Development of Broad-Spectrum Antiviral Agents—Inspiration from Immunomodulatory Natural Products
Abstract
:1. Introduction
1.1. Viral Infections and Current Treatment
1.2. Strategies for Developing Broad-Spectrum Antiviral Drugs
2. Fighting Viruses by Modulating Immunity
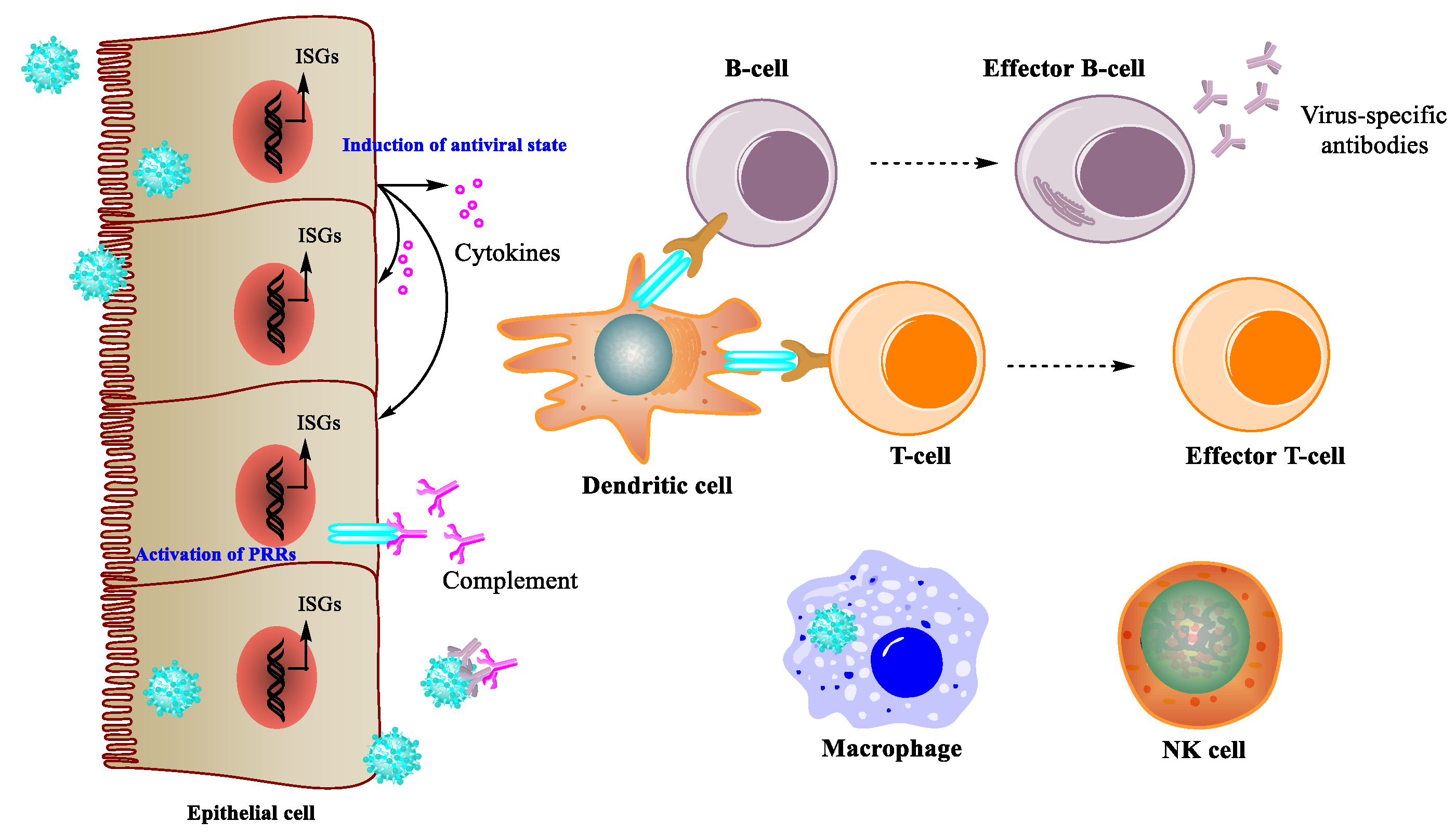
2.1. Immune Response during Viral Infection
2.2. Antiviral Effects of Immunomodulatory Natural Products
3. Natural Products That Enhance Immunity
3.1. Small Molecules
3.2. Peptides and Proteins
3.3. Polysaccharides
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [PubMed]
- Mirza, A.Z.; Shamshad, H.; Osra, F.A.; Habeebullah, T.M.; Morad, M. An overview of viruses discovered over the last decades and drug development for the current Pandemic. Eur. J. Pharmacol. 2021, 890, 173746. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Feldmann, H.; Geisbert, T.W. Ebola haemorrhagic fever. Lancet. 2011, 377, 849–862. [Google Scholar] [CrossRef] [Green Version]
- Report of a WHO/International Study Team. Ebola haemorrhagic fever in Sudan, 1976. Bull. World Health Organ. 1978, 56, 247. [Google Scholar]
- World Health Organization. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 29 May 2021).
- World Health Organization. Available online: https://www.who.int/health-topics/hepatitis#tab=tab_1 (accessed on 29 May 2021).
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695. [Google Scholar] [CrossRef] [Green Version]
- Kellam, P. Attacking pathogens through their hosts. Genome Biol. 2006, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Prussia, A.; Thepchatri, P.; Snyder, J.P.; Plemper, R. Systematic Approaches towards the Development of Host-Directed Antiviral Therapeutics. Int. J. Mol. Sci. 2011, 12, 4027–4052. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.P.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral drug discovery: Broad-spectrum drugs from nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef]
- Real, G.; Jiménez-Baranda, S.; Mira, E.; Lacalle, R.A.; Lucas, P.; Gómez-Moutón, C.; Alegret, M.; Peña, J.M.; Rodríguez-Zapata, M.; Alvarez-Mon, M.; et al. Statins inhibit HIV-1 infection by down-regulating rho activity. J. Exp. Med. 2004, 200, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahlne, A.; Larsson, P.A.; Horal, P.; Ahlmén, J.; Svennerholm, B.; Gronowitz, J.S.; Olofsson, S. Inhibition of herpes simplex virus production In Vitro by Cyclosporin, A. Arch. Virol. 1992, 122, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Damaso, C.R.; Keller, S.J. Cyclosporin A inhibits vaccinia virus replication In Vitro. Arch. Virol. 1994, 134, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Damaso, C.R.; Moussatché, N. Inhibition of vaccinia virus replication by cyclosporin A analogues correlates with their affinity for cellular cyclophilins. J. Gen. Virol. 1998, 79, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Mathur, M.; Bates, P.; Joshi, N.; Banerjee, A.K. Requirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J. Gen. Virol. 2003, 84, 1687–1699. [Google Scholar] [CrossRef]
- Wilde, A.H.; Zevenhoven-Dobbe, J.C.; Meer, Y.; Thiel, V.; Narayanan, K.; Makino, S.; Snijder, E.J.; Hemert, M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011, 92, 2542–2548. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Li, Z.; Xu, C.; Sun, L.; Chen, J.; Liu, W. Cyclosporin A inhibits the influenza virus replication through cyclophilin A-dependent and -independent pathways. PLoS ONE. 2012, 7, e37277. [Google Scholar] [CrossRef] [Green Version]
- Sauder, D.N. Imiquimod: Modes of action. Br. J. Dermatol. 2003, 149, 5–8. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Frazer-Abel, A.; Sepiashvili, L.; Mbughuni, M.M.; Willrich, M.A. Overview of laboratory testing and clinical presentations of complement deficiencies and dysregulation. Adv. Clin. Chem. 2016, 77, 1–75. [Google Scholar]
- Reis, E.S.; Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. New insights into the immune functions of complement. Adv. Clin. Chem. 2019, 19, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.; Essen, M.F.; Kooten, C.; Trouw, L.A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 2017, 188, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarovaya, O.I.; Salakhutdinov, N.F. Mono- and sesquiterpenes as a starting platform for the development of antiviral drugs. Russ. Chem. Rev. 2021, 90, 488–510. [Google Scholar] [CrossRef]
- Ge, H.; Wang, Y.F.; Xu, J.; Gu, Q.; Liu, H.B.; Xiao, P.G.; Zhou, J.; Liu, Y.; Yang, Z.; Su, H. Anti-influenza agents from Traditional Chinese Medicine. Nat. Prod. Rep. 2010, 27, 1758–1780. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.Y.; Song, C.Q.; Hu, Z.B. Effect of astragaloside IV on T, B lymphocyte proliferation and peritoneal macrophage function in mice. Acta Pharmacol. Sin. 2002, 23, 263–266. [Google Scholar]
- Zhang, Y.; Zhu, H.; Huang, C.; Cui, X.; Gao, Y.; Huang, Y.; Gong, W.; Zhao, Y.; Guo, S. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-γ. J. Cardiovasc. Pharmacol. 2006, 47, 190–195. [Google Scholar] [CrossRef]
- Gui, J.; Chen, R.; Xu, W.; Xiong, S. Remission of CVB3-induced myocarditis with Astragaloside IV treatment requires A20 (TNFAIP3) up-regulation. J. Cell. Mol. Med. 2015, 19, 850–864. [Google Scholar] [CrossRef]
- Kubota, T.; Hinoh, H. The constitution of saponins isolated from Bupleurum falcatum. Tetrahedron Lett. 1968, 9, 303–306. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kohno, H.; Tawara, M.; Odashima, S.; Abe, H. Effect of saikosaponin derivatives upon the immune response against T-dependent and T-independent antigens in mice. Int. J. Immunopharmacol. 1985, 7, 827–832. [Google Scholar] [CrossRef]
- Kumazawa, Y.; Takimoto, H.; Nishimura, C.; Kawakita, T.; Nomoto, K. Activation of murine peritoneal macrophages by saikosaponin a, saikosaponin d and saikogenin d. Int. J. Immunopharmacol. 1989, 11, 21–28. [Google Scholar] [CrossRef]
- Chen, J.; Duan, M.; Zhao, Y.; Ling, F.; Xiao, K.; Li, Q.; Li, B.; Lu, C.; Qi, W.; Zeng, Z.; et al. Saikosaponin A inhibits influenza A virus replication and lung immunopathology. Oncotarget 2015, 6, 42541–42556. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Jiang, C.; He, K.; Hu, Y. Antiviral and immunoregulatory role against PCV2 In Vivo of Chinese herbal medicinal ingredients. J. Vet. Res. 2017, 61, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Mao, A.; Tan, Y.; Zhao, Y.; He, K. Role of 5 Saponins in Secretion of Cytokines by PRRSV-induced Endothelial Cells. Drug Res. 2016, 66, 357–362. [Google Scholar] [CrossRef]
- Shibata, S.; Tanaka, O.; Ando, T.; Sado, M.; Tsushima, S.; Ohsawa, T. Chemical studies on oriental plant drugs. XIV. Protopanaxadiol, a genuine sapogenin of ginseng saponins. Chem. Pharm. Bull. 1966, 14, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.; Gao, H.; He, L.; Liu, Y.; Fan, H.; Xu, Q.; Yang, S. Ginsenoside Rb1 is an immune-stimulatory agent with antiviral activity against enterovirus 71. J. Ethnopharmacol. 2020, 266, 113401. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Park, S.J.; Kim, Y.M.; Lee, J.Y.; Seo, W.D.; Chang, J.S.; Park, K.H.; Rho, M.C.; Lee, W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010, 20, 1873–1876. [Google Scholar] [CrossRef]
- Youn, G.S.; Kwon, D.J.; Ju, S.M.; Rhim, H.; Bae, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol. Appl. Pharmacol. 2014, 280, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Wu, Y.H.; Hsieh, C.L.; Lee, J.C. Celastrol inhibits dengue virus replication via up-regulating type I interferon and downstream interferon-stimulated responses. Antivir. Res. 2017, 137, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; He, J.; Xu, H.; Hu, X.P.; Wu, X.L.; Wu, H.Q.; Liu, L.Z.; Liao, C.H.; Zeng, Y.; Li, Y.; et al. The antiviral effects of acteoside and the underlying IFN-γ-inducing action. Food Funct. 2016, 7, 3017–3030. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ding, Y.; Zhou, J.; Sun, X.; Wang, S. The In Vitro and In Vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 2009, 16, 146–155. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Ganju, L. Salidroside exhibits anti-dengue virus activity by upregulating host innate immune factors. Arch. Virol. 2016, 161, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Agbo, M.O.; Odimegwu, D.C.; Okoye, F.B.C.; Osadebe, P.O. Antiviral activity of Salidroside from the leaves of Nigerian mistletoe (Loranthus micranthus Linn) parasitic on Hevea brasiliensis against respiratory syncytial virus. Pak. J. Pharm. Sci. 2017, 30, 1251–1256. [Google Scholar]
- Hayashi, K.; Narutaki, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biol. Pharm. Bull. 2010, 33, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, W.; Jin, E.; He, Q.; Yan, W.; Yang, H.; Gong, S.; Guo, Y.; Fu, S.; Chen, X.; et al. The antiviral activity of arctigenin in traditional Chinese medicine on porcine circovirus type 2. Res. Vet. Sci. 2016, 106, 159–164. [Google Scholar] [CrossRef]
- Swarup, V.; Ghosh, J.; Mishra, M.K.; Basu, A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignin. J. Antimicrob. Chemother. 2008, 61, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.C.; Merz, H.; Steffen, R.; Müller, W.E.; Sarin, P.S.; Trumm, S.; Schulz, J.; Eich, E. Differential In Vitro anti-HIV activity of natural lignans. Z. Naturforsch. C 1990, 45, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hollenbaugh, J.A.; Kim, D.H.; Kim, B. Novel PI3K/Akt inhibitors screened by the cytoprotective function of human immunodeficiency virus type 1 Tat. PLoS ONE. 2011, 6, e21781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Chang, L.; Du, Q.; Huang, Y.; Zhang, X.; Wu, X.; Zhang, J.; Li, R.; Zhang, Z.; Zhang, W.; et al. Arctigenin induces an activation response in porcine alveolar macrophage through TLR6-NOX2-MAPKs signaling pathway. Front. Pharmacol. 2018, 9, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.C.; Chang, J.S.; Chiang, L.C.; Lin, C.C. 4-Methoxycinnamaldehyde inhibited human respiratory syncytial virus in a human larynx carcinoma cell line. Phytomedicine 2009, 16, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Hung, C.Y.; Hseih, Y.C.; Chang, C.S.; Velu, A.B.; He, Y.C.; Huang, Y.L.; Chen, T.A.; Chen, T.C.; Lin, C.Y.; et al. Effect of aloin on viral neuraminidase and hemagglutinin-specific T cell immunity in acute influenza. Phytomedicine 2019, 64, 152904. [Google Scholar] [CrossRef]
- Lin, C.W.; Wu, C.F.; Hsiao, N.W.; Chang, C.Y.; Li, S.W.; Wan, L.; Lin, Y.J.; Lin, W.Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef]
- Ji, S.; Li, R.; Wang, Q.; Miao, W.J.; Li, Z.W.; Si, L.L.; Qiao, X.; Yu, S.W.; Zhou, D.M.; Ye, M. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol. 2015, 176, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dou, J.; Teng, Z.; Yu, J.; Wang, T.; Lu, N.; Wang, H.; Zhou, C. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch. Virol. 2014, 159, 3269–3278. [Google Scholar] [CrossRef]
- Chu, M.; Xu, L.; Zhang, M.B.; Chu, Z.Y.; Wang, Y.D. Role of Baicalin in anti-influenza virus A as a potent inducer of IFN-gamma. Biomed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.K.; Weeratunga, P.; Lee, B.H.; Park, J.S.; Kim, C.J.; Ma, J.Y.; Lee, J.S. Epimedium koreanum Nakai displays broad spectrum of antiviral activity In Vitro and In Vivo by inducing cellular antiviral state. Viruses 2015, 7, 352–377. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, B.; Cho, S.Y.; Oh, K.S.; Kim, S.H.; Kim, Y.O.; Jeong, E.H.; Nguyen, T.T.; Kim, S.H.; Kim, I.S.; Kwon, J.; et al. Effectiveness of Periodic Treatment of Quercetin against Influenza A Virus H1N1 through Modulation of Protein Expression. J. Agric. Food Chem. 2016, 64, 4416–4425. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Chen, M.; Liu, L.; Wang, Q.; Liu, S.; Wang, L.; Wang, R. Anti-influenza A virus mechanism of three representative compounds from Flos Trollii via TLRs signaling pathways. J. Ethnopharmacol. 2020, 253, 112634. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhang, H.; Sun, P.; Khan, A.; Guo, J.; Zheng, X.; Sun, Y.; Fan, K.; Yin, W.; Li, H. Matrine exhibits antiviral activity in a PRRSV/PCV2 co-infected mouse model. Phytomedicine 2020, 77, 153289. [Google Scholar] [CrossRef]
- Yao, N.; Wang, X. In Vitro immunomodulatory activity of oxymatrine on Toll-like receptor 9 signal pathway in chronic hepatitis B. Am. J. Chin. Med. 2014, 42, 1399–1410. [Google Scholar] [CrossRef]
- Liu, H.; Zou, M.; Li, P.; Wang, H.; Lin, X.; Ye, J. Oxymatrine-mediated maturation of dendritic cells leads to activation of FOXP3+/CD4+ Treg cells and reversal of cisplatin-resistance in lung cancer cells. Mol. Med. Rep. 2019, 19, 4081–4090. [Google Scholar] [CrossRef]
- Ye, J.; Zou, M.M.; Li, P.; Lin, X.J.; Jiang, Q.W.; Yang, Y.; Huang, J.R.; Yuan, M.L.; Xing, Z.H.; Wei, M.N.; et al. Oxymatrine and Cisplatin Synergistically Enhance Anti-Tumor Immunity of CD8+ T Cells in Non-small Cell Lung Cancer. Front. Oncol. 2018, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, X.; Zhang, K.; Zhang, X.; Li, S.; Li, Y.; Fan, W.; Leng, F.; Yang, M.; Chen, J. Extraction kinetics, thermodynamics, rheological properties and anti-BVDV activity of the hot water assisted extraction of Glycyrrhiza polysaccharide. Food Funct. 2020, 11, 4067–4080. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ruan, S.; Gu, X.; Zhu, B. Antiviral activities of Radix Isatidis polysaccharide against type II herpes simplex virus In Vitro. Food Sci. Technol. 2018, 38, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, L.; Zhou, H.; Zeng, L.; Chen, T.; Chen, Q.; Zhou, B.; Wang, Y.; Chen, Q.; Hu, P.; et al. Radix isatidis polysaccharides inhibit influenza a virus and influenza a virus-induced inflammation via suppression of host tlr3 signaling In Vitro. Molecules 2017, 22, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X.; Zhuo, Y.; Si, C.; Yang, L.; Meng, L.; Zhu, B. Antiviral activity of a polysaccharide from Radix Isatidis (Isatis indigotica Fortune) against hepatitis B virus (HBV) In Vitro via activation of JAK/STAT signal pathway. J. Ethnopharmacol. 2020, 257, 112782. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In Vivo Anti-influenza Virus Activity of an Immunomodulatory Acidic Polysaccharide Isolated from Cordyceps militaris Grown on Germinated Soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.P.; Shao, M.M.; Song, X.; Wu, X.L.; Qi, L.; Zheng, K.; Fan, L.; Liao, C.H.; Li, C.Y.; He, J.; et al. Anti-influenza virus effects of crude phenylethanoid glycosides isolated from ligustrum purpurascens via inducing endogenous interferon-γ. J. Ethnopharmacol. 2016, 179, 128–136. [Google Scholar] [CrossRef]
- Yang, Z.G.; Sun, H.X.; Fang, W.H. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice. Vaccine 2005, 23, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.P.; Gao, L.X.; Hou, L.F.; Yang, X.Q.; He, P.L.; Yang, Y.F.; Tang, W.; Yue, J.M.; Li, J.; Zuo, J.P. Astragaloside II triggers T cell activation through regulation of CD45 protein tyrosine phosphatase activity. Acta Pharmacol. Sin. 2013, 34, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Yesilada, E.; Bedir, E.; Caliş, I.; Takaishi, Y.; Ohmoto, Y. Effects of triterpene saponins from Astragalus species on In Vitro cytokine release. J. Ethnopharmacol. 2005, 96, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Nalbantsoy, A.; Nesil, T.; Yılmaz-Dilsiz, O.; Aksu, G.; Khan, S.; Bedir, E. Evaluation of the immunomodulatory properties in mice and In Vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J. Ethnopharmacol. 2012, 139, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, K.; Watanabe, M.; Ozeki, T.; Tsurufuji, S. Pharmacological influence of saikosaponins on prostaglandin E2 production by peritoneal macrophages. Planta Med. 1985, 51, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ushio, Y.; Abe, H. The effects of saikosaponin on macrophage functions and lymphocyte proliferation. Planta Med. 1991, 57, 511–514. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, C.; Deng, Y.; Kanagasabai, R.; Ninh, T.N.; Tu, V.T.; Chai, H.B.; Soejarto, D.D.; Fuchs, J.R.; Yalowich, J.C.; et al. Cytotoxic and natural killer cell stimulatory constituents of Phyllanthus songboiensis. Phytochemistry 2015, 111, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Murakami, T.; Komatsu, H.; Matsuda, H. Medicinal foodstuffs. XII. Saponin constituents with adjuvant activity from hyacinth bean, the seeds of Dolichos lablab L. (1): Structures of lablabosides A, B, and C. Chem. Pharm. Bull. 1998, 29, 812–816. [Google Scholar] [CrossRef]
- Peng, L.N.; Li, L.; Qiu, Y.F.; Miao, J.H.; Gao, X.Q.; Zhou, Y.; Shi, Z.X.; Xu, Y.L.; Shao, D.H.; Wei, J.C.; et al. Glycyrrhetinic acid extracted from Glycyrrhiza uralensis Fisch. induces the expression of Toll-like receptor 4 in Ana-1 murine macrophages. J. Asian Nat. Prod. Res. 2011, 13, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Hu, Y.; Fan, Y.; Wang, D.; Yuan, J.; Xu, L.; Cui, L.; Jing, Z. Optimization on condition of glycyrrhetinic acid liposome by RSM and the research of its immunological activity. Int. J. Biol. Macromol. 2012, 51, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lezama-Davila, C.M.; Isaac-Marquez, A.P.; Calomeni, E.P.; Fuchs, J.R.; Satoskar, A.R.; Kinghorn, A.D. Sterols with antileishmanial activity isolated from the roots of Pentalinon andrieuxii. Phytochemistry 2012, 82, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Peine, K.J.; Abdelhamid, D.; Snider, H.; Shelton, A.B.; Rao, L.; Kotha, S.R.; Huntsman, A.C.; Varikuti, S.; Oghumu, S.; et al. A Novel Sterol Isolated from a Plant Used by Mayan Traditional Healers Is Effective in Treatment of Visceral Leishmaniasis Caused by Leishmania donovani. ACS Infect. Dis. 2015, 1, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Sheeja, K.; Kuttan, G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth In Vivo by Andrographis paniculata extract and andrographolide. Immunopharmacol. Immunotoxicol. 2007, 29, 81–93. [Google Scholar] [CrossRef]
- Xiong, W.B.; Shao, Z.J.; Xiong, Y.; Chen, J.; Sun, Y.; Zhu, L.; Zhou, L.M. Dehydroandrographolide enhances innate immunity of intestinal tract through up-regulation the expression of hBD-2. DARU J. Pharm. Sci. 2015, 23, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Guoruoluo, Y.; Tuo, Y.; Zhou, J.; Zhang, H.; Wang, W.; Xiang, M.; Aisa, H.A.; Yao, G. Cassiabudanols A and B, Immunostimulative Diterpenoids with a Cassiabudane Carbon Skeleton Featuring a 3 Oxatetracyclo[6.6.1.02,6.010,14]pentadecane Scaffold from Cassia Buds. Org. Lett. 2019, 21, 549–553. [Google Scholar] [CrossRef]
- Zhou, L.; Tuo, Y.; Hao, Y.; Guo, X.; Tang, W.; Xue, Y.; Zeng, J.; Zhou, Y.; Xiang, M.; Zuo, J.; et al. Cinnamomols A and B, Immunostimulative Diterpenoids with a New Carbon Skeleton from the Leaves of Cinnamomum cassia. Org. Lett. 2017, 19, 3029–3032. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, P.; Yao, Q.; Shao, B.; Yu, H.; Yu, K.; Li, Y. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019, 10, 3868–3879. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Zhang, L.; Tomita, Y. Studies of immunomodulating actions of carotenoids. II. Astaxanthin enhances In Vitro antibody production to T-dependent antigens without facilitating polyclonal B-cell activation. Nutr. Cancer 1993, 19, 269–280. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Zhang, L.; Gross, M.; Tomita, Y. Immunomodulating actions of carotenoids: Enhancement of In Vivo and In Vitro antibody production to T-dependent antigens. Nutr. Cancer 1994, 21, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cao, W.; Gao, Y.; Yang, R.; Zhang, X.; Xu, J.; Tang, Q. Astaxanthin (ATX) enhances the intestinal mucosal functions in immunodeficient mice. Food Funct. 2020, 11, 3371–3381. [Google Scholar] [CrossRef]
- Wang, X.; Willén, R.; Wadström, T. Astaxanthin-Rich Algal Meal and Vitamin C Inhibit Helicobacter pylori Infection in BALB/cA Mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.Z.; Luo, J.; Yin, Y.X.; Wei, Q. Effects of chlorogenic acid, an active compound activating calcineurin, purified from Flos Lonicerae on macrophage. Acta Pharmacol. Sin. 2004, 25, 1685–1692. [Google Scholar]
- Deng, Y.; Chu, J.; Ren, Y.; Fan, Z.; Ji, X.; Mundy-Bosse, B.; Yuan, S.; Hughes, T.; Zhang, J.; Cheema, B.; et al. The natural product phyllanthusmin C enhances IFN-gamma production by human NK cells through upregulation of TLR-mediated NF-kappaB signaling. J. Immunol. 2014, 193, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Rhew, K.Y.; Han, Y. Immunoadjuvant activity of ICA that induces Th1-type antibody in mice. Arch. Pharm. Res. 2012, 35, 1685–1691. [Google Scholar] [CrossRef]
- Li, L.; Peng, L.; Miao, J.; Qiu, Y.; Zhou, Y.; Gao, X.; Xu, Y.; Shi, Z.; Shao, D.; Ma, Z. Icariin induces the expression of toll-like receptor 9 in ana-1 murine macrophages. Phytother. Res. 2011, 25, 1732–1735. [Google Scholar] [CrossRef]
- Su, B.; Ye, H.; You, X.; Ni, H.; Chen, X.; Li, L. Icariin alleviates murine lupus nephritis via inhibiting NF-κB activation pathway and NLRP3 inflammasome. Life Sci. 2018, 208, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Shen, J.; Wang, M.; Cui, J.; Wang, Y.; Zhu, S.; Zhang, W.; Yang, H.; Xu, Y.; Geng, D. ICA protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial mode. Biomaterials 2015, 60, 92–99. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, B.; Sun, J.; Lv, Y.; Luo, Q.; Liu, F.; Dong, J. Regulation of Th17/Treg function contributes to the attenuation of chronic airway inflammation by icariin in ovalbumin-induced murine asthma model. Immunobiology 2015, 220, 789–797. [Google Scholar] [CrossRef]
- Chi, L.; Gao, W.; Shu, X.; Lu, X. A natural flavonoid glucoside, ICA, regulates Th17 and alleviates rheumatoid arthritis in a murine model. Mediat. Inflamm. 2014, 2014, 392062. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Deng, W.; Li, C.; Zeng, G. A natural flavonoid glucoside ICA inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2015, 24, 224–231. [Google Scholar] [CrossRef]
- Ajaghaku, D.L.; Akah, P.A.; Ilodigwe, E.E.; Umeokoli, B.O.; Nworu, C.S.; Okoye, F.B. Antioxidant and immune-enhancing potentials of leaf extract and active constituents of Millettia aboensis. Planta Med. 2015, 81, 1479–1480. [Google Scholar] [CrossRef]
- Kikuchi, H.; Isobe, M.; Sekiya, M.; Abe, Y.; Hoshikawa, T.; Ueda, K.; Kurata, S.; Katou, Y.; Oshima, Y. Structures of the dimeric and monomeric chromanones, gonytolides A–C, isolated from the fungus Gonytrichum sp. and their promoting activities of innate immune responses. Org. Lett. 2011, 13, 4624–4627. [Google Scholar] [CrossRef]
- Kikuchi, H.; Hoshikawa, T.; Kurata, S.; Katou, Y.; Oshima, Y. Design and synthesis of Structure-Simplified derivatives of Gonytolide for the promotion of innate immune responses. J. Nat. Prod. 2016, 79, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 2013, 145, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Bergman, M.; Bessler, H.; Punsky, I.; Djaldetti, M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int. J. Immunopharmacol. 1999, 21, 589–597. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef] [Green Version]
- Kuttan, G. Immunomodulatory effect of some naturally occuring sulphur-containing compounds. J. Ethnopharmacol. 2000, 72, 93–99. [Google Scholar] [CrossRef]
- Oghumu, S.; Varikuti, S.; Saljoughian, N.; Terrazas, C.; Huntsman, A.C.; Parinandi, N.L.; Fuchs, J.R.; Kinghorn, A.D.; Satoskar, A.R. Pentalinonsterol, a Constituent of Pentalinon andrieuxii, Possesses Potent Immunomodulatory Activity and Primes T Cell Immune Responses. J. Nat. Prod. 2017, 80, 2515–2523. [Google Scholar]
- Ota, N.; Takano, F.; Muroga, S.; Kawabata, T.; Ishigaki, Y.; Yahagi, N.; Ohta, T. Garlic extract and its selected organosulphur constituents promote ileal immune responses Ex Vivo. J. Funct. Foods 2012, 4, 243–252. [Google Scholar] [CrossRef]
- He, L.X.; Ren, J.W.; Liu, R.; Chen, Q.H.; Zhao, J.; Wu, X.; Zhang, Z.F.; Wang, J.B.; Pettinato, G.; Li, Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017, 8, 3523–3532. [Google Scholar] [CrossRef]
- Xu, G.; Yuan, G.; Lu, X.; An, L.; Sheng, Y.; Du, P. Study on the effect of regulation of Cordyceps militaris polypeptide on the immune function of mice based on a transcription factor regulatory network. Food Funct. 2020, 11, 6066–6077. [Google Scholar] [CrossRef]
- Tanaka, S.; Ko, K.; Kino, K.; Tsuchiya, K.; Yamashita, A.; Murasugi, A.; Sakuma, S.; Tsunoo, H. Complete amino acid sequence of an immunomodulatory protein, ling zhi-8 (LZ-8): An immunomodulator from a fungus, Ganoderma lucidium, having similarity to immunoglobulin variable regions. J. Biol. Chem. 1989, 264, 16372–16377. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, H.C.; Yang, J.J.; Chuang, W.I.; Sheu, F. Polysaccharides PS-G and Protein LZ-8 from Reishi (Ganoderma lucidum) Exhibit Diverse Functions in Regulating Murine Macrophages and T Lymphocytes. J. Agric. Food Chem. 2010, 58, 8535–8544. [Google Scholar] [CrossRef]
- Chien, C.M.; Cheng, J.L.; Chang, W.T.; Tien, M.H.; Tsao, C.M.; Chang, Y.H.; Chang, H.Y.; Hsieh, J.F.; Wong, C.H.; Chen, S.T. Polysaccharides of Ganoderma lucidum alter cell immunophenotypic expression and enhance CD56+ NK-cell cytotoxicity in cord blood. Bioorg. Med. Chem. 2004, 12, 5603–5609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Khoo, K.H.; Chen, S.T.; Lin, C.C.; Wong, C.H.; Lin, C.H. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg. Med. Chem. 2002, 10, 1057–1062. [Google Scholar] [CrossRef]
- Qi, B.; Wang, S.; Wang, Q.; Zhang, H.; Bai, X.Y.; He, H.N.; Sun, W.J.; Liu, L.; Zhao, D.Q. Characterization and immunostimulating effects on murine peritoneal macrophages of a novel protein isolated from Panax quinquefolius. J. Ethnopharmacol. 2016, 193, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Hirao, Y.; Sumioka, I.; Nakagami, S.; Yamamoto, M.; Hatono, S.; Yoshida, S.; Fuwa, T.; Nakagawa, S. Activation of immunoresponder cells by the protein fraction from aged garlic extract. Phytother. Res. 1987, 1, 161–164. [Google Scholar] [CrossRef]
- Morioka, N.; Sze, L.L.; Morton, D.L.; Irie, R.F. A protein fraction from aged garlic extract enhances cytotoxicity and proliferation of human lymphocytes mediated by interleukin-2 and concanavalin A. Cancer Immunol. Immunother. 1993, 37, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Clement, F.; Pramod, S.N.; Venkatesh, Y.P. Identity of the immunomodulatory proteins from garlic (Allium sativum) with the major garlic lectins or agglutinins. Int. Immunopharmacol. 2010, 10, 316–324. [Google Scholar] [CrossRef]
- Suzuki, I.; Saito, H.; Inoue, S.; Migita, S.; Takahashi, T. Purification and characterization of two lectins from Aloe arborescens Mill. J. Biochem. 1979, 85, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, K.I. Aloctin A, an active substance of Aloe arborescens Miller as an immunomodulator. Phytother. Res. 1993, 7, S20–S22. [Google Scholar] [CrossRef]
- Favero, J.; Miquel, F.; Dornand, J.; Mani, J.C. Determination of mitogenic properties and lymphocyte target sites of Dolichos lablab lectin (DLA): Comparative study with concanavalin A and galactose oxidase cell surface receptors. Cell. Immunol. 1988, 112, 302–314. [Google Scholar] [CrossRef]
- Ko, J.L.; Hsu, C.I.; Lin, R.H.; Kao, C.L.; Lin, J.Y. A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence. Eur. J. Biochem. 1995, 228, 244–249. [Google Scholar] [CrossRef]
- Wang, P.H.; Hsu, C.I.; Tang, S.C.; Huang, Y.L.; Lin, J.Y.; Ko, J.L. Fungal immunomodulatory protein from Flammulina velutipes induces interferon-γ production through p38 mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2004, 52, 2721–2725. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chang, S.H.; Sun, H.L.; Chang, Y.C.; Hsin, I.L.; Lue, K.H.; Ko, J.L. IFN-γ Induction on Carbohydrate Binding Module of Fungal Immunomodulatory Protein in Human Peripheral Mononuclear Cells. J. Agric. Food Chem. 2012, 60, 4914–4922. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04571645?term=DSTAT&draw=2&rank=2. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04389840?term=DSTAT&draw=2&rank=3 (accessed on 29 May 2021).
- Dai, Z.; Su, D.; Zhang, Y.; Sun, Y.; Hu, B.; Ye, H.; Jabbar, S.; Zeng, X. Immunomodulatory activity In Vitro and In Vivo of verbascose from mung beans (Phaseolus aureus). J. Agric. Food Chem. 2014, 62, 10727–10735. [Google Scholar] [CrossRef]
- Kahlon, J.B.; Kemp, M.C.; Carpenter, R.H.; McAnalley, B.H.; McDaniel, H.R.; Shannon, W.M. Inhibition of AIDS virus replication by acemannan In Vitro. Mol. Biother. 1991, 3, 127–135. [Google Scholar]
- Kahlon, J.B.; Kemp, M.C.; Yawei, N.; Carpenter, R.H.; Shannon, W.M.; McAnalley, B.H. In Vitro evaluation of the synergistic antiviral effects of acemannan in combination with azidothymidine and acyclovir. Mol. Biother. 1991, 3, 214–223. [Google Scholar]
- Sheets, M.A.; Unger, B.A.; Giggleman, G.F.; Tizard, I.R. Studies of the effect of acemannan on retrovirus infections: Clinical stabilization of feline leukemia virus-infected cats. Mol. Biother. 1991, 3, 41–45. [Google Scholar]
- Womble, D.; Helderman, J.H. Enhancement of allo-responsiveness of human lymphocytes by acemannan (Carrisyn). Int. J. Immunopharmacol. 1988, 10, 967–974. [Google Scholar] [CrossRef]
- Womble, D.; Helderman, J.H. The impact of acemannan on the generation and function of cytotoxic T-lymphocytes. Immunopharmacol. Immunotoxicol. 1992, 14, 63–77. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, M.K.; Yun, Y.P.; Kim, Y.; Kim, J.S.; Kim, Y.S.; Kim, K.; Han, S.S.; Lee, C.K. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int. Immunopharmacol. 2001, 1, 1275–1284. [Google Scholar] [CrossRef]
- Qiu, Z.; Jones, K.; Wylie, M.; Jia, Q.; Orndorff, S. Modified Aloe barbadensis polysaccharide with immunoregulatory activity. Planta Med. 2000, 66, 152–156. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, M.A.; Pasco, D.S. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 2001, 49, 1030–1034. [Google Scholar] [CrossRef]
- Yelithao, K.; Surayot, U.; Lee, J.H.; You, S. RAW264.7 cell activating glucomannans extracted from rhizome of Polygonatum sibiricum. Prev. Nutr. Food Sci. 2016, 21, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Xiao, H.T.; Bao, W.R.; Ma, D.L.; Leung, C.H.; Han, X.Q.; Ko, C.H.; Lau, C.B.; Wong, C.K.; Fung, K.P.; et al. TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J. Ethnopharmacol. 2016, 179, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Xiao, C.; Qu, J.; Wang, G. Structural characterization of low molecular weight polysaccharide from Astragalus membranaceus and its immunologic enhancement in recombinant protein vaccine against systemic candidiasis. Carbohydr. Polym. 2016, 145, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.; Mao, R.; Zou, Y.; Zheng, D.; Feng, W.; Ren, Y.; Wang, W.; Zheng, W.; Song, J.; et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) Baill. Food Chem. Toxicol. 2013, 55, 609–616. [Google Scholar] [CrossRef]
- Zhao, T.; Feng, Y.; Li, J.; Mao, R.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Chen, Y.; Yang, L.; et al. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int. J. Biol. Macromol. 2014, 65, 33–40. [Google Scholar] [CrossRef]
- Yu, J.; Cong, L.; Wang, C.; Li, H.; Zhang, C.; Guan, X.; Liu, P.; Xie, Y.; Chen, J.; Sun, J. Immunomodulatory effect of Schisandra polysaccharides in cyclophosphamide induced immunocompromised mice. Exp. Ther. Med. 2018, 15, 4755–4762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Guilbert, L.J.; Li, J.; Wu, Y.; Pang, P.; Basu, T.K.; Shan, J.J. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-γ productions in murine spleen cells induced by Con-A. Int. Immunopharmacol. 2004, 4, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, Y.S.; Jung, I.S.; Park, S.Y.; Chung, H.Y.; Lee, I.R.; Yun, Y.S. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998, 64, 110–115. [Google Scholar] [CrossRef]
- Shin, K.S.; Kiyohara, H.; Matsumoto, T.; Yamada, H. Rhamnogalacturonan II from the leaves of Panax ginseng CA Meyer as a macrophage Fc receptor expression-enhancing polysaccharide. Carbohydr. Res. 1997, 300, 239–249. [Google Scholar] [CrossRef]
- Tomoda, M.; Hirabayashi, K.; Shimizu, N.; Gonda, R.; Ohara, N.; Takada, K. Characterization of two novel polysaccharides having immunological activities from the root of Panax ginseng. Biol. Pharm. Bull. 1993, 16, 1087–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Guilbert, L.J.; Ling, L.; Li, J.; Wu, Y.; Xu, S.; Pang, P.; Shan, J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J. Pharm. Pharmacol. 2001, 53, 1515–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.Q.; Li, H.; Yue, X.L.; Xie, J.Y.; Zhang, Y.Y.; Di, H.Y.; Chen, D.F. Macrophage immunomodulatory activity of the polysaccharides from the roots of Bupleurum smithii var. parvifolium. J. Ethnopharmacol. 2010, 130, 363–368. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Li, H.; Wan, F.; Su, X.Y.; Lu, Y.; Chen, D.F.; Zhang, Y.Y. Polysaccharides extracted from the roots of Bupleurum chinense DC modulates macrophage functions. Chin. J. Nat. Med. 2017, 15, 889–898. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Zhang, J.; Chen, D. Isolation and characterization of an anti-complementary polysaccharide D3-S1 from the roots of Bupleurum smithii. J. Ethnopharmacol. 2007, 7, 175–182. [Google Scholar] [CrossRef]
- Fang, X.; Yu, M.M.; Yuen, W.H.; Zee, S.Y.; Chang, R.C. Immune modulatory effects of Prunella vulgaris L. on monocytes/macrophages. Int. J. Mol. Med. 2005, 16, 1109–1116. [Google Scholar] [CrossRef]
- Fang, X.; Yu, M.M.; Yuen, W.H.; Zee, S.Y.; Chang, R.C. Immune modulatory effects of Prunella vulgaris L. Int. J. Mol. Med. 2005, 15, 491–496. [Google Scholar] [CrossRef]
- Li, C.; You, L.; Fu, X.; Huang, Q.; Yu, S.; Liu, R.H. Structural characterization and immunomodulatory activity of a new heteropolysaccharide from Prunella vulgaris. Food Funct. 2015, 6, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, H.; Matsuzaki, T.; Yamada, H. Intestinal Peyer’s patch-immunomodulating glucomannans from rhizomes of Anemarrhena asphodeloides Bunge. Phytochemistry 2013, 96, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; An, L.; Zhang, J.; Li, Z.; Zhang, J.; Li, Y.; Tuerhong, M.; Ohizumi, Y.; Jin, J.; et al. A fructan from Anemarrhena asphodeloides Bunge showing neuroprotective and immunoregulatory effects. Carbohydr. Polym. 2020, 229, 115477. [Google Scholar] [CrossRef]
- Kim, H.M.; Han, S.B.; Lee, K.H.; Lee, C.W.; Kim, C.Y.; Lee, E.J.; Huh, H. Immunomodulating activity of a polysaccharide isolated from Mori Cortex Radicis. Arch. Pharm. Res. 2000, 23, 240–242. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, R.; Bi, Y.; Bilal, M.; Kuang, Z.; Iqbal, H.M.N.; Luo, Q. Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs. Animals 2020, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune activation of RAW264.7 macrophages by low molecular weight fucoidan extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Lin, Z.; Liao, W.; Ren, J. Physicochemical characterization of a polysaccharide fraction from Platycladus orientalis (L.) franco and its macrophage immunomodulatory and anti-hepatitis B virus activities. J. Agric. Food Chem. 2016, 64, 5813–5823. [Google Scholar] [CrossRef]
- Perera, N.; Yang, F.L.; Chang, C.M.; Lu, Y.T.; Zhan, S.H.; Tsai, Y.T.; Hsieh, J.F.; Li, L.H.; Hua, K.F.; Wu, S.H. Galactomannan from Antrodia cinnamomea enhances the phagocytic activity of macrophages. Org. Lett. 2017, 19, 3486–3489. [Google Scholar] [PubMed]
- Ma, X.K.; Ma, Y.; Peterson, E.C.; Guo, W.Y.; Li, Z.Y.; Li, Y. Structural characterization of two endopolysaccharides from Phellinus sp. and their immunologic effects by intragastric administration in a healthy mammalian model. Food Funct. 2018, 9, 1224–1234. [Google Scholar] [CrossRef]
- Han, L.; Meng, M.; Guo, M.; Cheng, D.; Shi, L.; Wang, X.; Wang, C. Immunomodulatory activity of a water-soluble polysaccharide obtained from highland barley on immunosuppressive mice models. Food Funct. 2019, 10, 304–314. [Google Scholar] [CrossRef]
- Guo, M.Z.; Meng, M.; Duan, S.Q.; Feng, C.C.; Wang, C.L. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int. J. Biol. Macromol. 2019, 126, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Meng, M.; Feng, C.C.; Wang, X.; Wang, C.L. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-κB pathway. Food Funct. 2019, 10, 4792–4801. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Dong, F.; Liu, X.; Lv, Q.; Ying, Y.; Liu, F.; Chen, L.; Wang, T.; Wang, Z.; Zhang, Y. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr. Polym. 2016, 140, 461–471. [Google Scholar] [CrossRef]
- Meng, M.; Wang, H.; Li, Z.; Guo, M.; Hou, L. Protective effects of polysaccharides from Cordyceps gunnii mycelia against cyclophosphamide-induced immunosuppression to TLR4/TRAF6/NF-κB signalling in BALB/c mice. Food Funct. 2019, 10, 3262–3271. [Google Scholar] [CrossRef]
- Cai, B.; Chen, H.; Sun, H.; Wan, P.; Sun, H.; Pan, J. Production of immunoregulatory polysaccharides from Crassostrea hongkongensis and their positive effects as a nutrition factor in modulating the effectiveness and toxicity of 5-FU chemotherapy in mice. Food Funct. 2016, 7, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, D.; Huang, W.; Ou, X.; Song, L.; Guo, Z.; Wang, H.; Liu, W.; Zhu, J.; Yu, R. Structural characterization of novel comb-like branched α-d-glucan from Arca inflata and its immunoregulatory activities In Vitro and In Vivo. Food Funct. 2019, 10, 6589–6603. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, J.; Meng, Y.; Kang, Y.; Han, Z.; Tai, G.; Zhou, Y.; Cheng, H. Immunomodulatory effects of Hericium erinaceus derived polysaccharides are mediated by intestinal immunology. Food Funct. 2017, 8, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef]
- Meng, M.; Guo, M.; Feng, C.; Wang, R.; Cheng, D.; Wang, C. Water-soluble polysaccharides from Grifola frondosa fruiting bodies protect against immunosuppression in cyclophosphamide-induced mice via JAK2/STAT3/SOCS signal transduction pathways. Food Funct. 2019, 10, 4998–5007. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Ma, Q.; Dong, L.; Su, D.; Chi, J.; Zhang, M. Longan pulp polysaccharide protects against cyclophosphamide-induced immunosuppression in mice by promoting intestinal secretory IgA synthesis. Food Funct. 2020, 11, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tang, N.; Jia, X.; Nirasawa, S.; Bian, X.; Zhang, P.; Cheng, Y. Isolation, physical, structural characterization and in vitro prebiotic activity of a galactomannan extracted from endosperm splits of Chinese Sesbania cannabina seeds. Int. J. Biol. Macromol. 2020, 162, 1217–1226. [Google Scholar] [CrossRef]
- Li, R.; Zhu, C.; Bian, X.; Jia, X.; Tang, N.; Cheng, Y. An antioxidative galactomannan extracted from Chinese Sesbania cannabina enhances immune activation of macrophage cells. Food Funct. 2020, 11, 10635–10644. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, L.; Sun, H.; Wang, Y.; Yang, Z.; Zhang, G.; Jiang, S.; Yang, W. Polysaccharide from alfalfa activates RAW 264.7 macrophages through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 126, 960–968. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Sun, H.; Shang, Q.; Wang, Y.; Zhang, G.; Yang, W.; Jiang, S. A polysaccharide extracted from alfalfa activates splenic B cells by TLR4 and acts primarily via the MAPK/p38 pathway. Food Funct. 2020, 11, 9035–9047. [Google Scholar] [CrossRef] [PubMed]
- Inngjerdingen, K.T.; Coulibaly, A.; Diallo, D.; Michaelsen, T.E.; Paulsen, B.S. A complement fixing polysaccharide from Biophytum p etersianum Klotzsch, a medicinal plant from Mali, west Africa. Biomacromolecules 2006, 7, 48–53. [Google Scholar] [CrossRef]
- Grønhaug, T.E.; Kiyohara, H.; Sveaass, A.; Diallo, D.; Yamada, H.; Paulsen, B.S. Beta-D-(1→4)-galactan-containing side chains in RG-I regions of pectic polysaccharides from Biophytum petersianum Klotzsch. contribute to expression of immunomodulating activity against intestinal Peyer’s patch cells and macrophages. Phytochemistry 2011, 72, 2139–2147. [Google Scholar] [CrossRef]
- Inngjerdingen, M.; Inngjerdingen, K.T.; Patel, T.R.; Allen, S.; Chen, X.; Rolstad, B.; Morris, G.A.; Harding, S.E.; Michaelsen, T.E.; Diallo, D.; et al. Pectic polysaccharides from Biophytum petersianum Klotzsch, and their activation of macrophages and dendritic cells. Glycobiology 2008, 18, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, G.; Wen, Y.; Liu, S.; Li, C.; Yang, R.; Li, W. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide. Mol. Nutr. Food Res. 2017, 61, 8. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Meng, X.; Guan, N.; Xiao, H.; Liu, T.; Yu, Y. The proliferative effects of alfalfa polysaccharides on the mouse immune cells. Life Sci. J. 2013, 10, 868–873. [Google Scholar]
- Kubli, S.P.; Berger, T.; Araujo, D.V.; Siu, L.L.; Mak, T.W. Beyond immune checkpoint blockade: Emerging immunological strategie. Nat. Rev. Drug Discov. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
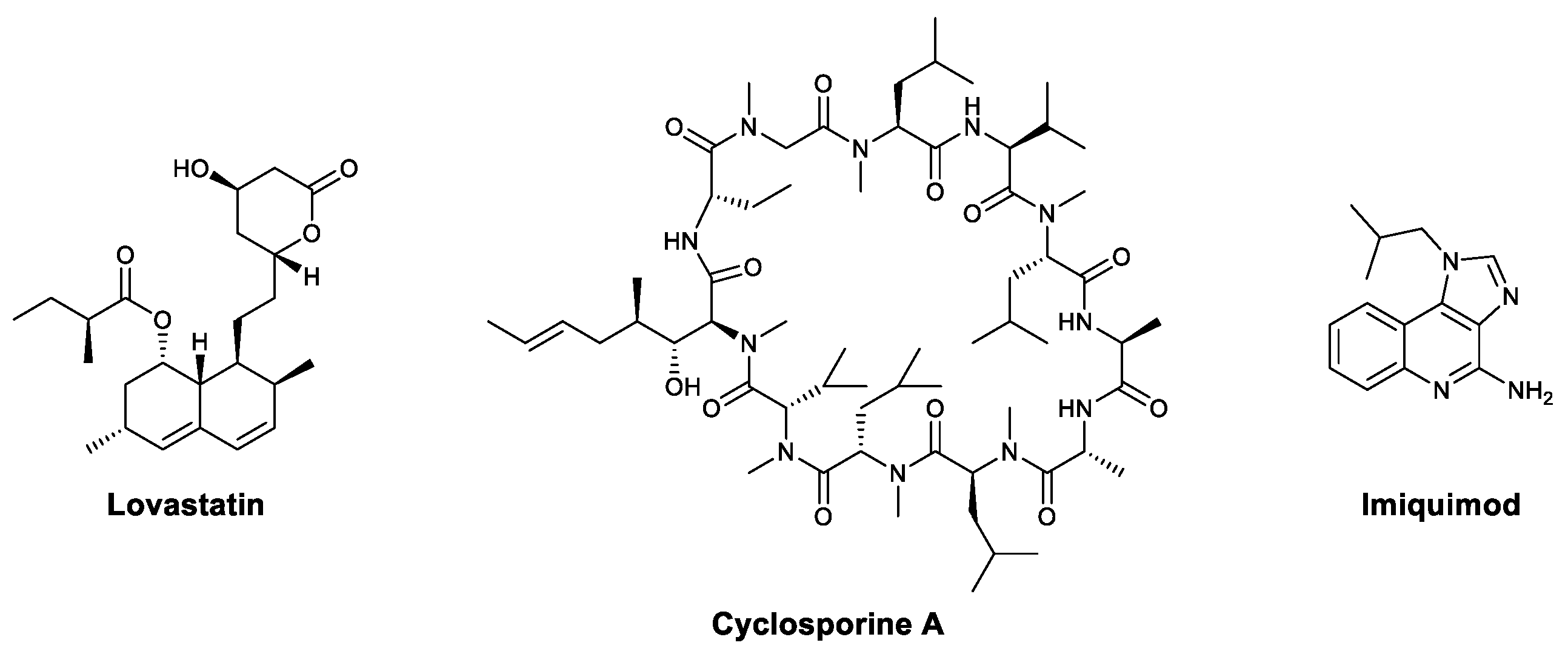
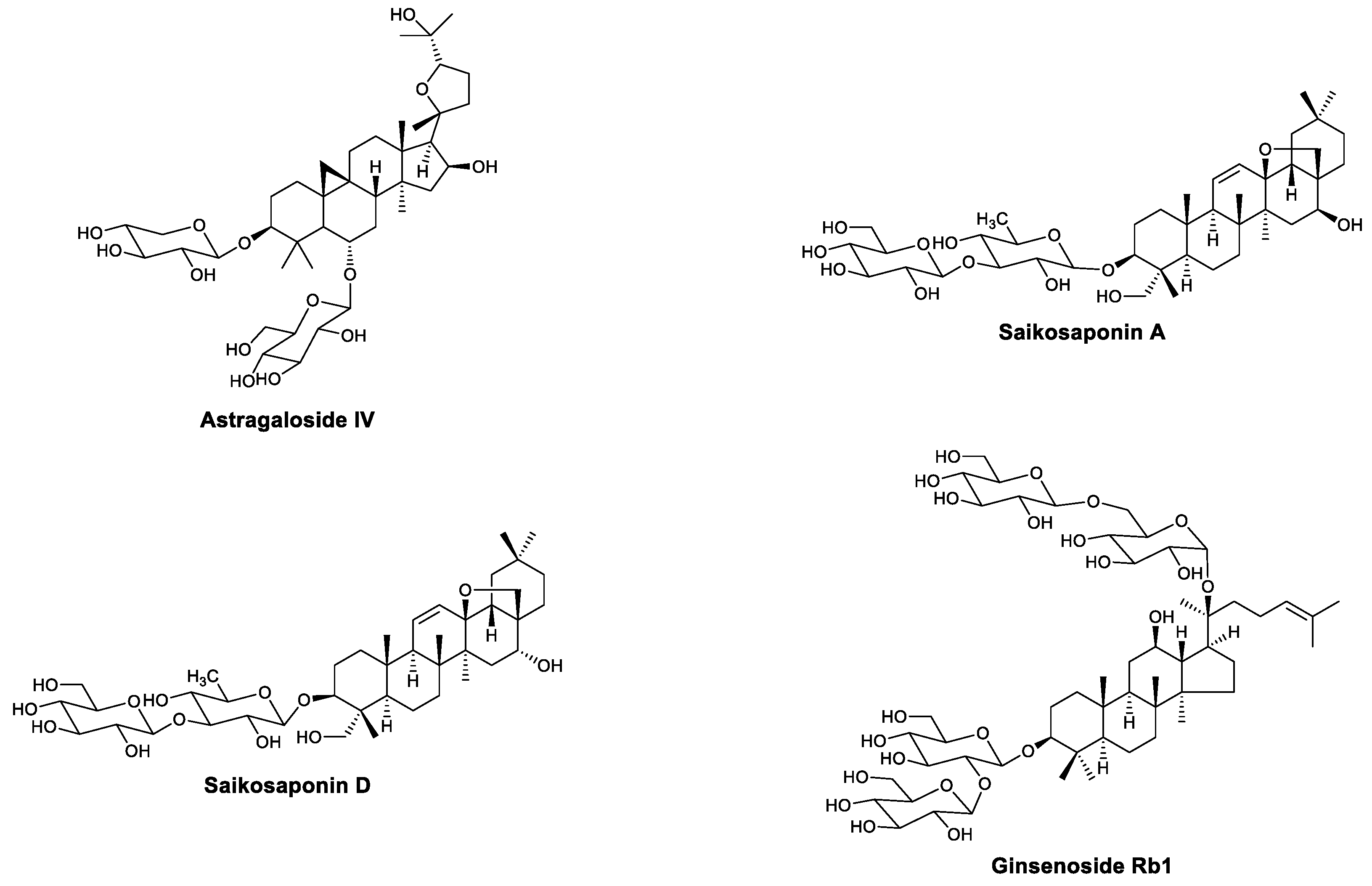


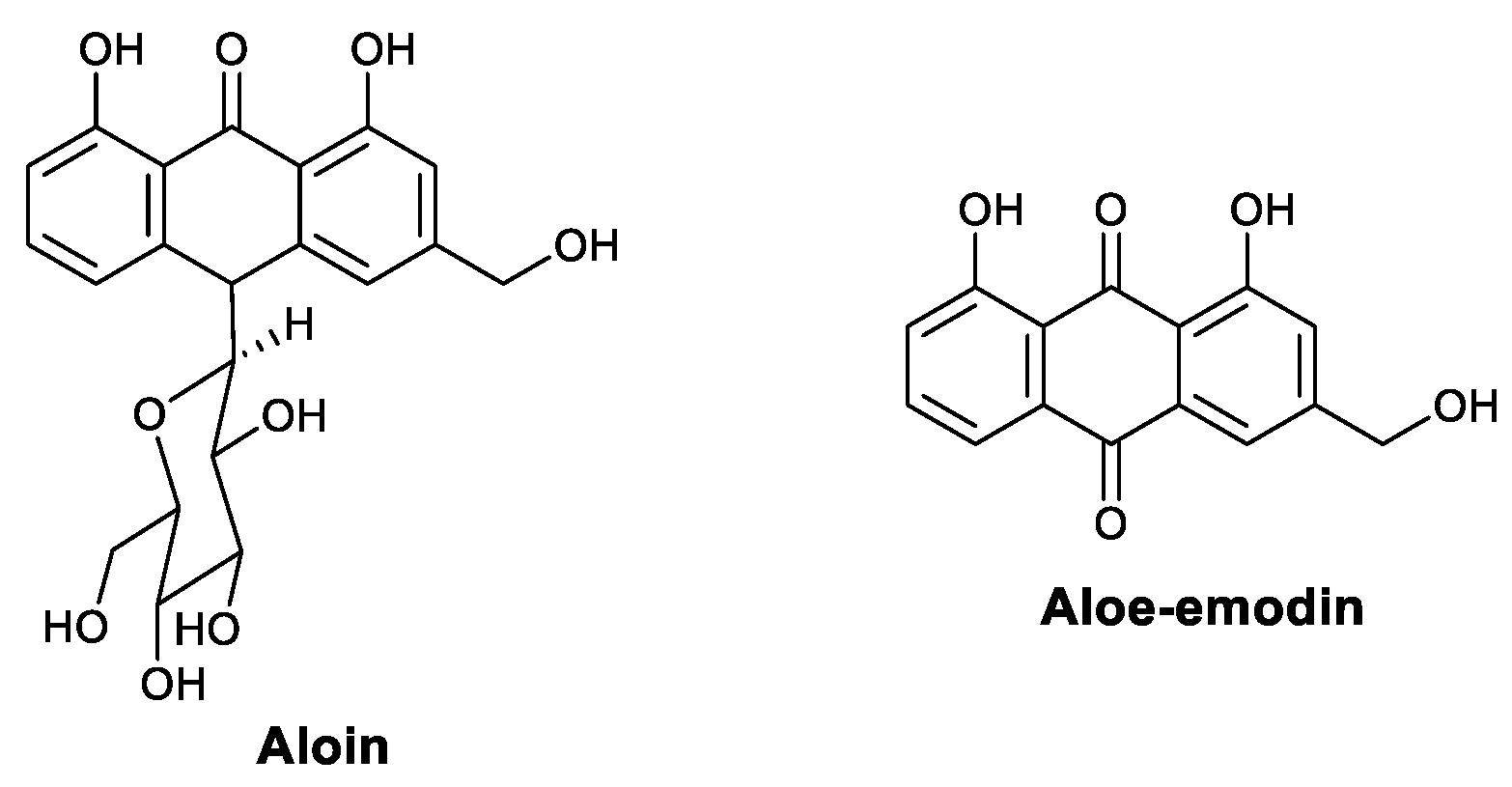



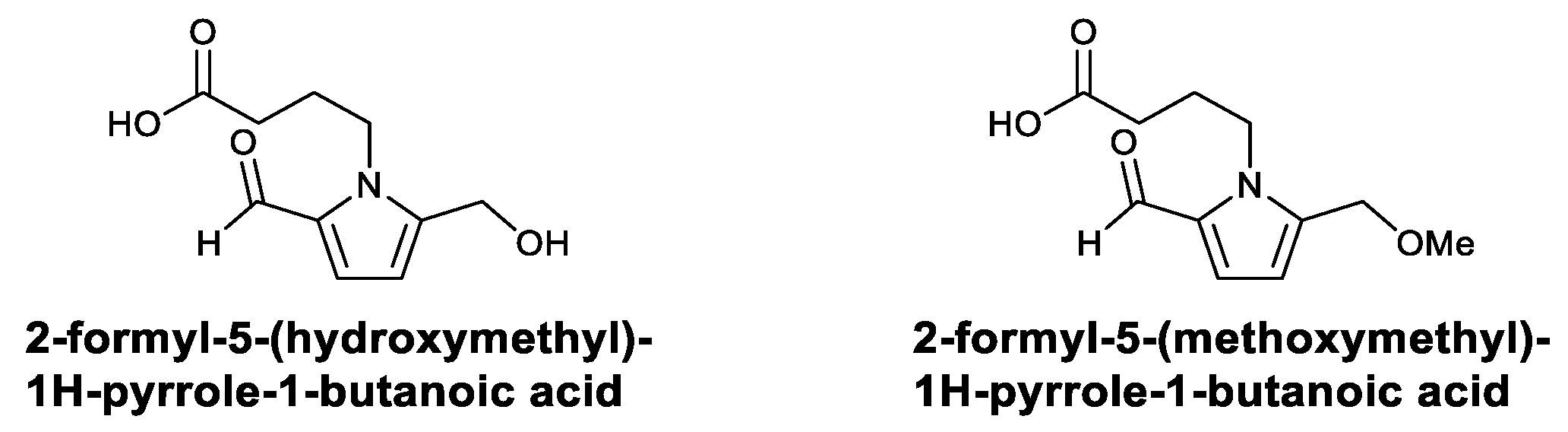
| Chemical Type | Compound Name | Antiviral Against | Mechanisms | Reference |
|---|---|---|---|---|
| Saponins | Astragaloside IV | CVB3 | Upregulated IFN-γ, inhibited NF-κB | [30,31,32] |
| Saikosaponin A | Influenza virus, PCV2 | Downregulated NF-κB and promoted neutrophil and monocyte recruitment; upregulated IL-2 and IFN-γ and increased IgG and WBCs in serum | [33,34,35,36,37,38] | |
| Saikosaponin D | PCV2 | Upregulated IL-2 and IFN-γ and increased IgG and WBCs in serum; activated macrophages | [35,36,37,38] | |
| Ginsenoside Rb1 | EV71 | Increased TNF-α, IL-10, IFNs, and antibodies in serum | [39,40] | |
| Terpenoids | Celastrol | SARS-CoV, HIV, DENV | Activated the JAK/STAT pathway and increased IFN-α | [41,42,43] |
| Phenylpropanoids | Acteoside | VSV, influenza virus | Activated T-bet and increased IFN-γ; activated ERK to promote proliferation of lymphocytes | [44] |
| Salidroside | CVB3, DENV, RSV | Increased IL-10 and IFN-γ, decreased TNF-α and IL-2; increased the expression of RIG-I and upregulated IRFs | [45,46] | |
| Arctigenin | H1N1, PCV2, JEV, HIV, HSV | Activated the TLR6/NOX2/MAPK signaling pathway and activated macrophages | [47,48,49,50,51,52,53] | |
| Arctiin | Influenza A | Increased antibody levels in serum | [48] | |
| 4-Methoxy-cinnamaldehyde | RSV | Increased the secretion of IFN | [54] | |
| Anthraquinones | Aloin | Influenza | Activated STAT, T-bet, and IFN-γ; increased the number of antigen-specific T cells | [55] |
| Aloe-emodin | JEV, EV71 | Increased IFN-α | [56] | |
| Flavonoids | Baicalin | H1N1 | Promoted the secretion of IFN-γ in T cells and NK cells | [57,58,59] |
| Quercetin | HSV, influenza A, NDV, VSV | Increased type I IFN and IL-6 | [60,61] | |
| Vitexin | Influenza virus | Upregulated TLR4 and increased IFN-β | [62] | |
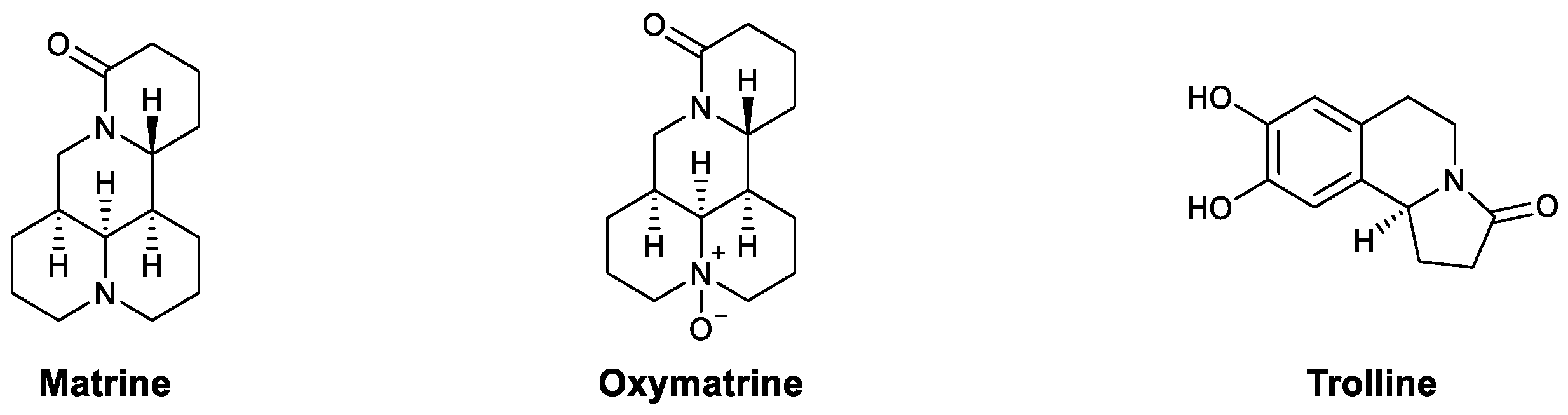
| Alkaloids | Matrine | PRRSV, PCV2 | Enhanced phagocytosis and lymphocyte proliferation | [63] |
| Oxymatrine | HBV | Upregulated the TLR9 pathway, promoted maturation of dendritic cells, and increased IFN-γ, TNF-α, and IL-2 | [64,65,66] | |
| Trolline | Influenza virus | Upregulated TLR4 and increased IFN-β | [62] | |
| Polysaccharides | GP | BVDB | Promoted the expression of IRFs | [67] |
| RIP | HSV-2, influenza A, HBV | Activated the JAK/STAT pathway and upregulated IFN-α | [68,69,70] | |
| APS | Influenza A | Enhanced IL-1β, IL-10, IL-6, and TNF-α levels | [71] |
| Chemical Type | Compound Names | Immunomodulatory Effects | Reference |
|---|---|---|---|
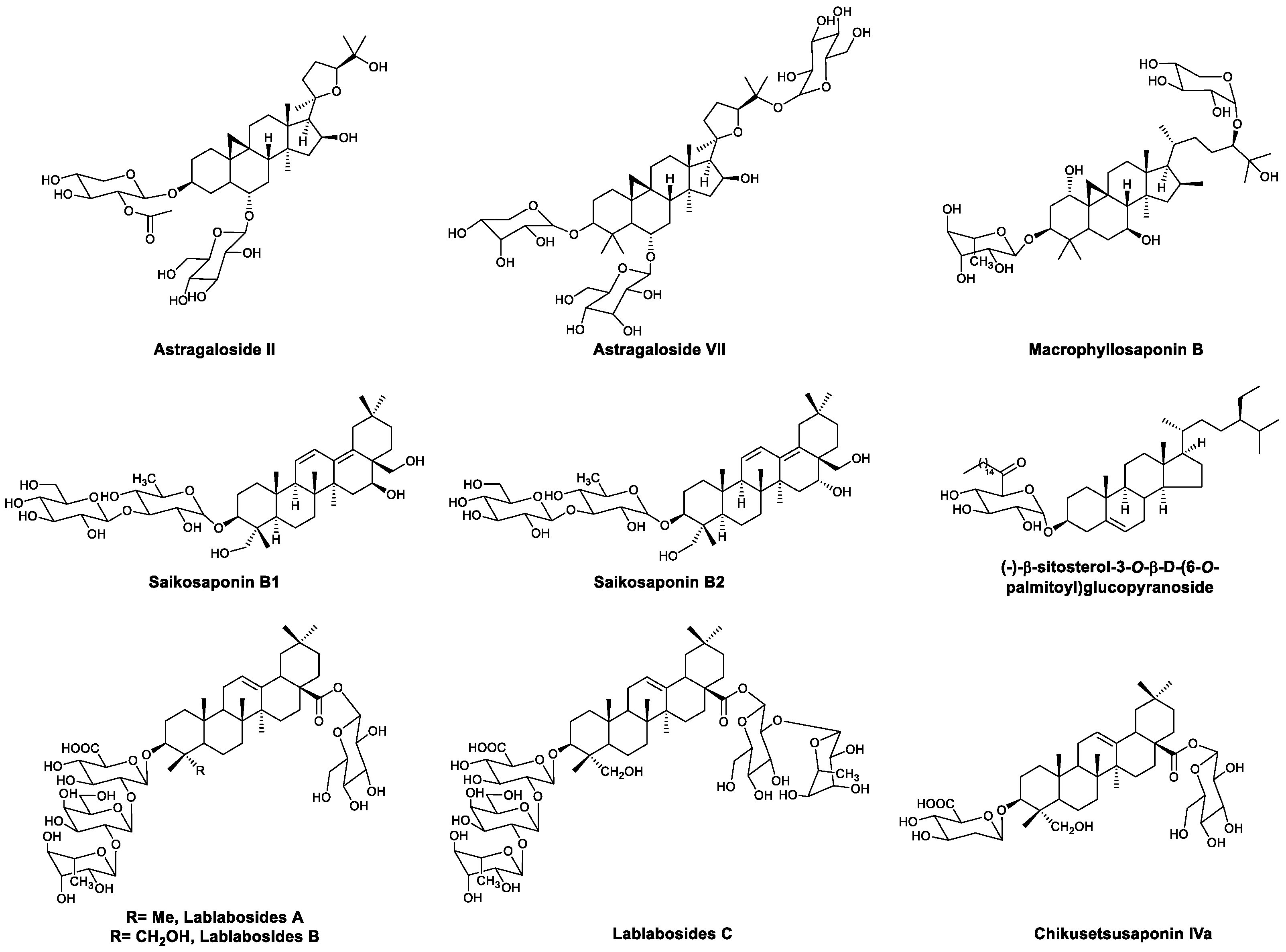
| Saponins | Astragaloside II | Increased IFN-γ and IL-2 and promoted the proliferation and activation of T cells | [73,74] |
| Astragaloside VII, macrophyllosaponin B | Increased IL-2 and IFN-γ and promoted the proliferation of splenocytes | [75,76] | |
| Saikosaponins B1 and B2 | Activated macrophages | [77,78] | |
| (−)-β-Sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside | Promoted the secretion of IFN-γ in NK cells | [79] | |
| Lablabosides A, B, and C | Adjuvant activity | [80] | |
| Chikusetsusaponin IVa | Adjuvant activity | [80] | |
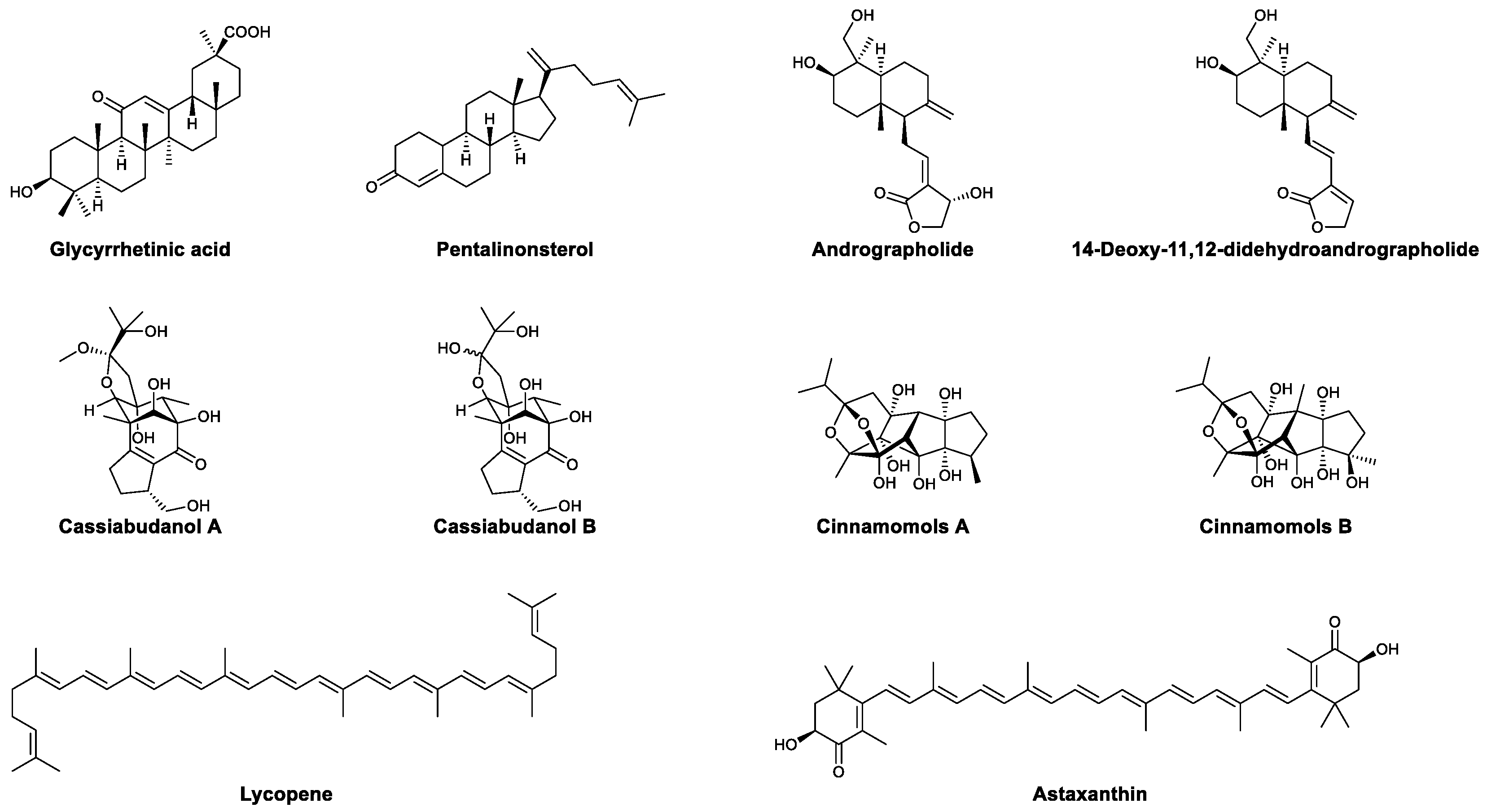
| Terpenoids | Glycyrrhetinic acid | Promoted the expression of TLRs and upregulated downstream MyD88, IL-6, and IFN-β; enhanced the proliferation of lymphocytes | [81,82] |
| Pentalinonsterol | Activated macrophages and DCs, increased proinflammatory cytokines, and increased antibodies | [83,84] | |
| Andrographolide | Enhanced the formation of specific cytotoxic T lymphocytes | [85] | |
| 14-Deoxy-11,12-didehydroandrographolide | Enhanced the innate immunity of intestinal epithelial cells | [86] | |
| Cassiabudanols A and B | Promoted the proliferation of splenocytes and increased the ratio of CD4+ and CD8+ T cells | [87] | |
| Cinnamomols A and B | Promoted the proliferation of T cells | [88] | |
| Lycopene | Increased the proportion of CD4+ and CD8+ T cells and increased IL-2, TNF-α, and IFN-γ | [89] | |
| Astaxanthin | Promoted the proliferation of lymphocytes, enhanced the activity of NK cells, and increased the production of antibodies | [90,91,92,93] | |
| Phenylpropanoids | Chlorogenic acid | Activated macrophages via the calcineurin/NF-ATc/IL-2 pathway | [94,95] |
| Phyllanthusmin C | Increased IFN-γ via the TLR/NF-κB pathway | [96] | |
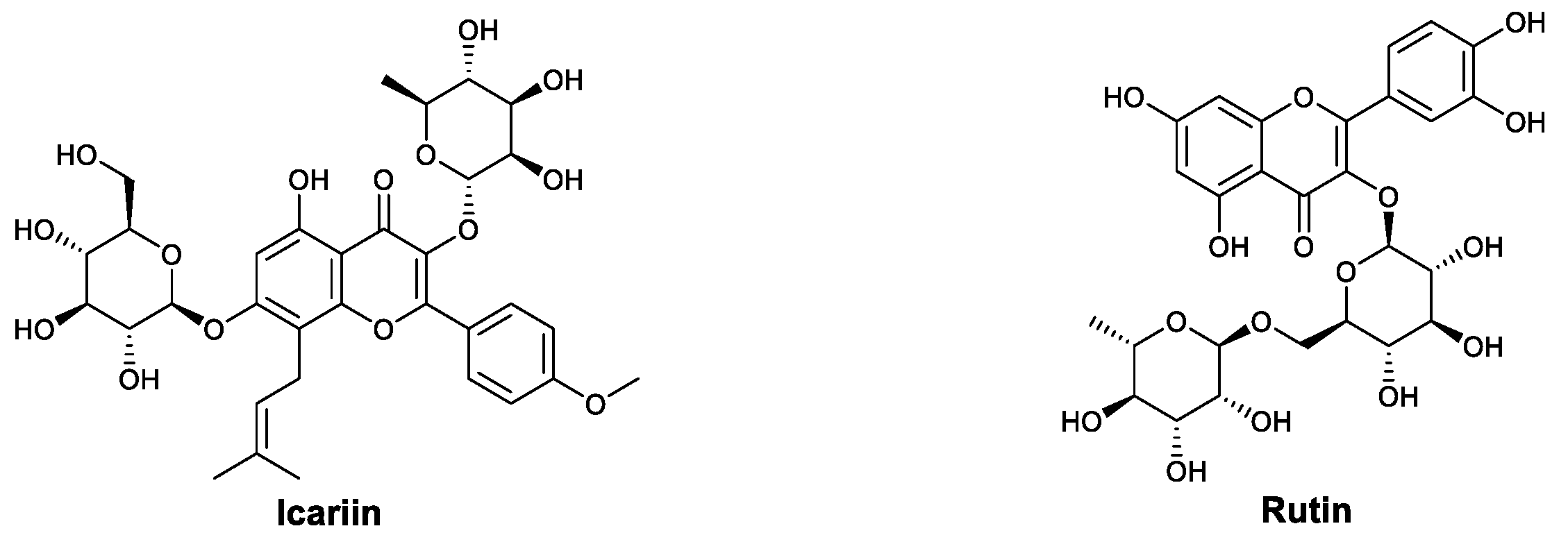
| Flavonoids | Icariin | Increased the production of antibodies, promoted the expression of TLR9 in macrophages, and downregulated inflammatory cytokines | [97,98,99,100,101,102,103] |
| Rutin | Increased the population of CD4+ lymphocytes and promoted the secretion of IFN-γ | [104] | |
| Chromanone | Gonytolide A | Activated innate immune system and increased the production of IL-8 | [105,106] |
| the Alkaloids | 2-Formyl-5-(hydroxymethyl)-1H-pyrrole-1-butanoic acid; 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoic acid | Activated macrophages and increased TNF-α, IL-12, and NO | [107] |
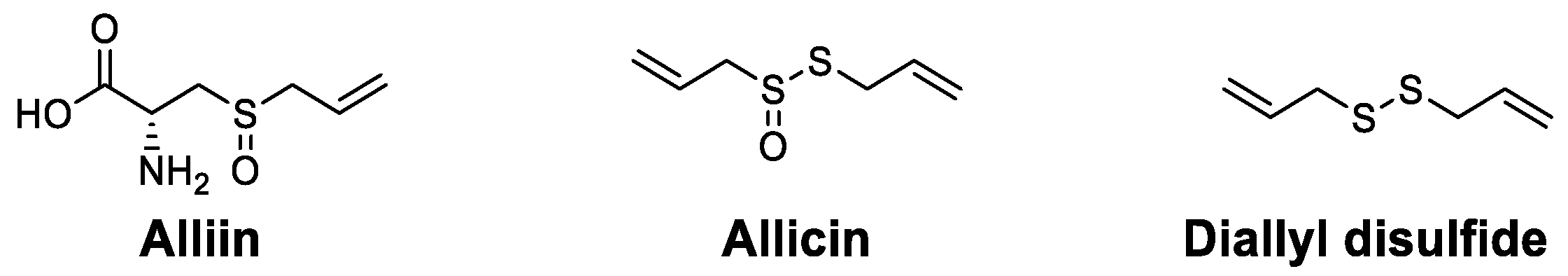
| Organosulfur compounds | Alliin | Promoted the proliferation of mononuclear cells and increased IL-1β | [108] |
| Allicin | Increased proinflammatory cytokines and increased the populations of macrophages, DCs and CD4+ T cells | [109] | |
| Diallyl disulfide | Increased WBCs and antibodies and led to more effector B cells | [110] |
| Plant | Polysaccharide | Molecular Weight | Constituents | Reference |
|---|---|---|---|---|
| Phaseolus aureus | Verbascose | 0.83 kDa | Glu, Gal | [130] |
| Acemannan | 1.66 kDa | Man, Gal | [131,132,133,134,135,136] | |
| Aloe | MAP | 8 kDa | Man, Gal, Glu | [137] |
| Aloeride | 4000–7000 kDa | Glu (37.2%), Gal (23.9%), Man (19.5%), Ara (10.3%) | [138] | |
| Polygonatum sibiricum | F1 | 103 kDa | Man, Glu, Gal, Ara | [139] |
| F2 | 628 kDa | [139] | ||
| PSP | - | Major: Gal, Rha Minor: Man, Glu, Xyl | [140] | |
| Astragalus membranaceus (Fisch.) Bge. | - | 1334 kDa | Rha, Ara, Glc, Gal, Gal (0.03:1.00:0.27:0.36:0.30) | [141] |
| - | 5.6 kDa | Glc, Gal, Ara, Xyl, Gal (10.0: 1.3: 1.7: 1.0: 0.9) | [142] | |
| Schisandra chinensis (Turcz.) Baill | SCPP11 | 3.4 kDa | Man, Glu, Gal (1:11.38:3.55) | [143,144] |
| Crude | - | Glu (38.0%), Gal (36.7%), galacturonic acid (12.0%), Ara (7.3%), Rha (4.0%), Man (1.2%), glucuronic acid (0.6%). | [145] | |
| Panax quinquefolium L. | CVT-E002 | - | Poly-furanosyl-pyranosyl-saccharides | [146,147] |
| GL-4IIb2 | 11 kDa | 15 different monosaccharides | [148,149,150] | |
| Bupleurum smithii var. parvifolium | Crude | - | Major: Ara:Gal:Glu:Rha = 6.35:3.15:1.47:1 Minor: Man, Xyl | [151,152] |
| D3-S1 | 2000 kDa | Major: Ara:Gal = 2.6:1.0 Minor: Rha, Glu, Xyl, Man | [153] | |
| Prunella vulgaris L | PV2IV | - | Ara, Xyl, Man, Gal, Glu | [154,155] |
| P1 | - | Ara (28.37%), Xyl (54.67%), Man (5.61%), Glu (5.46%), Gal (5.89) | [156] | |
| Anemarrhena asphodeloides Bunge | AS-1 | - | Man, Gal, Ara, Glu | [157] |
| AAP70-1 | 2.72 kDa | Glu, Fru | [158] | |
| Morus alba | PMA | - | - | [159] |
| Crude | - | Glu:Man:Ara:Gal:Xyl:Rha:ribose = 250:66:6:3.25:2.5:1.25:1 | [160] | |
| Undaria pinnatifida | Fucoidan | <10 kDa | Sugar (72.16% ± 0.31%), uronic acid (1.42% ± 0.03%), amino acids (7.04% ± 0.47%), sulfate (16.62% ± 1.31%) | [161] |
| Ganoderma lucidum | PS-G | - | Major: Glu, Man Minor: Fuc, N-acetylglucosamine, Xyl, Rha | [119] |
| Platycladus orientalis (L.) Franco | POP1 | 8.1 kDa | Rha (5.74%), Ara (12.58%), Man (10.97%), Glu (64.96%), Gal (6.55%) | [162] |
| Antrodia cinnamomea | ACP | 70 kDa | - | [163] |
| Phellinus sp. | SHIP-1 | - | Man:Glu:Gal:Ara:l-fucose = 1.92:1.00:2.37:0.44:1.13 | [164] |
| SHIP-2 | - | Glu:Gal:l-fucose = 1.0:0.61:0.83 | [164] | |
| Hordeum vulgare L. | BP-1 | 67 kDa | - | [165] |
| C. cornucopioides. | CCP | 1970 kDa | Man (48.73%), Gal (17.37%), Glu (15.97%), Xyl (17.93%) | [166,167] |
| Cordyceps gunnii mycelia | PPS | - | Man, Glu, Gal | [168,169] |
| Oyster C. hongkongensis | C30–60% | - | Glu (96.76%), Man (2.91%), Ara (0.24%), Ribose (0.04%), Gal (0.04%), Xyl (0.01%) | [170] |
| Arca inflata | JNY2PW | 52500 kDa | - | [171] |
| Hericium erinaceus | HEP | - | Man (2.5%), glucuronic acid (1.1%), Glu (60.9%), Gal (28.0%), Fru (7.5%) | [172] |
| Grifola Frondosa | GFP | 155 kDa | Rha:Xyl:Man:Glu = 1.00:1.04:1.11:6.21 | [173,174] |
| Longan (Dimocarpus longan Lour.) | LP | 377 kDa | Gal (40.88%), Ara (38.26%), Glu (9.00%), Rha (5.49%), Xyl (1.60%), ribose (1.05%), Fuc (0.91%) | [175,176] |
| Sesbania cannabina | Galactomannan | 216 kDa | Gal:Man = 1.6:1 | [176,177] |
| Medicago sativa L. | APS | - | Fuc, Ara, Gal, Glu, Xyl, Man, galacturonic acid, glucuronic acid | [178,179,180] |
| Biophytum petersianum Klotzsch | BP100 III.1 | 24 kDa | Ara:Rha:Fuc:Xyl:Man:Gal:GalA = 7.9:22.6:1.1:5.0:2.0:20.0:38.5 | [180] |
| BP1002 | 64 kDa | Rha (9.8%), Gal (10.5%), Ara (15.6%), Xyl (10.8%), galacturonic acid (45.8%) | [181] | |
| BPII | - | Major: GalA (65.1%) Minor: Gal, Rha, Xyl, Ara, Fuc, Glc, Man, GlcA | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhong, J.; Xiong, Y.; Song, X.; Li, C.; He, Z. Development of Broad-Spectrum Antiviral Agents—Inspiration from Immunomodulatory Natural Products. Viruses 2021, 13, 1257. https://doi.org/10.3390/v13071257
Zhang M, Zhong J, Xiong Y, Song X, Li C, He Z. Development of Broad-Spectrum Antiviral Agents—Inspiration from Immunomodulatory Natural Products. Viruses. 2021; 13(7):1257. https://doi.org/10.3390/v13071257
Chicago/Turabian StyleZhang, Mengxun, Jiaqing Zhong, Yongai Xiong, Xun Song, Chenyang Li, and Zhendan He. 2021. "Development of Broad-Spectrum Antiviral Agents—Inspiration from Immunomodulatory Natural Products" Viruses 13, no. 7: 1257. https://doi.org/10.3390/v13071257
APA StyleZhang, M., Zhong, J., Xiong, Y., Song, X., Li, C., & He, Z. (2021). Development of Broad-Spectrum Antiviral Agents—Inspiration from Immunomodulatory Natural Products. Viruses, 13(7), 1257. https://doi.org/10.3390/v13071257






