Screening for Hepatocellular Carcinoma in Patients with Hepatitis B
Abstract
:1. Introduction
2. Epidemiology of Liver Cancer and HBV
3. Screening for Hepatocellular Carcinoma
3.1. Rationale for Screening
3.2. Current Society Guidelines for HCC Screening
3.2.1. Asian Pacific Association for the Study of the Liver (APASL)
3.2.2. American Association for the Study of Liver Diseases (AASLD)
3.2.3. European Association for the Study of the Liver (EASL)
3.2.4. Canadian Association for the Study of the Liver (CASL)
3.2.5. Guideline Based Approach to Co-Infection
3.3. Cost Effectiveness of Screening Strategies for HCC
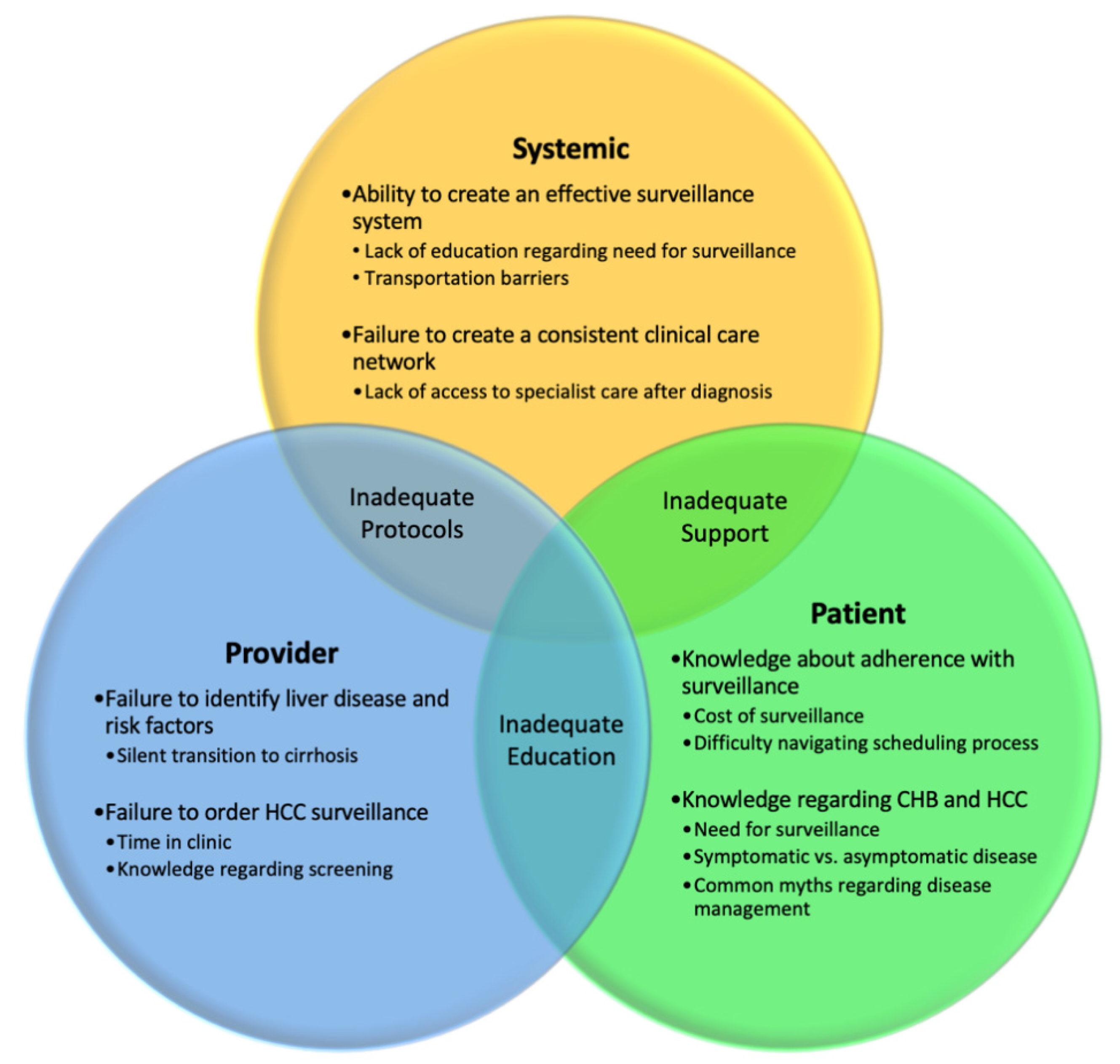
4. Challenges with Screening Adherence
Methods to Improve Adherence
5. Risk Stratification Systems for HCC Risk in Patients with HBV
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Clark, P.J.; Petrick, J.L.; McGlynn, K.A.; Bray, F. Projections of Primary Liver Cancer to 2030 in 30 Countries Worldwide. Hepatology 2018, 67, 600–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, S.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma: Consider the Population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Gill, U.S.; Kennedy, P.T.F. Prioritisation and the Initiation of HCC Surveillance in CHB Patients: Lessons to Learn from the COVID-19 Crisis. Gut 2020, 69, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.-I.; Khan, S.-A.; Toledano, M.-B.; Waked, I.; Taylor-Robinson, S.-D. Hepatocellular Carcinoma: Epidemiology, Risk Factors and Pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, E.; Macera, M.; Russo, A.; Coppola, N.; Sagnelli, C. Epidemiological and Etiological Variations in Hepatocellular Carcinoma. Infection 2020, 48, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Wanich, N.; Vilaichone, R.K.; Chotivitayatarakorn, P.; Siramolpiwat, S. High Prevalence of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Infection in Thailand. Asian Pac. J. Cancer Prev. 2016, 17, 2857–2860. [Google Scholar]
- Lemoine, M.; Nayagam, S.; Thursz, M. Viral Hepatitis in Resource-Limited Countries and Access to Antiviral Therapies: Current and Future Challenges. Future Virol. 2013, 8, 371–380. [Google Scholar] [CrossRef] [Green Version]
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-H.; Chen, C.-J.; Lai, M.-S.; Hsu, H.-M.; Wu, T.-C.; Kong, M.-S.; Liang, D.-C.; Shau, W.-Y.; Chen, D.-S. Universal Hepatitis B Vaccination in Taiwan and the Incidence of Hepatocellular Carcinoma in Children. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.E.; Plitt, S.S.; Osiowy, C.; Surynicz, K.; Kouadjo, E.; Preiksaitis, J.; Lee, B. Factors Associated with Vaccine Failure and Vertical Transmission of Hepatitis B among a Cohort of Canadian Mothers and Infants. J. Viral Hepat. 2011, 18, 468–473. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, Y.; Holmberg, S.D.; Moorman, A.C.; Spradling, P.R.; Teshale, E.H.; Boscarino, J.A.; Daida, Y.G.; Schmidt, M.A.; Li, J.; et al. Trends in Diagnosed Chronic Hepatitis B in a US Health System Population, 2006–2015. Open Forum Infect. Dis. 2019, 6, ofz286. [Google Scholar] [CrossRef]
- Lim, J.K.; Nguyen, M.H.; Kim, W.R.; Gish, R.; Perumalswami, P.; Jacobson, I.M. Prevalence of Chronic Hepatitis B Virus Infection in the United States. Am. J. Gastroenterol. 2020, 115, 1429–1438. [Google Scholar] [CrossRef]
- Yang, J.D.; Mohammed, H.A.; Harmsen, W.S.; Enders, F.; Gores, G.J.; Roberts, L.R. Recent Trends in the Epidemiology of Hepatocellular Carcinoma in Olmsted County, Minnesota: A US Population-Based Study. J. Clin. Gastroenterol. 2017, 51, 742–748. [Google Scholar] [CrossRef]
- Herman, C. What Makes a Screening Exam “Good”? Virtual Mentor VM 2006, 8, 34–37. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Gandhi, S.; Khubchandani, S.; Iyer, R. Quality of Life and Hepatocellular Carcinoma. J. Gastrointest. Oncol. 2014, 5, 296–317. [Google Scholar] [CrossRef]
- Coffin, C.S.; Fung, S.K.; Alvarez, F.; Cooper, C.L.; Doucette, K.E.; Fournier, C.; Kelly, E.; Ko, H.H.; Ma, M.M.; Martin, S.R.; et al. Management of Hepatitis B Virus Infection: 2018 Guidelines from the Canadian Association for the Study of Liver Disease and Association of Medical Microbiology and Infectious Disease Canada. Can. Liver J. 2018, 1, 156–217. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef] [Green Version]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma: A 2017 Update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhang, B.; Xu, Y.; Wang, W.; Shen, Y.; Zhang, A.; Xu, Z. Prospective Study of Early Detection for Primary Liver Cancer. J. Cancer Res. Clin. Oncol. 1997, 123, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-H.; Yang, B.-H.; Tang, Z.-Y. Randomized Controlled Trial of Screening for Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kansagara, D.; Papak, J.; Pasha, A.S.; O’Neil, M.; Freeman, M.; Relevo, R.; Quiñones, A.; Motu’apuaka, M.; Jou, J.H. Screening for Hepatocellular Carcinoma in Chronic Liver Disease: A Systematic Review. Ann. Intern. Med. 2014, 161, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-G.; Parkin, D.M.; Chen, Q.-G.; Lu, J.-H.; Shen, Q.-J.; Zhang, B.-C.; Zhu, Y.-R. Screening for Liver Cancer: Results of a Randomised Controlled Trial in Qidong, China. J. Med. Screen. 2003, 10, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-Y.; Ren, Z.-G.; Zhou, J.; Fan, J.; Gao, Q. 2019 Chinese Clinical Guidelines for the Management of Hepatocellular Carcinoma: Updates and Insights. Hepatobiliary Surg. Nutr. 2020, 9, 452–463. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-Analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Thein, H.-H.; Yi, Q.; Dore, G.J.; Krahn, M.D. Natural History of Hepatitis C Virus Infection in HIV-Infected Individuals and the Impact of HIV in the Era of Highly Active Antiretroviral Therapy: A Meta-Analysis. AIDS Lond. Engl. 2008, 22, 1979–1991. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.G.; Tiro, J.; Li, X.; Adams-Huet, B.; Chubak, J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-Based Integrated Health Care Delivery System. J. Clin. Gastroenterol. 2017, 51, 650–655. [Google Scholar] [CrossRef]
- Choi, D.T.; Kum, H.-C.; Park, S.; Ohsfeldt, R.L.; Shen, Y.; Parikh, N.D.; Singal, A.G. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 976–987. [Google Scholar] [CrossRef]
- Tong, M.J.; Rosinski, A.A.; Huynh, C.T.; Raman, S.S.; Lu, D.S.K. Long-Term Survival after Surveillance and Treatment in Patients with Chronic Viral Hepatitis and Hepatocellular Carcinoma. Hepatol. Commun. 2017, 1, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Moon, A.M.; Weiss, N.S.; Beste, L.A.; Su, F.; Ho, S.B.; Jin, G.-Y.; Lowy, E.; Berry, K.; Ioannou, G.N. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology 2018, 155, 1128–1139. [Google Scholar] [CrossRef]
- PDQ. Screening and Prevention Editorial Board Liver (Hepatocellular) Cancer Screening (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goutté, N.; Sogni, P.; Bendersky, N.; Barbare, J.C.; Falissard, B.; Farges, O. Geographical Variations in Incidence, Management and Survival of Hepatocellular Carcinoma in a Western Country. J. Hepatol. 2017, 66, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Taketa, K. Alpha-Fetoprotein: Reevaluation in Hepatology. Hepatology 1990, 12, 1420–1432. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and Significance of Alpha-fetoprotein in Hepatocellular Carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [Green Version]
- Takikawa, Y.; Suzuki, K. Is AFP a New Reliable Marker of Liver Regeneration in Acute Hepatic Failure? J. Gastroenterol. 2002, 37, 681–682. [Google Scholar] [CrossRef]
- Patil, M.; Sheth, K.A.; Adarsh, C.K. Elevated Alpha Fetoprotein, No Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2013, 3, 162–164. [Google Scholar] [CrossRef] [Green Version]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-Analysis. Gastroenterology 2018, 154, 1706–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenette, C.T.; Isaacson, A.J.; Bargellini, I.; Saab, S.; Singal, A.G. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases Management of Hepatocellular Carcinoma: An Update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Alfaiate, D.; Clément, S.; Gomes, D.; Goossens, N.; Negro, F. Chronic Hepatitis D and Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. J. Hepatol. 2020, 73, 533–539. [Google Scholar] [CrossRef]
- Farci, P.; Niro, G.A.; Zamboni, F.; Diaz, G. Hepatitis D Virus and Hepatocellular Carcinoma. Viruses 2021, 13, 830. [Google Scholar] [CrossRef]
- Cho, L.Y.; Yang, J.J.; Ko, K.-P.; Park, B.; Shin, A.; Lim, M.K.; Oh, J.-K.; Park, S.; Kim, Y.J.; Shin, H.-R.; et al. Coinfection of Hepatitis B and C Viruses and Risk of Hepatocellular Carcinoma: Systematic Review and Meta-Analysis. Int. J. Cancer 2011, 128, 176–184. [Google Scholar] [CrossRef]
- Kim, H.N. Chronic Hepatitis B and HIV Coinfection: A Continuing Challenge in the Era of Antiretroviral Therapy. Curr. Hepatol. Rep. 2020, 19, 345–353. [Google Scholar] [CrossRef]
- Merchante, N.; Rodríguez-Fernández, M.; Pineda, J.A. Screening for Hepatocellular Carcinoma in HIV-Infected Patients: Current Evidence and Controversies. Curr. HIV/AIDS Rep. 2020, 17, 6–17. [Google Scholar] [CrossRef]
- Nguyen, A.L.T.; Nguyen, H.T.T.; Yee, K.C.; Palmer, A.J.; Blizzard, C.L.; de Graaff, B. A Systematic Review and Narrative Synthesis of Health Economic Evaluations of Hepatocellular Carcinoma Screening Strategies. Value Health 2021, 24, 733–743. [Google Scholar] [CrossRef]
- Sangmala, P.; Chaikledkaew, U.; Tanwandee, T.; Pongchareonsuk, P. Economic Evaluation and Budget Impact Analysis of the Surveillance Program for Hepatocellular Carcinoma in Thai Chronic Hepatitis B Patients. Asian Pac. J. Cancer Prev. 2014, 15, 8993–9004. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Lee, T.P.; Yap, I.; Lun, K.C. Analysis of Cost-Effectiveness of Different Strategies for Hepatocellular Carcinoma Screening in Hepatitis B Virus Carriers. J. Gastroenterol. Hepatol. 1992, 7, 463–468. [Google Scholar] [CrossRef]
- Lam, C. Screening for Hepatocellular Carcinoma (HCC): Is It Cost-Effective? Hong Kong Pract. 2000, 22, 546–551. [Google Scholar]
- Robotin, M.C.; Kansil, M.; Howard, K.; George, J.; Tipper, S.; Dore, G.J.; Levy, M.; Penman, A.G. Antiviral Therapy for Hepatitis B-Related Liver Cancer Prevention Is More Cost-Effective than Cancer Screening. J. Hepatol. 2009, 50, 990–998. [Google Scholar] [CrossRef]
- Chang, Y.; Lairson, D.R.; Chan, W.; Lu, S.-N.; Aoki, N. Cost-Effectiveness of Screening for Hepatocellular Carcinoma among Subjects at Different Levels of Risk. J. Eval. Clin. Pract. 2011, 17, 261–267. [Google Scholar] [CrossRef]
- Parikh, N.D.; Singal, A.G.; Hutton, D.W.; Tapper, E.B. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am. J. Gastroenterol. 2020, 115, 1642–1649. [Google Scholar] [CrossRef]
- Wolf, E.; Rich, N.E.; Marrero, J.A.; Parikh, N.D.; Singal, A.G. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology 2021, 73, 713–725. [Google Scholar] [CrossRef]
- Singal, A.G.; Yopp, A.C.; Gupta, S.; Skinner, C.S.; Halm, E.A.; Okolo, E.; Nehra, M.; Lee, W.M.; Marrero, J.A.; Tiro, J.A. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev. Res. Phila. Pa 2012, 5, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, P.; Liu, P.; Immergluck, J.; Olivares, J.; Arroyo, A.; Rich, N.E.; Parikh, N.D.; Yopp, A.C.; Singal, A.G. Hepatocellular Carcinoma Screening Process Failures in Patients with Cirrhosis. Hepatol. Commun. 2021. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Gohel, T.D. Perspectives of Physicians Regarding Screening Patients at Risk of Hepatocellular Carcinoma. Gastroenterol. Rep. 2016, 4, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.G.; Li, X.; Tiro, J.; Kandunoori, P.; Adams-Huet, B.; Nehra, M.S.; Yopp, A. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. Am. J. Med. 2015, 128, 90.e1–90.e7. [Google Scholar] [CrossRef]
- Farvardin, S.; Patel, J.; Khambaty, M.; Yerokun, O.A.; Mok, H.; Tiro, J.A.; Yopp, A.C.; Parikh, N.D.; Marrero, J.A.; Singal, A.G. Patient-Reported Barriers Are Associated with Lower Hepatocellular Carcinoma Surveillance Rates in Patients with Cirrhosis. Hepatology 2017, 65, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.S.; Taddei, T.H.; Serper, M.; Mehta, R.; Dieperink, E.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Pedrosa, M.; et al. Identifying Barriers to Hepatocellular Carcinoma Surveillance in a National Sample of Patients with Cirrhosis. Hepatology 2017, 65, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Simmons, O.L.; Feng, Y.; Parikh, N.D.; Singal, A.G. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clin. Gastroenterol. Hepatol. 2019, 17, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Dalton-Fitzgerald, E.; Tiro, J.; Kandunoori, P.; Halm, E.A.; Yopp, A.; Singal, A.G. Practice Patterns and Attitudes of Primary Care Providers and Barriers to Surveillance of Hepatocellular Carcinoma in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.G.; Tiro, J.A.; Murphy, C.C.; Marrero, J.A.; McCallister, K.; Fullington, H.; Mejias, C.; Waljee, A.K.; Pechero Bishop, W.; Santini, N.O.; et al. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients With Cirrhosis: A Randomized Clinical Trial. Hepatology 2019, 69, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Nazareth, S.; Leembruggen, N.; Tuma, R.; Chen, S.-L.; Rao, S.; Kontorinis, N.; Cheng, W. Nurse-Led Hepatocellular Carcinoma Surveillance Clinic Provides an Effective Method of Monitoring Patients with Cirrhosis. Int. J. Nurs. Pract. 2016, 22 (Suppl. 2), S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, N.A.; Rodgers, A.; Altus, R.; McCormick, R.; Wundke, R.; Wigg, A.J. Optimisation of Hepatocellular Carcinoma Surveillance in Patients with Viral Hepatitis: A Quality Improvement Study. Intern. Med. J. 2013, 43, 772–777. [Google Scholar] [CrossRef]
- Farrell, C.; Halpen, A.; Cross, T.J.S.; Richardson, P.D.; Johnson, P.; Joekes, E.C. Ultrasound Surveillance for Hepatocellular Carcinoma: Service Evaluation of a Radiology-Led Recall System in a Tertiary-Referral Centre for Liver Diseases in the UK. Clin. Radiol. 2017, 72, 338.e11–338.e17. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, N.; Sun, F.; Zhou, J.; Wu, X.; Sun, Y.; Wang, B.; Zhan, S.; Kong, Y.; Jia, J.; et al. Hepatocellular Carcinoma Prediction Models in Chronic Hepatitis B: A Systematic Review of 14 Models and External Validation. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Congly, S.E.; Brownfield, K.A. Distinguishing between Sex and Gender Is Critical for Research in Transplantation. Transplantation 2019, 104, e57. [Google Scholar] [CrossRef]
- Han, K.-H.; Ahn, S.H. How to Predict HCC Development in Patients with Chronic B Viral Liver Disease? Intervirology 2005, 48, 23–28. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Chan, S.L.; Mo, F.; Chan, T.C.; Loong, H.H.F.; Wong, G.L.H.; Lui, Y.Y.N.; Chan, A.T.C.; Sung, J.J.Y.; Yeo, W.; et al. Clinical Scoring System to Predict Hepatocellular Carcinoma in Chronic Hepatitis B Carriers. J. Clin. Oncol. 2010, 28, 1660–1665. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.L.Y.; Hui, A.Y.; Wong, M.L.; Tse, A.M.L.; Hung, L.C.T.; Wong, V.W.S.; Sung, J.J.Y. Genotype C Hepatitis B Virus Infection Is Associated with an Increased Risk of Hepatocellular Carcinoma. Gut 2004, 53, 1494–1498. [Google Scholar] [CrossRef] [Green Version]
- Abu-Amara, M.; Cerocchi, O.; Malhi, G.; Sharma, S.; Yim, C.; Shah, H.; Wong, D.K.; Janssen, H.L.A.; Feld, J.J. The Applicability of Hepatocellular Carcinoma Risk Prediction Scores in a North American Patient Population with Chronic Hepatitis B Infection. Gut 2016, 65, 1347–1358. [Google Scholar] [CrossRef]
- Yip, T.C.-F.; Hui, V.W.-K.; Tse, Y.-K.; Wong, G.L.-H. Statistical Strategies for HCC Risk Prediction Models in Patients with Chronic Hepatitis B. Hepatoma Res. 2021, 2021, 7. [Google Scholar] [CrossRef]
- Wong, G.L.-H.; Chan, H.L.-Y.; Wong, C.K.-Y.; Leung, C.; Chan, C.Y.; Ho, P.P.-L.; Chung, V.C.-Y.; Chan, Z.C.-Y.; Tse, Y.-K.; Chim, A.M.-L.; et al. Liver Stiffness-Based Optimization of Hepatocellular Carcinoma Risk Score in Patients with Chronic Hepatitis B. J. Hepatol. 2014, 60, 339–345. [Google Scholar] [CrossRef]
- Ganne-Carrié, N.; Ziol, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Castera, L.; Dhumeaux, D.; Trinchet, J.-C.; Beaugrand, M. Accuracy of Liver Stiffness Measurement for the Diagnosis of Cirrhosis in Patients with Chronic Liver Diseases. Hepatology 2006, 44, 1511–1517. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Janssen, H.L.A. Can We Use HCC Risk Scores to Individualize Surveillance in Chronic Hepatitis B Infection? J. Hepatol. 2015, 63, 722–732. [Google Scholar] [CrossRef] [Green Version]
- Yuen, M.-F.; Tanaka, Y.; Fong, D.Y.-T.; Fung, J.; Wong, D.K.-H.; Yuen, J.C.-H.; But, D.Y.-K.; Chan, A.O.-O.; Wong, B.C.-Y.; Mizokami, M.; et al. Independent Risk Factors and Predictive Score for the Development of Hepatocellular Carcinoma in Chronic Hepatitis B. J. Hepatol. 2009, 50, 80–88. [Google Scholar] [CrossRef]
- Yang, H.I.; Yuen, M.F.; Chan, H.L.Y.; Han, K.H.; Chen, P.J.; Kim, D.Y.; Ahn, S.H.; Chen, C.J.; Wong, V.W.S.; Seto, W.K. Risk Estimation for Hepatocellular Carcinoma in Chronic Hepatitis B (REACH-B): Development and Validation of a Predictive Score. Lancet Oncol. 2011, 12, 568–574. [Google Scholar] [CrossRef]
- Chen, T.M.; Chang, C.C.; Huang, P.T.; Wen, C.F.; Lin, C.C. Performance of Risk Estimation for Hepatocellular Carcinoma in Chronic Hepatitis B (REACH-B) Score in Classifying Treatment Eligibility under 2012 Asian Pacific Association for the Study of the Liver (APASL) Guideline for Chronic Hepatitis B Patients. Aliment. Pharmacol. Ther. 2013, 37, 243–251. [Google Scholar] [CrossRef]
- Magalhães-Costa, P.; Lebre, L.; Peixe, P.; Santos, S.; Chagas, C. Carcinoma Hepatocelular Em Doentes Com Infecção Crónica Pelo Vírus Da Hepatite B Sob Análogos Dos Nucleós(t)Idos de 3a Geração: Factores de Risco e o Desempenho de Um Score de Risco. GE Port. J. Gastroenterol. 2016, 23, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. AMAP Risk Score Predicts Hepatocellular Carcinoma Development in Patients with Chronic Hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Alempijevic, T.M.; Stojkovic Lalosevic, M.; Dumic, I.; Jocic, N.; Markovic, A.P.; Dragasevic, S.; Jovicic, I.; Lukic, S.; Popovic, D.; Milosavljevic, T. Diagnostic Accuracy of Platelet Count and Platelet Indices in Noninvasive Assessment of Fibrosis in Nonalcoholic Fatty Liver Disease Patients. Can. J. Gastroenterol. Hepatol. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Shiha, G.; Mikhail, N.; Soliman, R. External Validation of AMAP Risk Score in Patients with Chronic Hepatitis C Genotype 4 and Cirrhosis Who Achieved SVR Following DAAs. J. Hepatol. 2020, 74, 994–996. [Google Scholar] [CrossRef]
- Poh, Z.; Shen, L.; Yang, H.-I.; Seto, W.-K.; Wong, V.W.; Lin, C.Y.; Goh, B.-B.G.; Chang, P.-E.J.; Chan, H.L.-Y.; Yuen, M.-F.; et al. Real-World Risk Score for Hepatocellular Carcinoma (RWS-HCC): A Clinically Practical Risk Predictor for HCC in Chronic Hepatitis B. Gut 2016, 65, 887–888. [Google Scholar] [CrossRef]
- Yang, H.-I.; Sherman, M.; Su, J.; Chen, P.-J.; Liaw, Y.-F.; Iloeje, U.H.; Chen, C.-J. Nomograms for Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Virus Infection. J. Clin. Oncol. 2010, 28, 2437–2444. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Kim, B.K. Liver Stiffness-Based Model for Prediction of Hepatocellular Carcinoma in Chronic Hepatitis B Virus Infection: Comparison with Histological Fibrosis. Liver Int. 2015, 35, 1054–1062. [Google Scholar] [CrossRef]
- Kim, B.K.; Park, Y.N.; Kim, D.Y.; Park, J.Y.; Chon, C.Y.; Han, K.-H.; Ahn, S.H. Risk Assessment of Development of Hepatic Decompensation in Histologically Proven Hepatitis B Viral Cirrhosis Using Liver Stiffness Measurement. Digestion 2012, 85, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, M.; Gan, Y.; Chen, T.; Sun, Y.; Lu, J.; Wang, J.; Jin, Y.; Lu, J.; Qian, G.; et al. A Simple AGED Score for Risk Classification of Primary Liver Cancer: Development and Validation with Long-Term Prospective HBsAg-Positive Cohorts in Qidong, China. Gut 2019, 68, 948–949. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Lee, J.-H.; Kim, K.; Ahn, J.H.; Lee, J.H.; Kim, J.H.; Lee, D.H.; Yoon, J.-H.; Kang, W.; Gwak, G.-Y.; et al. A Novel Model for Predicting Hepatocellular Carcinoma Development in Patients with Chronic Hepatitis B and Normal Alanine Aminotransferase Levels. Gut Liver 2017, 11, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Papatheodoridis, G.; Dalekos, G.; Sypsa, V.; Yurdaydin, C.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; Mangia, G.; et al. PAGE-B Predicts the Risk of Developing Hepatocellular Carcinoma in Caucasians with Chronic Hepatitis B on 5-Year Antiviral Therapy. J. Hepatol. 2016, 64, 800–806. [Google Scholar] [CrossRef]
- Kirino, S.; Tamaki, N.; Kaneko, S.; Kurosaki, M.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Watakabe, K.; et al. Validation of Hepatocellular Carcinoma Risk Scores in Japanese Chronic Hepatitis B Cohort Receiving Nucleot(s)Ide Analog. J. Gastroenterol. Hepatol. Aust. 2020, 35, 1595–1601. [Google Scholar] [CrossRef]
- Yip, T.C.F.; Wong, G.L.H.; Wong, V.W.S.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.K.; Lee, H.W.; Lui, G.C.Y.; Chan, H.L.Y. Reassessing the Accuracy of PAGE-B-Related Scores to Predict Hepatocellular Carcinoma Development in Patients with Chronic Hepatitis B. J. Hepatol. 2020, 72, 847–854. [Google Scholar] [CrossRef]
- Kim, M.N.; Hwang, S.G.; Rim, K.S.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Kim, S.U. Validation of PAGE-B Model in Asian Chronic Hepatitis B Patients Receiving Entecavir or Tenofovir. Liver Int. 2017, 37, 1788–1795. [Google Scholar] [CrossRef]
- Lee, H.W.; Yoo, E.J.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H. Prediction of Development of Liver-Related Events by Transient Elastography in Hepatitis B Patients With Complete Virological Response on Antiviral Therapy. Am. J. Gastroenterol. 2014, 109, 1241–1249. [Google Scholar] [CrossRef]
- Seo, Y.S.; Jang, B.K.; Um, S.H.; Hwang, J.S.; Han, K.-H.; Kim, S.G.; Lee, K.S.; Kim, S.U.; Kim, Y.S.; Lee, J.I. Validation of Risk Prediction Models for the Development of HBV-Related HCC: A Retrospective Multi-Center 10-Year Follow-up Cohort Study. Oncotarget 2017, 8, 113213–113224. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Suh, Y.J.; Jin, Y.-J.; Heo, N.-Y.; Jang, J.W.; You, C.R.; An, H.Y.; Lee, J.-W. Prediction Model for Hepatocellular Carcinoma Risk in Treatment-Naive Chronic Hepatitis B Patients Receiving Entecavir/Tenofovir. Eur. J. Gastroenterol. Hepatol. 2019, 31, 865–872. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Yip, T.C.-F.; Ho, H.J.; Wong, V.W.-S.; Huang, Y.-T.; El-Serag, H.B.; Lee, T.-Y.; Wu, M.-S.; Lin, J.-T.; Wong, G.L.-H.; et al. Development of a Scoring System to Predict Hepatocellular Carcinoma in Asians on Antivirals for Chronic Hepatitis B. J. Hepatol. 2018, 69, 278–285. [Google Scholar] [CrossRef]
- Kim, S.U.; Seo, Y.S.; Lee, H.A.; Kim, M.N.; Kim, E.H.; Kim, H.Y.; Lee, Y.R.; Lee, H.W.; Park, J.Y.; Kim, D.Y.; et al. Validation of the CAMD Score in Patients With Chronic Hepatitis B Virus Infection Receiving Antiviral Therapy. Clin. Gastroenterol. Hepatol. 2020, 18, 693–699. [Google Scholar] [CrossRef]
- Yang, H.-I.; Yeh, M.-L.; Wong, G.L.; Peng, C.-Y.; Chen, C.-H.; Trinh, H.N.; Cheung, K.-S.; Xie, Q.; Su, T.-H.; Kozuka, R.; et al. Real-World Effectiveness From the Asia Pacific Rim Liver Consortium for HBV Risk Score for the Prediction of Hepatocellular Carcinoma in Chronic Hepatitis B Patients Treated With Oral Antiviral Therapy. J. Infect. Dis. 2020, 221, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Cho, J.-Y.; Kim, J.H.; Lee, J.I.; Kim, H.J.; Woo, M.-A.; Jung, S.-H.; Paik, Y.-H. Risk Score Model for the Development of Hepatocellular Carcinoma in Treatment-Naïve Patients Receiving Oral Antiviral Treatment for Chronic Hepatitis B. Clin. Mol. Hepatol. 2017, 23, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Güzelbulut, F.; Gökçen, P.; Can, G.; Adalı, G.; Değirmenci Saltürk, A.G.; Bahadır, Ö.; Özdil, K.; Doğanay, H.L. Validation of the HCC-RESCUE Score to Predict Hepatocellular Carcinoma Risk in Caucasian Chronic Hepatitis B Patients under Entecavir or Tenofovir Therapy. J. Viral Hepat. 2021, 28, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lee, C.-M.; Lai, H.-C.; Hu, T.-H.; Su, W.-P.; Lu, S.-N.; Lin, C.-H.; Hung, C.-H.; Wang, J.-H.; Lee, M.-H.; et al. Prediction Model of Hepatocellular Carcinoma Risk in Asian Patients with Chronic Hepatitis B Treated with Entecavir. Oncotarget 2017, 8, 92431–92441. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Axley, P.; Mudumbi, S.; Sarker, S.; Kuo, Y.-F.; Singal, A. Patients with Stage 3 Compared to Stage 4 Liver Fibrosis Have Lower Frequency of and Longer Time to Liver Disease Complications. PLoS ONE 2018, 13, e0197117. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis–2021 Update. J. Hepatol. 2021. [Google Scholar] [CrossRef]
- Udompap, P.; Kim, W.R. Development of Hepatocellular Carcinoma in Patients With Suppressed Viral Replication: Changes in Risk Over Time. Clin. Liver Dis. 2020, 15, 85–90. [Google Scholar] [CrossRef]
- Chiang, H.-H.; Lee, C.-M.; Hu, T.-H.; Hung, C.-H.; Wang, J.-H.; Lu, S.-N.; Lai, H.-C.; Su, W.-P.; Lin, C.-H.; Peng, C.-Y.; et al. A Combination of the On-Treatment FIB-4 and Alpha-Foetoprotein Predicts Clinical Outcomes in Cirrhotic Patients Receiving Entecavir. Liver Int. 2018, 38, 1997–2005. [Google Scholar] [CrossRef]
- Demir, M.; Grünewald, F.; Lang, S.; Schramm, C.; Bowe, A.; Mück, V.; Kütting, F.; Goeser, T.; Steffen, H.-M. Elevated Liver Fibrosis Index FIB-4 Is Not Reliable for HCC Risk Stratification in Predominantly Non-Asian CHB Patients. Medicine (Baltimore) 2016, 95, e4602. [Google Scholar] [CrossRef]
- Metwally, K.; Elsabaawy, M.; Abdel-Samiee, M.; Morad, W.; Ehsan, N.; Abdelsameea, E. FIB-5 versus FIB-4 Index for Assessment of Hepatic Fibrosis in Chronic Hepatitis B Affected Patients. Clin. Exp. Hepatol. 2020, 6, 335–338. [Google Scholar] [CrossRef]
| 1 | APASL 2017 | AASLD 2018 | CASL 2019 | EASL 2018 |
|---|---|---|---|---|
| Screening Strategy | ||||
| Abdominal ultrasound (US) | Recommended every 6 months | |||
| Alpha-fetoprotein (AFP) | Recommend AFP every 6 months with US | Can consider AFP every 6 months with US | AFP use not recommended | |
| Patients for Which Screening is Recommended | ||||
| Patients with cirrhosis | Yes | Child Pugh A/B | Yes | Child Pugh A/B |
| Asian men with chronic hepatitis B | >40 years old | PAGE-B ≥ 10 | ||
| Asian women with chronic hepatitis B | >50 years old | |||
| African men/women with chronic hepatitis B | >20 | >40 | >20 | |
| Family history of hepatocellular carcinoma | Yes | Yes | Yes, >40 years old | No |
| Co-infected with hepatitis delta virus (HDV) | No | Yes | No | No |
| Co-infected with human immunodeficiency virus (HIV) | No | No | >40 years old | No |
| Name 1 | Components | Strengths | Weaknesses |
|---|---|---|---|
| IPM [73] | Cirrhosis Age Chronic HCV infection AFP CHB infection Chronic hepatitis Alcohol consumption Alcohol history Sex ALT |
|
|
| CU-HCC [74] | Age Albumin Bilirubin HBV DNA Cirrhosis |
| |
| LSM-HCC [78] | Age Albumin HBV DNA LSM |
|
|
| GAG-HCC [81] | Version 1: Gender Age HBV DNA BCP mutations Cirrhosis Version 2: Gender Age HBV DNA Cirrhosis |
|
|
| REACH-B [82] | Gender Age ALT HBeAg HBV DNA |
| |
| aMAP score [85] | Age Sex Albumin Bilirubin Platelet |
| |
| RWS-HCC [88] | Sex Age Cirrhosis AFP |
|
|
| NGM1-HCC [89] | Gender Age Family history of HCC Alcohol consumption ALT HBeAg |
|
|
| NGM2-HCC [89] | Gender Age Family history of HCC Alcohol consumption ALT HBV DNA level |
|
|
| LSPS [90] |
|
| |
| AGED [92] | Age Gender HBeAg HBV DNA |
|
|
| D2AS Risk score [93] | HBV DNA Sex Age |
|
|
| Name 1 | Components | Strengths | Weaknesses |
|---|---|---|---|
| PAGE-B [94] | Age Sex Platelets |
|
|
| mREACH-B [98] | Gender Age ALT HBeAg LSM |
|
|
| AASL-HCC [100] | Age Albumin Sex Cirrhosis |
|
|
| CAMD [101] | Cirrhosis Age Sex Diabetes |
|
|
| REAL-B [103] | Sex Age Alcohol Cirrhosis Diabetes Baseline Platelet Count Baseline AFP |
|
|
| HCC-RESCUE [104] | Age Gender Cirrhosis |
|
|
| APA-B [106] | Age Platelet Count Baseline AFP |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachar, Y.; Brahmania, M.; Dhanasekaran, R.; Congly, S.E. Screening for Hepatocellular Carcinoma in Patients with Hepatitis B. Viruses 2021, 13, 1318. https://doi.org/10.3390/v13071318
Sachar Y, Brahmania M, Dhanasekaran R, Congly SE. Screening for Hepatocellular Carcinoma in Patients with Hepatitis B. Viruses. 2021; 13(7):1318. https://doi.org/10.3390/v13071318
Chicago/Turabian StyleSachar, Yashasavi, Mayur Brahmania, Renumathy Dhanasekaran, and Stephen E. Congly. 2021. "Screening for Hepatocellular Carcinoma in Patients with Hepatitis B" Viruses 13, no. 7: 1318. https://doi.org/10.3390/v13071318