Phylogenetic Networks and Parameters Inferred from HIV Nucleotide Sequences of High-Risk and General Population Groups in Uganda: Implications for Epidemic Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. HIV DNA Sequencing and Sequence Editing
2.3. Phylogenetic Analysis
2.4. HIV Subtyping and Bayesian Phylogenetic Inference in BEAST v1.8.4
2.5. Phylodynamic Analysis and Network Generation
2.6. Assessing for Power Law Distributions and Estimating Network Parameters
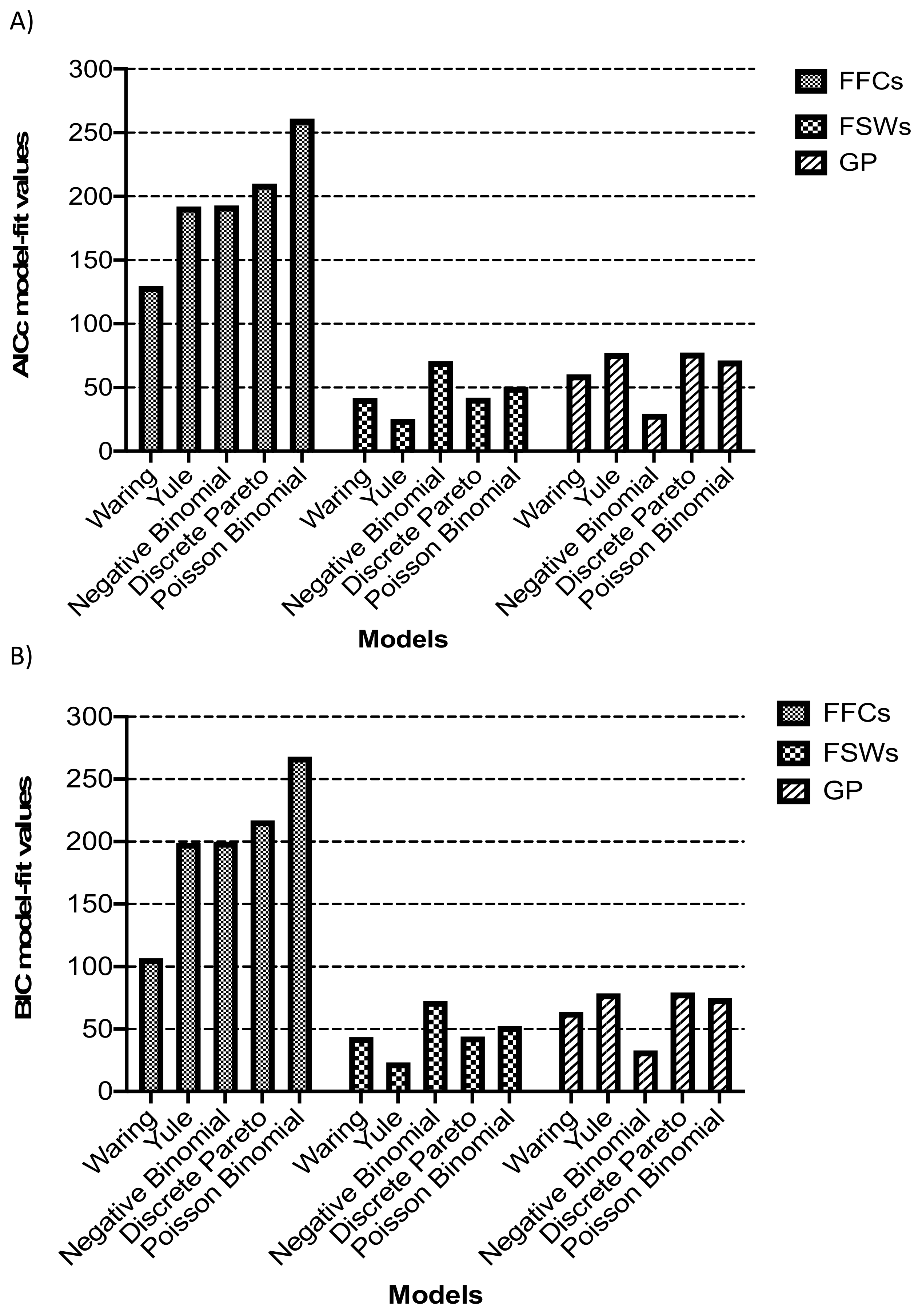
2.7. Model Fitting
3. Results
3.1. Network Generation
Cluster Size Distribution and Assortativity Coefficient
3.2. Network Parameters
3.3. Model Fitting to Degree Distributions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wabwire-Mangen, F.; Odiit, M.; Kirungi, W.; Kisitu, D.K.; Wanyama, J.O. HIV Modes of Transmission and Prevention Response Analysis; Uganda National AIDS Commission: Kampala, Uganda, 2009.
- Gysels, M.; Pool, R.; Bwanika, K. Truck Drivers, Middlemen and Commercial Sex Workers: AIDS and the Mediation of Sex in South West Uganda. AIDS Care 2001, 13, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Vandepitte, J.; Bukenya, J.; Weiss, H.A.; Nakubulwa, S.; Francis, S.C.; Hughes, P.; Hayes, R.; Grosskurth, H. HIV and Other Sexually Transmitted Infections in a Cohort of Women Involved in High-Risk Sexual Behavior in Kampala, Uganda. Sex. Transm. Dis. 2011, 38, 316–323. [Google Scholar] [CrossRef]
- Hladik, W.; Barker, J.; Ssenkusu, J.M.; Opio, A.; Tappero, J.W.; Hakim, A.; Serwadda, D.; Crane Survey Group. HIV Infection among Men Who Have Sex with Men in Kampala, Uganda—A Respondent Driven Sampling Survey. PLoS ONE 2012, 7, e38143. [Google Scholar] [CrossRef] [PubMed]
- Uganda AIDS Commision and Ministry of Health. Multi-Sectoral HIV Programming for MARPS in Uganda: Review of Profiles, Sizes and Programme Coverage: Study Review Report; Ministry of Health and Uganda AIDS Commission: Kampala, Uganda, 2014.
- Gopalappa, C. Model-Based Estimation of Sources of New Infections in Uganda. HIV Modes of Transmission Synthesis; Uganda AIDS Commision: Kampala, Uganda, 2014.
- Kamali, A.; Nsubuga, R.N.; Ruzagira, E.; Bahemuka, U.; Asiki, G.; Price, M.A.; Newton, R.; Kaleebu, P.; Fast, P. Heterogeneity of HIV Incidence: A Comparative Analysis between Fishing Communities and in a Neighbouring Rural General Population, Uganda, and Implications for HIV Control. Sex. Transm. Infect. 2016, 92, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kasamba, I.; Nash, S.; Seeley, J.; Weiss, H.A. HIV Incidence among Women at High Risk of HIV Infection Attending a Dedicated Clinic in Kampala, Uganda: 2008–2017. Sex. Transm. Dis. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasamba, I.; Nash, S.; Shahmanesh, M.; Baisley, K.; Todd, J.; Kamacooko, O.; Mayanja, Y.; Seeley, J.; Weiss, H.A. Missed Study Visits and Subsequent HIV Incidence Among Women in a Predominantly Sex Worker Cohort Attending a Dedicated Clinic Service in Kampala, Uganda. J. Acquir. Immune Defic. Syndr. 2019, 82, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health Uganda. Population-based HIV impact assessement (UPHIA) 2016–2017. In Summary Sheet: Preliminary Findings; Ministry of Health Uganda: Kampala, Uganda, 2017. [Google Scholar]
- Ratmann, O.; Kagaayi, J.; Hall, M.; Golubchick, T.; Kigozi, G.; Xi, X.; Wymant, C.; Nakigozi, G.; Abeler-Dörner, L.; Bonsall, D.; et al. Quantifying HIV Transmission Flow between High-Prevalence Hotspots and Surrounding Communities: A Population-Based Study in Rakai, Uganda. Lancet HIV 2020. [Google Scholar] [CrossRef] [Green Version]
- Bbosa, N.; Ssemwanga, D.; Nsubuga, R.N.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Nanyonjo, M.; Kuteesa, M.; Seeley, J.; Kiwanuka, N.; Bagaya, B.S.; et al. Phylogeography of HIV Suggests That Ugandan Fishing Communities Are a Sink for, Not a Source of, Virus from General Populations. Sci. Rep. 2019, 9, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiwuwa-Muyingo, S.; Nazziwa, J.; Ssemwanga, D.; Ilmonen, P.; Njai, H.; Ndembi, N.; Parry, C.; Kitandwe, P.K.; Gershim, A.; Mpendo, J.; et al. HIV Transmission Networks in High Risk Fishing Communities on the Shores of Lake Victoria in Uganda: A Phylogenetic and Epidemiological Approach. PLoS ONE 2017, 12, e0185818. [Google Scholar] [CrossRef] [Green Version]
- Little, S.J.; Kosakovsky Pond, S.L.; Anderson, C.M.; Young, J.A.; Wertheim, J.O.; Mehta, S.R.; May, S.; Smith, D.M. Using HIV Networks to Inform Real Time Prevention Interventions. PLoS ONE 2014, 9, e98443. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Leigh Brown, A.J.; Hepler, N.L.; Mehta, S.R.; Richman, D.D.; Smith, D.M.; Kosakovsky Pond, S.L. The Global Transmission Network of HIV. J. Infect. Dis. 2014, 209, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Wertheim, J.O.; Kosakovsky Pond, S.L.; Forgione, L.A.; Mehta, S.R.; Murrell, B.; Shah, S.; Smith, D.M.; Scheffler, K.; Torian, L.V. Social and Genetic Networks of HIV Transmission in New York City. PLoS Pathog. 2017, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, M.K.; Lessler, J.; Redd, A.D.; Kagaayi, J.; Laeyendecker, O.; Ndyanabo, A.; Nelson, M.I.; Cummings, D.A.T.; Bwanika, J.B.; Mueller, A.C.; et al. The Role of Viral Introductions in Sustaining Community-Based HIV Epidemics in Rural Uganda: Evidence from Spatial Clustering, Phylogenetics, and Egocentric Transmission Models. PLoS Med. 2014, 11, e1001610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kate Grabowski, M.; Lessler, J.; Bazaale, J.; Nabukalu, D.; Nankinga, J.; Nantume, B.; Ssekasanvu, J.; Reynolds, S.J.; Ssekubugu, R.; Nalugoda, F.; et al. Migration, Hotspots, and Dispersal of HIV Infection in Rakai, Uganda. Nat. Commun. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bbosa, N.; Ssemwanga, D.; Ssekagiri, A.; Xi, X.; Mayanja, Y.; Bahemuka, U.; Seeley, J.; Pillay, D.; Abeler-Dörner, L.; Golubchik, T.; et al. Phylogenetic and Demographic Characterization of Directed HIV Transmission Using Deep Sequences from High-Risk and General Population Cohorts/Groups in Uganda. Viruses 2020, 12, 331. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.R.; Murrell, B.; Anderson, C.M.; Kosakovsky Pond, S.L.; Wertheim, J.O.; Young, J.A.; Freitas, L.; Richman, D.D.; Mathews, W.C.; Scheffler, K.; et al. Using HIV Sequence and Epidemiologic Data to Assess the Effect of Self-Referral Testing for Acute HIV Infection on Incident Diagnoses in San Diego, California. Clin. Infect. Dis. 2016, 63, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yebra, G.; Ragonnet-Cronin, M.; Ssemwanga, D.; Parry, C.M.; Logue, C.H.; Cane, P.A.; Kaleebu, P.; Brown, A.J.L. Analysis of the History and Spread of HIV in Uganda Using Phylodynamics. J. Gen. Virol. 2015, 96, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Nazziwa, J.; Njai, H.F.; Ndembi, N.; Birungi, J.; Lyagoba, F.; Gershim, A.; Nakiyingi-Miiro, J.; Nielsen, L.; Mpendo, J.; Nanvubya, A.; et al. Short Communication: HIV Type 1 Transmitted Drug Resistance and Evidence of Transmission Clusters among Recently Infected Antiretroviral-Naive Individuals from Ugandan Fishing Communities of Lake Victoria. AIDS Res. Hum. Retrovir. 2013, 29, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Ssemwanga, D.; Ndembi, N.; Lyagoba, F.; Bukenya, J.; Seeley, J.; Vandepitte, J.; Grosskurth, H.; Kaleebu, P. HIV Type 1 Subtype Distribution, Multiple Infections, Sexual Networks, and Partnership Histories in Female Sex Workers in Kampala, Uganda. AIDS Res. Hum. Retrovir. 2012, 28, 357–365. [Google Scholar] [CrossRef]
- Wertheim, J.O.; Oster, A.M.; Hernandez, A.L.; Saduvala, N.; Bañez Ocfemia, M.C.; Hall, H.I. The International Dimension of the U.S. HIV Transmission Network and Onward Transmission of HIV Recently Imported into the United States. AIDS Res. Hum. Retrovir. 2016, 32, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, R.M.; Liang, R.H.; Poon, A.F.Y. Reconstructing Contact Network Parameters from Viral Phylogenies. Virus Evol. 2016, 2, vew029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giardina, F.; Romero-Severson, E.O.; Albert, J.; Britton, T.; Leitner, T. Inference of Transmission Network Structure from HIV Phylogenetic Trees. PLoS Comput. Biol. 2017, 13, e1005316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leigh Brown, A.J.; Lycett, S.J.; Weinert, L.; Hughes, G.J.; Fearnhill, E.; Dunn, D.T.; UK HIV Drug Resistance Collaboration. Transmission Network Parameters Estimated from HIV Sequences for a Nationwide Epidemic. J. Infect. Dis. 2011, 204, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthélemy, M.; Barrat, A.; Pastor-Satorras, R.; Vespignani, A. Dynamical Patterns of Epidemic Outbreaks in Complex Heterogeneous Networks. J. Theor. Biol. 2005, 235, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Schneeberger, A.; Mercer, C.H.; Gregson, S.A.J.; Ferguson, N.M.; Nyamukapa, C.A.; Anderson, R.M.; Johnson, A.M.; Garnett, G.P. Scale-Free Networks and Sexually Transmitted Diseases: A Description of Observed Patterns of Sexual Contacts in Britain and Zimbabwe. Sex. Transm. Dis. 2004, 31, 380–387. [Google Scholar] [CrossRef]
- Ma, J.; van den Driessche, P.; Willeboordse, F.H. The Importance of Contact Network Topology for the Success of Vaccination Strategies. J. Theor. Biol. 2013, 325, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kuteesa, M.O.; Weiss, H.A.; Abaasa, A.; Nash, S.; Nsubuga, R.N.; Newton, R.; Seeley, J.; Kamali, A. Feasibility of Conducting HIV Combination Prevention Interventions in Fishing Communities in Uganda: A Pilot Cluster Randomised Trial. PLoS ONE 2019, 14, e0210719. [Google Scholar] [CrossRef]
- Bahemuka, U.M.; Abaasa, A.; Ruzagira, E.; Lindan, C.; Price, M.A.; Kamali, A.; Fast, P. Retention of Adults from Fishing Communities in an HIV Vaccine Preparedness Study in Masaka, Uganda. PLoS ONE 2019, 14, e0198460. [Google Scholar] [CrossRef] [Green Version]
- Nampijja, M.; Webb, E.L.; Kaweesa, J.; Kizindo, R.; Namutebi, M.; Nakazibwe, E.; Oduru, G.; Kabuubi, P.; Kabagenyi, J.; Kizito, D.; et al. The Lake Victoria Island Intervention Study on Worms and Allergy-Related Diseases (LaVIISWA): Study Protocol for a Randomised Controlled Trial. Trials 2015, 16, 187. [Google Scholar] [CrossRef] [Green Version]
- Sanya, R.E.; Nkurunungi, G.; Hoek Spaans, R.; Nampijja, M.; O’Hara, G.; Kizindo, R.; Oduru, G.; Kabuubi Nakawungu, P.; Niwagaba, E.; Abayo, E.; et al. The Impact of Intensive versus Standard Anthelminthic Treatment on Allergy-Related Outcomes, Helminth Infection Intensity and Helminth-Related Morbidity in Lake Victoria Fishing Communities, Uganda: Results from the LaVIISWA Cluster Randomised Trial. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Woods, C.K.; Brumme, C.J.; Liu, T.F.; Chui, C.K.S.; Chu, A.L.; Wynhoven, B.; Hall, T.A.; Trevino, C.; Shafer, R.W.; Harrigan, P.R. Automating HIV Drug Resistance Genotyping with RECall, a Freely Accessible Sequence Analysis Tool. J. Clin. Microbiol. 2012, 50, 1936–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- HIV Drug Resistance Database. Available online: https://hivdb.stanford.edu/ (accessed on 15 January 2021).
- Brooks, J.I.; Niznick, H.; Ofner, M.; Merks, H.; Angel, J.B. Local Phylogenetic Analysis Identifies Distinct Trends in Transmitted HIV Drug Resistance: Implications for Public Health Interventions. BMC Infect. Dis. 2013, 13, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castor, D.; Low, A.; Evering, T.; Karmon, S.; Davis, B.; Figueroa, A.; LaMar, M.; Garmon, D.; Mehandru, S.; Markowitz, M. Transmitted Drug Resistance and Phylogenetic Relationships among Acute and Early HIV Infected Individuals in New York City. J. Acquir. Immune Defic. Syndr. 2012, 61, 1–8. [Google Scholar] [CrossRef] [Green Version]
- ElimDupes. Available online: https://www.hiv.lanl.gov/content/sequence/ELIMDUPES/elimdupes.html (accessed on 15 January 2021).
- Stamatakis, A.; Hoover, P.; Rougemont, J. A Rapid Bootstrap Algorithm for the RAxML Web Servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. JModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Ragonnet-Cronin, M.; Hodcroft, E.; Hué, S.; Fearnhill, E.; Delpech, V.; Brown, A.J.L.; Lycett, S. UK HIV Drug Resistance Database. Automated Analysis of Phylogenetic Clusters. BMC Bioinform. 2013, 14, 317. [Google Scholar] [CrossRef] [Green Version]
- FigTree Is Designed as a Graphical Viewer of Phylogenetic Trees and as a Program for Producing Publication-Ready Figures. Available online: http://Tree.Bio.Ed.Ac.Uk/Software/Figtree/ (accessed on 15 January 2021).
- Struck, D.; Lawyer, G.; Ternes, A.-M.; Schmit, J.-C.; Bercoff, D.P. COMET: Adaptive Context-Based Modeling for Ultrafast HIV Subtype Identification. Nucleic Acids Res. 2014, 42, e144. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Posada, D.; Stawiski, E.; Chappey, C.; Poon, A.F.Y.; Hughes, G.; Fearnhill, E.; Gravenor, M.B.; Leigh Brown, A.J.; Frost, S.D.W. An Evolutionary Model-Based Algorithm for Accurate Phylogenetic Breakpoint Mapping and Subtype Prediction in HIV. PLoS Comput. Biol. 2009, 5, e1000581. [Google Scholar] [CrossRef] [Green Version]
- Pineda-Peña, A.-C.; Faria, N.R.; Imbrechts, S.; Libin, P.; Abecasis, A.B.; Deforche, K.; Gómez-López, A.; Camacho, R.J.; de Oliveira, T.; Vandamme, A.-M. Automated Subtyping of HIV Genetic Sequences for Clinical and Surveillance Purposes: Performance Evaluation of the New REGA Version 3 and Seven Other Tools. Infect. Genet. Evol. 2013, 19, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Lam, T.T.; Max Carvalho, L.; Pybus, O.G. Exploring the Temporal Structure of Heterochronous Sequences Using TempEst (Formerly Path-O-Gen). Virus Evol. 2016, 2, vew007. [Google Scholar] [CrossRef] [Green Version]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, B.; Rambaut, A.; Drummond, A.J. Choosing Appropriate Substitution Models for the Phylogenetic Analysis of Protein-Coding Sequences. Mol. Biol. Evol. 2006, 23, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Baele, G.; Li, W.L.S.; Drummond, A.J.; Suchard, M.A.; Lemey, P. Accurate Model Selection of Relaxed Molecular Clocks in Bayesian Phylogenetics. Mol. Biol. Evol. 2013, 30, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Tracer Is a Program for Analysing the Trace Files Generated by Bayesian MCMC Runs (That Is, the Continuous Parameter Values Sampled from the Chain). Available online: http://Tree.Bio.Ed.Ac.Uk/Software/Tracer/ (accessed on 15 January 2021).
- Bayesian Evolutionary Analysis Sampling Trees. Available online: http://Beast.Bio.Ed.Ac.Uk/TreeAnnotator/ (accessed on 15 January 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available online: https://www.R-Project.Org/ (accessed on 15 January 2021).
- Jacob, R.; Harikrishnan, K.P.; Misra, R.; Ambika, G. Measure for Degree Heterogeneity in Complex Networks and Its Application to Recurrence Network Analysis. R. Soc. Open Sci. 2017, 4, 160757. [Google Scholar] [CrossRef] [Green Version]
- White, E.P.; Enquist, B.J.; Green, J.L. On Estimating the Exponent of Power-Law Frequency Distributions. Ecology 2008, 89, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Barabasi, A.-L.; Albert, R. Emergence of Scaling in Random Networks. Science 1999, 286, 509–512. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, C.S. Fitting Heavy Tailed Distributions: The PoweRlaw Package. arXiv 2014, arXiv:1407.3492. [Google Scholar]
- Clauset, A.; Shalizi, C.R.; Newman, M.E.J. Power-Law Distributions in Empirical Data. SIAM Rev. 2009, 51, 661–703. [Google Scholar] [CrossRef] [Green Version]
- Handcock, M.S.; Hunter, D.R.; Butts, C.T.; Goodreau, S.M.; Morris, M. Statnet: Software Tools for the Representation, Visualization, Analysis and Simulation of Network Data. J. Stat. Softw. 2008, 24, 1548–7660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, D.T.; Handcock, M.S.; Morris, M. Degree Distributions in Sexual Networks: A Framework for Evaluating Evidence. Sex. Transm. Dis. 2008, 35, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, J.O. The Place of Mathematics in Medical and Biological Statistics. J. R. Stat. Soc. 1963, 126, 1–41. [Google Scholar] [CrossRef]
- Handcock, M.S.; Jones, J.H. Likelihood-Based Inference for Stochastic Models of Sexual Network Formation. Theor. Popul. Biol. 2004, 65, 413–422. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Novitsky, V.; Moyo, S.; Lei, Q.; DeGruttola, V.; Essex, M. Impact of Sampling Density on the Extent of HIV Clustering. AIDS Res. Hum. Retrovir. 2014, 30, 1226–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Lands, Housing and Urban Development. Slum Settlements in Kampala. Available online: https://askyourgov.org/ (accessed on 12 December 2018).
- Krejcie, R.V.; Morgan, D.W. Determining Sample Size for Research Activities. Educ. Psychol. Meas. 1970, 30, 607–610. [Google Scholar] [CrossRef]
- Latora, V.; Nyamba, A.; Simpore, J.; Sylvette, B.; Diane, S.; Sylvére, B.; Musumeci, S. Network of Sexual Contacts and Sexually Transmitted HIV Infection in Burkina Faso. J. Med. Virol. 2006, 78, 724–729. [Google Scholar] [CrossRef]
- Jones, J.H.; Handcock, M.S. An Assessment of Preferential Attachment as a Mechanism for Human Sexual Network Formation. Proc. Biol. Sci. 2003, 270, 1123–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, I.A.; Padian, N.S.; Marlow, C.; Aral, S.O. Determinants and Consequences of Sexual Networks as They Affect the Spread of Sexually Transmitted Infections. J. Infect. Dis. 2005, 191 (Suppl. 1), S42–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.M.; May, R.M. Epidemiological Parameters of HIV Transmission. Nature 1988, 333, 514–519. [Google Scholar] [CrossRef]
- Kiwanuka, N.; Ssetaala, A.; Mpendo, J.; Wambuzi, M.; Nanvubya, A.; Sigirenda, S.; Nalutaaya, A.; Kato, P.; Nielsen, L.; Kaleebu, P.; et al. High HIV Prevalence, Risk Behaviours, and Willingness to Participate in HIV Vaccine Trials in Fishing Communities on Lake Victoria, Uganda. J. Int. AIDS Soc. 2013, 16, 18621. [Google Scholar] [CrossRef]
- Pickering, H.; Okongo, M.; Bwanika, K.; Nnalusiba, B.; Whitworth, J. Sexual Behaviour in a Fishing Community on Lake Victoria, Uganda. Health Transit. Rev. 1997, 7, 13–20. [Google Scholar] [PubMed]
- Mafigiri, R.; Matovu, J.K.B.; Makumbi, F.E.; Ndyanabo, A.; Nabukalu, D.; Sakor, M.; Kigozi, G.; Nalugoda, F.; Wanyenze, R.K. HIV Prevalence and Uptake of HIV/AIDS Services among Youths (15–24 Years) in Fishing and Neighboring Communities of Kasensero, Rakai District, South Western Uganda. BMC Public Health 2017, 17, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubega, M.; Nakyaanjo, N.; Nansubuga, S.; Hiire, E.; Kigozi, G.; Nakigozi, G.; Lutalo, T.; Nalugoda, F.; Serwadda, D.; Gray, R.; et al. Understanding the Socio-Structural Context of High HIV Transmission in Kasensero Fishing Community, South Western Uganda. BMC Public Health 2015, 15, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubega, M.; Nakyaanjo, N.; Nansubuga, S.; Hiire, E.; Kigozi, G.; Nakigozi, G.; Lutalo, T.; Nalugoda, F.; Serwadda, D.; Gray, R.; et al. Risk Denial and Socio-Economic Factors Related to High HIV Transmission in a Fishing Community in Rakai, Uganda: A Qualitative Study. PLoS ONE 2015, 10, e0132740. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, B.F.; Svensson, A.; Liljeros, F. Preferential Attachment in Sexual Networks. Proc. Natl. Acad. Sci. USA 2007, 104, 10762–10767. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cluster Size | Total | ||||
|---|---|---|---|---|---|
| TD (years) | 2 | 3 | 4 | 6 | |
| ≤5 | 106 | 21 | 8 | 6 | 141 |
| 5−10 | 34 | 3 | _ | _ | 37 |
| 10−20 | 82 | 24 | 4 | _ | 110 |
| 20−25 | 6 | 9 | _ | _ | 15 |
| Total | 228 | 57 | 12 | 6 | 303 |
| Cluster Size | ||||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | Total | ||
| Population | ||||||
| FFCs | 21 | 5 | 1 | 1 | 28 | |
| GP | 15 | -- | -- | -- | 15 | |
| FSWs | 13 | -- | 1 | -- | 14 | |
| FFCs/GP | 1 | 1 | -- | -- | 2 | |
| FFCs/FSWs | 2 | 1 | -- | -- | 3 | |
| GP/FSWs | 1 | -- | -- | -- | 1 | |
| Total | 53 | 7 | 2 | 1 | 63 | |
| Assortativity Coefficient | 0.83 | 0.59 | 0.47 | −0.2 | 0.69 | |
| Population | 95% Confidence Intervals | No. of Bootstraps | ||
|---|---|---|---|---|
| FFCs | 1 | 2.38 | 2.35−3.47 | 5000 |
| FSWs | 1 | 3.51 | 3.22−4.21 | 5000 |
| GP | 1 | 4.03 | 3.84−4.73 | 5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bbosa, N.; Ssemwanga, D.; Nsubuga, R.N.; Kiwanuka, N.; Bagaya, B.S.; Kitayimbwa, J.M.; Ssekagiri, A.; Yebra, G.; Kaleebu, P.; Leigh-Brown, A. Phylogenetic Networks and Parameters Inferred from HIV Nucleotide Sequences of High-Risk and General Population Groups in Uganda: Implications for Epidemic Control. Viruses 2021, 13, 970. https://doi.org/10.3390/v13060970
Bbosa N, Ssemwanga D, Nsubuga RN, Kiwanuka N, Bagaya BS, Kitayimbwa JM, Ssekagiri A, Yebra G, Kaleebu P, Leigh-Brown A. Phylogenetic Networks and Parameters Inferred from HIV Nucleotide Sequences of High-Risk and General Population Groups in Uganda: Implications for Epidemic Control. Viruses. 2021; 13(6):970. https://doi.org/10.3390/v13060970
Chicago/Turabian StyleBbosa, Nicholas, Deogratius Ssemwanga, Rebecca N. Nsubuga, Noah Kiwanuka, Bernard S. Bagaya, John M. Kitayimbwa, Alfred Ssekagiri, Gonzalo Yebra, Pontiano Kaleebu, and Andrew Leigh-Brown. 2021. "Phylogenetic Networks and Parameters Inferred from HIV Nucleotide Sequences of High-Risk and General Population Groups in Uganda: Implications for Epidemic Control" Viruses 13, no. 6: 970. https://doi.org/10.3390/v13060970







