone | Serology/Human | [115] | ||
| 2006/8 | Sierra Leone | Serology/Human | [80] | ||
| 2012/13 | Sierra Leone | Serology/Human | [82] | ||
| 1975/77 | Sierra Leone | Serology/Human | [108] | ||
| 1977 | Liberia | Serology/Human | [108] | ||
| Semliki Forest | 1971 | Senegal | Serology/Horses | [116] | |
| 1951/55 | Nigeria | Serology/Human | [86] | ||
| 2014 * | Nigeria | Serology/Human | [117] | ||
| Sindbis | 2012/13 | Senegal | Culex sp | [83] | |
| 1972/75 | Senegal | Serology | [84] | ||
| Ndumu | 2012/13 | Senegal | Several mosquitoes | [84] | |
| Onyongnyong | 1989 * | Nigeria | Serology/Horses | [90] | |
| 1974 | Nigeria | Serology/Human | [108] | ||
| 1975 | Nigeria | Serology/Human | [108] | ||
| 1954 | Ghana | Serology/Human | [108] | ||
| 1975/77 | Sierra Leone | Serology/Human | [108] | ||
| Phenuiviridae | |||||
| Rift Valley fever | 1993/96 | Burkina Faso | Aedes sp | [60] | |
| 1987 | Burkina Faso | Serology/Sheep | [118] | ||
| 2005/7 | Burkina Faso | Serology/livestock | [119] | ||
| 1985/87 | Burkina Faso | Serology/livestock | [120] | ||
| 2006/8 | Sierra Leone | Serology/Human | [80] | ||
| 1987 * | Guinea | Serology/Bats | [121] | ||
| 1978/91 | Guinea | Mosquitoes | [79] | ||
| 2016 | Mali | Serology/Human | [122] | ||
| 2015 | Mali | Serology/Human | [123] | ||
| 2005/14 | Mali | Serology/Bovine | [124] | ||
| 2016 | Niger | Serology/Human/ Livestock | [125] | ||
| 1959 | Nigeria | Serology/Sheep | [126] | ||
| 2016 | Nigeria | Serology/Camels | [127] | ||
| 1989 | Nigeria | Seroloy/Horses | [90] | ||
| 1998 | Senegal | Culex sp, Serology | [128] | ||
| 2012/13 | Senegal | Aedes ochraceus | [83] | ||
| 1998/99 | Senegal | Culex poicilipes | [85] | ||
| 1998 | Mauritania | Culex poicilipes | [85] | ||
| 2015 | Mauritania | Serology/Human | [55] | ||
| Peribunyaviridae | |||||
| Zinga | 1975/1977 | Nigeria | Serology/Human | [108] | |
| Bunyamwera | 1978 * | Senegal | Serology/Human | [84] | |
| 1951/55 | Nigeria | Serology/Human | [86] | ||
| 1963 | Nigeria | M. africana | [109] | ||
| 1978/91 | Guinea | Mosquitoes | [79] | ||
| Arumowot | 1968/69 | Nigeria | Serology/Human | [87] | |
| Bwamba | 1969/72 | Nigeria | Serology/Human | [129] | |
| 1951/55 | Nigeria | Serology/Human | [86] | ||
| Ngari | 2010 | Mauritania | Serology/Goat | [130] | |
| Nyando | 1972 * | Senegal | Serology/Human | [131] | |
| Pongola | 1963 | Nigeria | M. africana | [109] | |
| Akabane | 2015 | Nigeria | Serology/Livestock | [132] | |
| M’Poko | 1978/91 | Guinea | Mosquitoes | [79] | |
| Rhabdoviridae | |||||
| Mossuril | 1978/91 | Guinea | Mosquitoes | [79] | |
| Reoviridae | |||||
| Acado | 2012/13 | Senegal | Culex sp | [83] | |
| Sanar | 2012/13 | Senegal | Culex neavei, M. uniformis | [83] | |
| 1998/99 | Senegal | Culex poicilipes | [85] | ||
| Kindia | 1978/91 | Guinea | Mosquitoes | [79] | |
| African horse sickeness | 1971 * | Nigeria | Serology/Horse | [105] | |
| 1993 * | Nigeria | Serology/Camels/Donkey/ Dogs/Horses | [133] | ||
| 1993 * | Nigeria | Serology/Horse | [134] | ||
| 1995 * | Nigeria | Serology/Horse | [135] | ||
| Flaviviridae | Mosquito-specific viruses | ||||
| Culex flavivirus | 2016 | Ghana | Culex sp | [136] | |
| Cell fusing agent virus | 2016 | Ghana | Aedes aegypti | [136] | |
| Anopheles flavivirus-like 2 | 2012 | Senegal | Anopheles sp | [13] | |
| Anopheles flavivirus-like 1 | 2012 | Senegal | Anopheles sp | [13] | |
| Nienokoue | 2004 | Côte d’Ivoire | Culex species mosquitoes | [137] | |
| Nounane | 2004 | Côte d’Ivoire | Uranotaenia mashonaensis | [138] | |
| Togaviridae | |||||
| Taï Forest alphavirus | 2004 | Côte d’Ivoire | Culex decens | [139] | |
| Peribunyaviridae | |||||
| Ferak | 2004 | Côte d’Ivoire | Culex decens | [140] | |
| Jonchet | 2004 | Côte d’Ivoire | Culex sp | [140] | |
| Herbert | 2004 | Côte d’Ivoire | Culex nebulosus | [141] | |
| Tai | 2004 | Côte d’Ivoire | Culex sp | [141] | |
| Rhabdoviridae | |||||
| Moussa | 2004 | Côte d’Ivoire | Culex decens | [142] | |
| Mesoniviridae | |||||
| Odorna | 2016 | Ghana | Aedes aegypti | [136] | |
| Dianke | 2013 | Senegal | Culex poicilipes | [143] | |
| Cavally | 2016 | Ghana | Aedes aegypti | [136] | |
| Cavally | 2004 | Côte d’Ivoire | Aedes harrisoni | [144] | |
| Nse | 2004 | Côte d’Ivoire | Culex nebulosus | [145] | |
| Meno | 2004 | Côte d’Ivoire | Uranotaenia chorleyi | [145] | |
| Hana | 2004 | Côte d’Ivoire | Culex sp | [145] | |
| Moumo | 2004 | Côte d’Ivoire | Culex sp | [145] | |
| Totiviridae | |||||
| Aedes aegypti totivirus | 2016 | Ghana | Aedes aegypti | [136] | |
| Reoviridae | |||||
| Aedes pseudoscutellaris reovirus | 2015 | Ghana | Aedes aegypti | [136] | |
| Cimodo | 2008 | Côte d’Ivoire | Culex nebulosus | [146] | |
| Phenuiviridae | |||||
| Phasi Charoen-like phasivirus | 2016 | Ghana | Aedes aegypti | [136] | |
| Gouleako | 2004 | Côte d’Ivoire | Culex sp | [147] | |
| Negeviruses ** | |||||
| Dezidougou | 1984 | Côte d’Ivoire | Aedes aegypti | [148] | |
| Iflaviridae | |||||
| Aedes vexans iflavirus | 2017 | Senegal | Aedes vexans | [149] | |
| Permutotetraviridae | |||||
| Culex permutotetra-like virus | 2016 | Ghana | Culex sp | [136] | |
| Nodaviridae | |||||
| Mosinovirus | 2004 | Côte d’Ivoire | Culicidae | [150] | |
| Xinmoviridae | |||||
| Bolahun virus variant 2 | 2012/15 | Liberia | Anopheles gambiae | [13] | |
| Unclassified *** | |||||
| Unclassified Riboviria | Bolahun virus variant 1 | 2012/15 | Burkina Faso | Anopheles gambiae | [13] |
| Unclassified Riboviria | Aedes aegypti virga-like virus | 2016 | Ghana | Aedes aegypti | [136] |
| Unclassified Riboviria | West Accra | 2015 | Ghana | Aedes aegypti | [136] |
| Unclassified Riboviria | Mole Culex | 2016 | Ghana | Culex sp | [136] |
| Unclassified Riboviria | Goutanap | 2004 | Côte d’Ivoire | Culex nebulosus | [151] |
| Unclassified Riboviria | Goutanap | 2016 | Ghana | Culex sp | [136] |
| Unclassified Riboviria | Tesano Aedes | 2016 | Ghana | Aedes aegypti | [136] |
| Unclassified Riboviria | Korle-bu Aedes | 2016 | Ghana | Aedes aegypti | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agboli, E.; Zahouli, J.B.Z.; Badolo, A.; Jöst, H. Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa. Viruses 2021, 13, 891. https://doi.org/10.3390/v13050891
Agboli E, Zahouli JBZ, Badolo A, Jöst H. Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa. Viruses. 2021; 13(5):891. https://doi.org/10.3390/v13050891
Chicago/Turabian StyleAgboli, Eric, Julien B. Z. Zahouli, Athanase Badolo, and Hanna Jöst. 2021. "Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa" Viruses 13, no. 5: 891. https://doi.org/10.3390/v13050891








