Elicitation of Neutralizing Antibody Responses to HIV-1 Immunization with Nanoparticle Vaccine Platforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Antigen Expression and Purification
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Mouse Immunizations
2.5. Guinea Pig Immunizations
2.6. TZM-bl Neutralization Assays
2.7. Statistical Analysis
3. Results
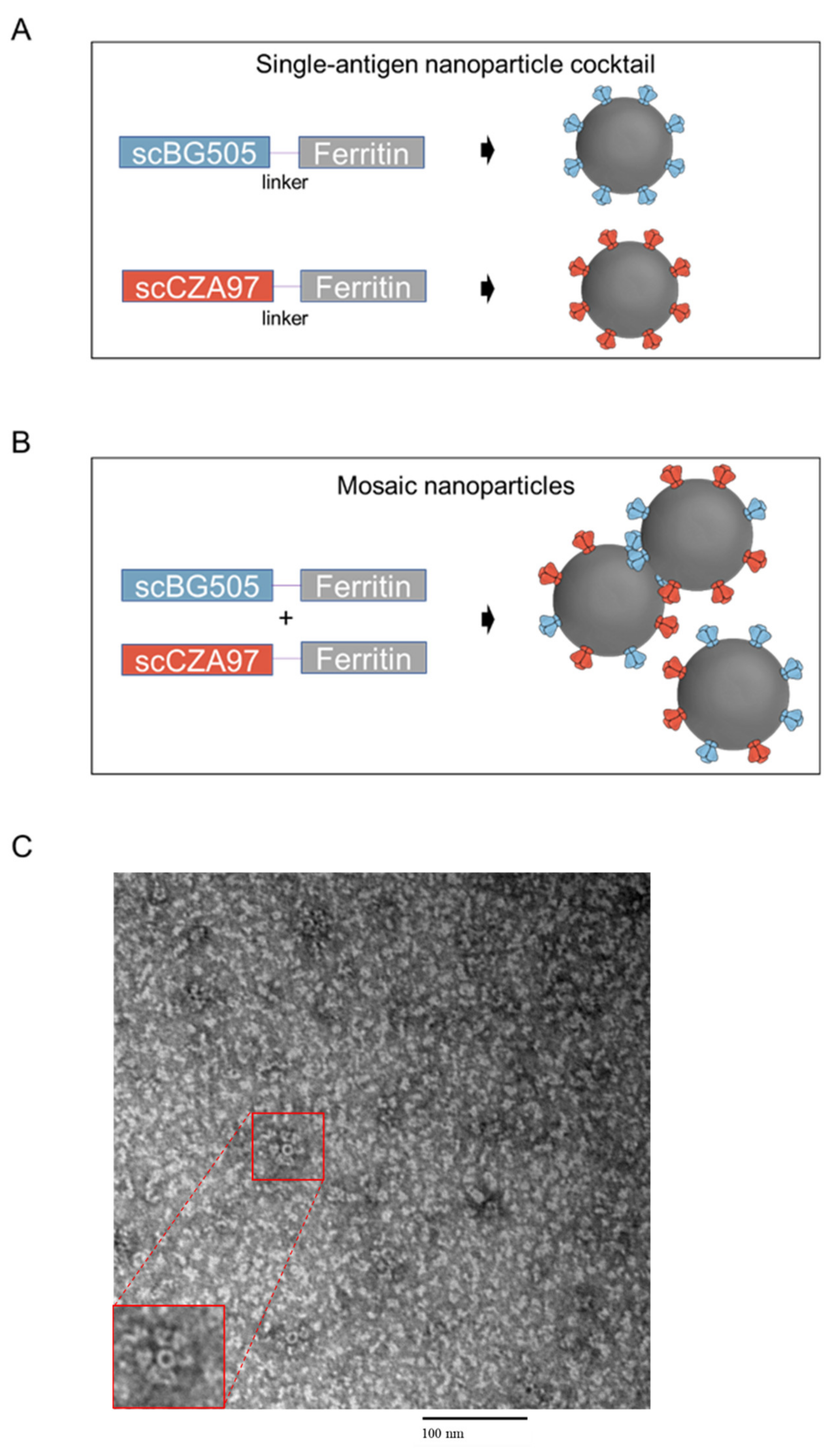
3.1. Design and Characterization of HIV-1 Nanoparticle Immunogens
3.2. Single—Antigen and Mosaic HIV-1 Nanoparticle Immunogens Elicit Comparable Responses in Mice
3.3. HIV-1 Neutralization by Vaccine—Elicited Antibody Responses in Mice
3.4. Serum from Guinea Pigs Immunized with Cocktails of Nanoparticles and Cotransfected Nanoparticles Recognize Heterologous gp140 Envs
3.5. Single—Antigen and Mosaic HIV-1 Nanoparticle Immunogens ELICIT Autologous and Heterologous Virus Neutralizing Antibodies
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, S. Adaptation in the env Gene of HIV-1 and Evolutionary Theories of Disease Progression. Mol. Biol. Evol. 2003, 20, 1318–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, J.M.; Geller, R.; Garijo, R.; López-Aldeguer, J.; Sanjuán, R. Extremely High Mutation Rate of HIV-1 In Vivo. PLoS Biol. 2015, 13, e1002251. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, R.; Durell, S.; Viard, M. HIV entry and envelope glycoprotein-mediated fusion. J. Biol. Chem. 2012, 287, 40841–40849. [Google Scholar] [CrossRef] [Green Version]
- Escolano, A.; Steichen, J.M.; Dosenovic, P.; Kulp, D.W.; Golijanin, J.; Sok, D.; Freund, N.T.; Gitlin, A.D.; Oliveira, T.; Araki, T.; et al. Sequential Immunization Elicits Broadly Neutralizing anti-HIV-1 Antibodies in Ig Knock-in Mice. Cell 2016, 166, 1445–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klasse, P.J.; Labranche, C.C.; Ketas, T.J.; Ozorowski, G.; Cupo, A.; Pugach, P.; Ringe, R.P.; Golabek, M.; van Gils, M.J.; Guttman, M.; et al. Sequential and Simultaneous Immunization of Rabbits with HIV-1 Envelope Glycoprotein SOSIP.664 Trimers from Clades A, B and C. PLoS Pathog. 2016, 12, e1005864. [Google Scholar] [CrossRef]
- De la Peña, A.T.; de Taeye, S.W.; Sliepen, K.; LaBranche, C.C.; Burger, J.A.; Schermer, E.E.; Montefiori, D.C.; Moore, J.P.; Klasse, P.J.; Sanders, R.W. Immunogenicity in Rabbits of HIV-1 SOSIP Trimers from Clades A, B, and C, Given Individually, Sequentially, or in Combination. J. Virol. 2018, 92, e01957-17. [Google Scholar]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, K.; Ozorowski, G.; Burger, J.A.; van Montfort, T.; Stunnenberg, M.; LaBranche, C.; Montefiori, D.C.; Moore, J.P.; Ward, A.B.; Sanders, R.W. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 2015, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.T.; Cottrell, C.A.; Antanasijevic, A.; Carnathan, D.G.; Cossette, B.J.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; Fischinger, S.; et al. Targeting HIV Env immunogens to B cell follicles in nonhuman primates through immune complex or protein nanoparticle formulations. Npj Vaccines 2020, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Zinkernagel, R.M. Neutralizing Antiviral B Cell Responses. Annu. Rev. Immunol. 1997, 15, 235–270. [Google Scholar] [CrossRef] [PubMed]
- He, L.; de Val, N.; Morris, C.D.; Vora, N.; Thinnes, T.C.; Kong, L.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016, 7, 12041. [Google Scholar] [CrossRef]
- Li, C.Q.; Soistman, E.; Carter, D.C. Ferritin nanoparticle technology… A new platform for antigen presentation and vaccine development. Ind. Biotechnol. 2006, 2, 143–147. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; Mctamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef] [PubMed]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. CSBJ 2016, 14, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Cui, K.; Wang, H.; Liu, F.; Huang, K.; Duan, Z.; Wang, F.; Shi, D.; Liu, Q.J. A milk-based self-assemble rotavirus VP6–ferritin nanoparticle vaccine elicited protection against the viral infection. Nanobiotechnology 2019, 17, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Bu, W.; Joyce, M.G.; Meng, G.; Whittle, J.R.R.; Baxa, U.; Yamamoto, T.; Narpala, S.; Todd, J.P.; Rao, S.S.; et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015, 162, 1090–1100. [Google Scholar] [CrossRef] [Green Version]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef]
- Cohen, A.A.; Gnanapragasam, P.N.P.; Lee, Y.E.; Hoffman, P.R.; Ou, S.; Kakutani, L.M.; Keeffe, J.R.; Wu, H.J.; Howarth, M.; West, A.P.; et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 2021, 371, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Z.Y.; Li, Y.; Hogerkorp, C.M.; Schief, W.R.; Seaman, M.S.; Zhou, T.; Schmidt, S.D.; Wu, L.; Xu, L.; et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010, 329, 856–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzotti-Kelsoe, M.; Bailer, R.T.; Turk, E.; Lin, C.L.; Bilska, M.; Greene, K.M.; Gao, H.; Todd, C.A.; Ozaki, D.A.; Seaman, M.S.; et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, I.S.; Joyce, M.G.; Yang, Y.; Sastry, M.; Zhang, B.; Baxa, U.; Chen, R.E.; Druz, A.; Lees, C.R.; Narpala, S.; et al. Single-Chain Soluble BG505.SOSIP gp140 Trimers as Structural and Antigenic Mimics of Mature Closed HIV-1 Env. J. Virol. 2015, 89, 5318–5329. [Google Scholar] [CrossRef] [Green Version]
- Korber, B.T.; Foley, B.T.; Kuiken, C.L.; Pillai, S.K.; Sodroski, J.G. Numbering Positions in HIV Numbering Positions in HIV Relative to HXB2CG. Hum. Retrovir. AIDS 1998, 3, 102–111. [Google Scholar]
- Sanders, R.W.R.R.W.; Derking, R.; Cupo, A.; Julien, J.-P.P.; Yasmeen, A.; de Val, N.; Kim, H.J.; Blattner, C.; de la Peña, A.T.; Korzun, J.; et al. A Next-Generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, Expresses Multiple Epitopes for Broadly Neutralizing but Not Non-Neutralizing Antibodies. PLoS Pathog. 2013, 9, e1003618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.K.; Crampton, J.C.; Cupo, A.; Ketas, T.; van Gils, M.J.; Sliepen, K.; de Taeye, S.W.; Sok, D.; Ozorowski, G.; Deresa, I.; et al. Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity. J. Virol. 2015, 89, 10383–10398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Acharya, P.; Kong, R.; Cheng, C.; Chuang, G.Y.; Liu, K.; Louder, M.K.; O’Dell, S.; Rawi, R.; Sastry, M.; et al. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 2018, 24, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Bricault, C.A.; Yusim, K.; Seaman, M.S.; Yoon, H.; Theiler, J.; Giorgi, E.E.; Wagh, K.; Theiler, M.; Hraber, P.; Macke, J.P.; et al. HIV-1 Neutralizing Antibody Signatures and Application to Epitope-Targeted Vaccine Design. Cell Host Microbe. Cell Host Microbe 2019, 25, 59–72.e8. [Google Scholar] [CrossRef] [Green Version]
- Pauthner, M.; Havenar-Daughton, C.; Sok, D.; Nkolola, J.P.; Bastidas, R.; Boopathy, A.V.; Carnathan, D.G.; Chandrashekar, A.; Cirelli, K.M.; Cottrell, C.A.; et al. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 2017, 46, 1073–1088.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murji, A.A.; Qin, J.S.; Hermanus, T.; Morris, L.; Georgiev, I.S. Elicitation of Neutralizing Antibody Responses to HIV-1 Immunization with Nanoparticle Vaccine Platforms. Viruses 2021, 13, 1296. https://doi.org/10.3390/v13071296
Murji AA, Qin JS, Hermanus T, Morris L, Georgiev IS. Elicitation of Neutralizing Antibody Responses to HIV-1 Immunization with Nanoparticle Vaccine Platforms. Viruses. 2021; 13(7):1296. https://doi.org/10.3390/v13071296
Chicago/Turabian StyleMurji, Amyn A., Juliana S. Qin, Tandile Hermanus, Lynn Morris, and Ivelin S. Georgiev. 2021. "Elicitation of Neutralizing Antibody Responses to HIV-1 Immunization with Nanoparticle Vaccine Platforms" Viruses 13, no. 7: 1296. https://doi.org/10.3390/v13071296
APA StyleMurji, A. A., Qin, J. S., Hermanus, T., Morris, L., & Georgiev, I. S. (2021). Elicitation of Neutralizing Antibody Responses to HIV-1 Immunization with Nanoparticle Vaccine Platforms. Viruses, 13(7), 1296. https://doi.org/10.3390/v13071296





