Human APOBEC3 Variations and Viral Infection
Abstract
:1. Introduction
2. APOBEC3 Variations
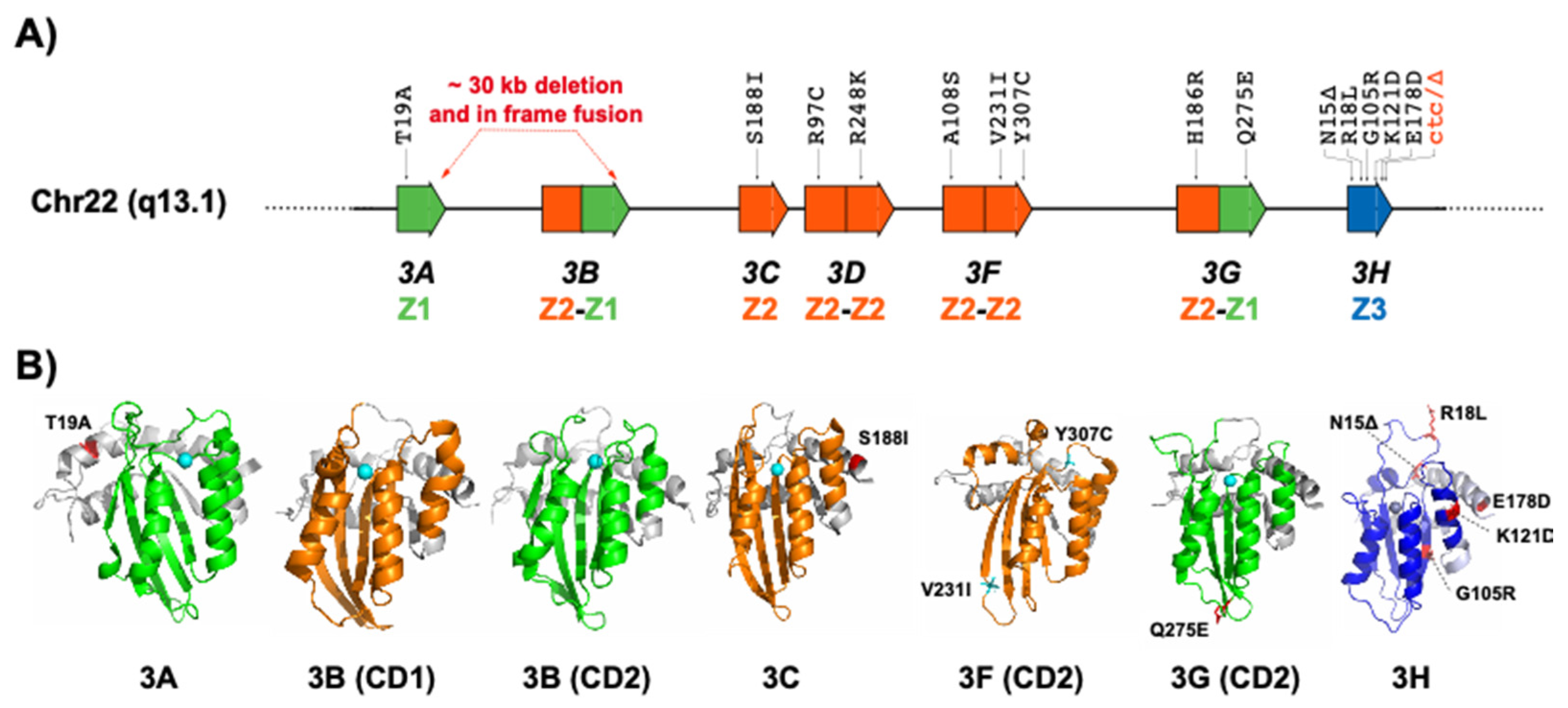
2.1. APOBEC3A and APOBEC3B
2.2. APOBEC3C
2.3. APOBEC3D
2.4. APOBEC3F
2.5. APOBEC3G
2.6. APOBEC3H
3. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uriu, K.; Kosugi, Y.; Ito, J.; Sato, K. The Battle between Retroviruses and APOBEC3 Genes: Its Past and Present. Viruses 2021, 13, 124. [Google Scholar] [CrossRef]
- Xu, W.K.; Byun, H.; Dudley, J.P. The Role of APOBECs in Viral Replication. Microorganisms 2020, 8, 1899. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Ross, S.R. APOBEC3 Proteins in Viral Immunity. J. Immunol. 2015, 195, 4565–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goila-Gaur, R.; Strebel, K. HIV-1 Vif, APOBEC, and Intrinsic Immunity. Retrovirology 2008, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.S.; Dudley, J.P. APOBECs and virus restriction. Virology 2015, 479, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.S.; Liddament, M.T. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 2004, 4, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-L.; Greene, W.C. The APOBEC3 Cytidine Deaminases: An Innate Defensive Network Opposing Exogenous Retroviruses and Endogenous Retroelements. Annu. Rev. Immunol. 2008, 26, 317–353. [Google Scholar] [CrossRef]
- Okada, A.; Iwatani, Y. APOBEC3G-Mediated G-to-A Hypermutation of the HIV-1 Genome: The Missing Link in Antiviral Molecular Mechanisms. Front. Microbiol. 2016, 7, 2027. [Google Scholar] [CrossRef] [Green Version]
- Hultquist, J.; Lengyel, J.A.; Refsland, E.W.; LaRue, R.S.; Lackey, L.; Brown, W.L.; Harris, R.S. Human and Rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H Demonstrate a Conserved Capacity to Restrict Vif-Deficient HIV-1. J. Virol. 2011, 85, 11220–11234. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, J.; Geller, R.; Garijo, R.; López-Aldeguer, J.; Sanjuán, R. Extremely High Mutation Rate of HIV-1 In Vivo. PLoS Biol. 2015, 13, e1002251. [Google Scholar] [CrossRef] [Green Version]
- Iwatani, Y.; Chan, D.S.; Wang, F.; Stewart-Maynard, K.; Sugiura, W.; Gronenborn, A.M.; Rouzina, I.; Williams, M.C.; Musier-Forsyth, K.; Levin, J.G. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 2007, 35, 7096–7108. [Google Scholar] [CrossRef]
- Holmes, R.K.; Malim, M.H.; Bishop, K.N. APOBEC-mediated viral restriction: Not simply editing? Trends Biochem. Sci. 2007, 32, 118–128. [Google Scholar] [CrossRef]
- Chen, X. Insights into the Structures and Multimeric Status of APOBEC Proteins Involved in Viral Restriction and Other Cellular Functions. Viruses 2021, 13, 497. [Google Scholar] [CrossRef]
- Navarro, F.; Bollman, B.; Chen, H.; König, R.; Yu, Q.; Chiles, K.; Landau, N.R. Complementary function of the two catalytic domains of APOBEC3G. Virology 2005, 333, 374–386. [Google Scholar] [CrossRef] [Green Version]
- Salter, J.D.; Bennett, R.P.; Smith, H.C. The APOBEC Protein Family: United by Structure, Divergent in Function. Trends Biochem. Sci. 2016, 41, 578–594. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, S.; Ode, H.; Iwatani, Y. Structural Features of Antiviral APOBEC3 Proteins Are Linked to Their Functional Activities. Front. Microbiol. 2011, 2, 258. [Google Scholar] [CrossRef] [Green Version]
- Delviks-Frankenberry, K.A.; Desimmie, B.A.; Pathak, V.K. Structural Insights into APOBEC3-Mediated Lentiviral Restriction. Viruses 2020, 12, 587. [Google Scholar] [CrossRef]
- Yang, H.; Ito, F.; Wolfe, A.D.; Li, S.; Mohammadzadeh, N.; Love, R.P.; Yan, M.; Zirkle, B.; Gaba, A.; Chelico, L.; et al. Understanding the structural basis of HIV-1 restriction by the full length double-domain APOBEC3G. Nat. Commun. 2020, 11, 632. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, D.; Alinejad-Rokny, H.; Davenport, M.P. Insights into the Motif Preference of APOBEC3 Enzymes. PLoS ONE 2014, 9, e87679. [Google Scholar] [CrossRef]
- Armitage, A.; Katzourakis, A.; de Oliveira, T.; Welch, J.J.; Belshaw, R.; Bishop, K.N.; Kramer, B.; McMichael, A.J.; Rambaut, A.; Iversen, A.K.N. Conserved Footprints of APOBEC3G on Hypermutated Human Immunodeficiency Virus Type 1 and Human Endogenous Retrovirus HERV-K(HML2) Sequences. J. Virol. 2008, 82, 8743–8761. [Google Scholar] [CrossRef] [Green Version]
- Salamango, D.J.; Harris, R.S. Dual Functionality of HIV-1 Vif in APOBEC3 Counteraction and Cell Cycle Arrest. Front. Microbiol. 2021, 11, 622012. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, A.M.; Gaddis, N.; Choi, J.D.; Malim, M. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nat. Cell Biol. 2002, 418, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Desimmie, B.A.; Delviks-Frankenberrry, K.A.; Burdick, R.C.; Qi, D.F.; Izumi, T.; Pathak, V.K. Multiple APOBEC3 Restriction Factors for HIV-1 and One Vif to Rule Them All. J. Mol. Biol. 2014, 426, 1220–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malim, M.H. Natural resistance to HIV infection: The Vif–APOBEC interaction. Comptes Rendus Biol. 2006, 329, 871–875. [Google Scholar] [CrossRef]
- Liddament, M.T.; Brown, W.L.; Schumacher, A.J.; Harris, R.S. APOBEC3F Properties and Hypermutation Preferences Indicate Activity against HIV-1 In Vivo. Curr. Biol. 2004, 14, 1385–1391. [Google Scholar] [CrossRef] [Green Version]
- Refsland, E.W.; Hultquist, J.; Luengas, E.M.; Ikeda, T.; Shaban, N.M.; Law, E.K.; Brown, W.L.; Reilly, C.; Emerman, M.; Harris, R.S. Natural Polymorphisms in Human APOBEC3H and HIV-1 Vif Combine in Primary T Lymphocytes to Affect Viral G-to-A Mutation Levels and Infectivity. PLoS Genet. 2014, 10, e1004761. [Google Scholar] [CrossRef]
- Ooms, M.; Brayton, B.; Letko, M.; Maio, S.M.; Pilcher, C.D.; Hecht, F.M.; Barbour, J.D.; Simon, V. HIV-1 Vif Adaptation to Human APOBEC3H Haplotypes. Cell Host Microbe 2013, 14, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Misawa, N.; Juarez-Fernandez, G.; Moriwaki, M.; Nakaoka, S.; Funo, T.; Yamada, E.; Soper, A.; Yoshikawa, R.; Ebrahimi, D.; et al. HIV-1 competition experiments in humanized mice show that APOBEC3H imposes selective pressure and promotes virus adaptation. PLoS Pathog. 2017, 13, e1006348. [Google Scholar]
- Wang, X.; Ao, Z.; Chen, L.; Kobinger, G.; Peng, J.; Yao, X. The Cellular Antiviral Protein APOBEC3G Interacts with HIV-1 Reverse Transcriptase and Inhibits Its Function during Viral Replication. J. Virol. 2012, 86, 3777–3786. [Google Scholar] [CrossRef] [Green Version]
- Pollpeter, D.; Parsons, M.; Sobala, A.E.; Coxhead, S.; Lang, R.D.; Bruns, A.M.; Papaioannou, S.; McDonnell, J.M.; Apolonia, L.; Chowdhury, J.A.; et al. Deep sequencing of HIV-1 reverse transcripts reveals the multifaceted antiviral functions of APOBEC3G. Nat. Microbiol. 2018, 3, 220–233. [Google Scholar] [CrossRef]
- Adolph, M.B.; Webb, J.; Chelico, L. Retroviral Restriction Factor APOBEC3G Delays the Initiation of DNA Synthesis by HIV-1 Reverse Transcriptase. PLoS ONE 2013, 8, e64196. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, D.; Richards, C.M.; Carpenter, M.A.; Wang, J.; Ikeda, T.; Becker, J.T.; Cheng, A.Z.; McCann, J.L.; Shaban, N.M.; Salamango, D.J.; et al. Genetic and mechanistic basis for APOBEC3H alternative splicing, retrovirus restriction, and counteraction by HIV-1 protease. Nat. Commun. 2018, 9, 4137. [Google Scholar] [CrossRef] [Green Version]
- Ara, A.; Love, R.; Chelico, L. Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms. PLoS Pathog. 2014, 10, e1004024. [Google Scholar] [CrossRef] [Green Version]
- Delviks-Frankenberry, K.A.; Nikolaitchik, O.A.; Burdick, R.C.; Gorelick, R.J.; Keele, B.F.; Hu, W.-S.; Pathak, V.K. Minimal Contribution of APOBEC3-Induced G-to-A Hypermutation to HIV-1 Recombination and Genetic Variation. PLoS Pathog. 2016, 12, e1005646. [Google Scholar] [CrossRef] [Green Version]
- Armitage, A.E.; Deforche, K.; Welch, J.J.; Van Laethem, K.; Camacho, R.; Rambaut, A.; Iversen, A.K. Possible footprints of APOBEC3F and/or other APOBEC3 deaminases, but not APOBEC3G, on HIV-1 from patients with acute/early and chronic infections. J. Virol. 2014, 88, 12882–12894. [Google Scholar] [CrossRef] [Green Version]
- Bélanger, K.; Langlois, M.-A. Comparative analysis of the gene-inactivating potential of retroviral restriction factors APOBEC3F and APOBEC3G. J. Gen. Virol. 2015, 96, 2878–2887. [Google Scholar] [CrossRef]
- Sato, K.; Takeuchi, J.S.; Misawa, N.; Izumi, T.; Kobayashi, T.; Kimura, Y.; Iwami, S.; Takaori-Kondo, A.; Hu, W.-S.; Aihara, K.; et al. APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model. PLoS Pathog. 2014, 10, e1004453. [Google Scholar] [CrossRef] [Green Version]
- Adolph, M.B.; Love, R.P.; Chelico, L. Biochemical Basis of APOBEC3 Deoxycytidine Deaminase Activity on Diverse DNA Substrates. ACS Infect. Dis. 2018, 4, 224–238. [Google Scholar] [CrossRef] [Green Version]
- Simon, V.; Zennou, V.; Murray, D.; Huang, Y.; Ho, D.D.; Bieniasz, P.D. Natural Variation in Vif: Differential Impact on APOBEC3G/3F and a Potential Role in HIV-1 Diversification. PLoS Pathog. 2005, 1, e6. [Google Scholar] [CrossRef] [Green Version]
- Anwar, F.; Davenport, M.P.; Ebrahimi, D. Footprint of APOBEC3 on the Genome of Human Retroelements. J. Virol. 2013, 87, 8195–8204. [Google Scholar] [CrossRef] [Green Version]
- Alteri, C.; Surdo, M.; Bellocchi, M.C.; Saccomandi, P.; Continenza, F.; Armenia, D.; Parrotta, L.; Carioti, L.; Costa, G.; Fourati, S.; et al. Incomplete APOBEC3G/F Neutralization by HIV-1 Vif Mutants Facilitates the Genetic Evolution from CCR5 to CXCR4 Usage. Antimicrob. Agents Chemother. 2015, 59, 4870–4881. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-Y.; Lorenzo-Redondo, R.; Little, S.J.; Chung, Y.-S.; Phalora, P.K.; Berry, I.M.; Archer, J.; Penugonda, S.; Fischer, W.; Richman, D.D.; et al. Human APOBEC3 Induced Mutation of Human Immunodeficiency Virus Type-1 Contributes to Adaptation and Evolution in Natural Infection. PLoS Pathog. 2014, 10, e1004281. [Google Scholar] [CrossRef] [Green Version]
- Wood, N.; Bhattacharya, T.; Keele, B.F.; Giorgi, E.; Liu, M.; Gaschen, B.; Daniels, M.; Ferrari, G.; Haynes, B.F.; McMichael, A.; et al. HIV Evolution in Early Infection: Selection Pressures, Patterns of Insertion and Deletion, and the Impact of APOBEC. PLoS Pathog. 2009, 5, e1000414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.; Larijani, M. Evasion of adaptive immunity by HIV through the action of host APOBEC3G/F enzymes. AIDS Res. Ther. 2017, 14, 44. [Google Scholar] [CrossRef] [Green Version]
- Jern, P.; Russell, R.A.; Pathak, V.K.; Coffin, J.M. Likely Role of APOBEC3G-Mediated G-to-A Mutations in HIV-1 Evolution and Drug Resistance. PLoS Pathog. 2009, 5, e1000367. [Google Scholar] [CrossRef] [PubMed]
- Sadler, H.A.; Stenglein, M.D.; Harris, R.S.; Mansky, L.M. APOBEC3G Contributes to HIV-1 Variation through Sublethal Mutagenesis. J. Virol. 2010, 84, 7396–7404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, M.M.; Fahrny, A.; Jayaprakash, A.; Gers-Huber, G.; Dillon-White, M.; Audigé, A.; Mulder, L.C.F.; Sachidanandam, R.; Speck, R.F.; Simon, V. Impact of Suboptimal APOBEC3G Neutralization on the Emergence of HIV Drug Resistance in Humanized Mice. J. Virol. 2020, 94, 5. [Google Scholar] [CrossRef]
- Mulder, L.C.F.; Harari, A.; Simon, V. Cytidine deamination induced HIV-1 drug resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 5501–5506. [Google Scholar] [CrossRef] [Green Version]
- Fourati, S.; Malet, I.; Binka, M.; Boukobza, S.; Wirden, M.; Sayon, S.; Simon, A.; Katlama, C.; Simon, V.; Calvez, V.; et al. Partially active HIV-1 Vif alleles facilitate viral escape from specific antiretrovirals. AIDS 2010, 24, 2313–2321. [Google Scholar] [CrossRef]
- Tzou, P.L.; Pond, S.L.K.; Avila-Rios, S.; Holmes, S.P.; Kantor, R.; Shafer, R.W. Analysis of unusual and signature APOBEC-mutations in HIV-1 pol next-generation sequences. PLoS ONE 2020, 15, e0225352. [Google Scholar] [CrossRef] [Green Version]
- Poulain, F.; Lejeune, N.; Willemart, K.; Gillet, N.A. Footprint of the host restriction factors APOBEC3 on the genome of human viruses. PLoS Pathog. 2020, 16, e1008718. [Google Scholar] [CrossRef]
- Turelli, P.; Liagre-Quazzola, A.; Mangeat, B.; Verp, S.; Jost, S.; Trono, D. APOBEC3-Independent Interferon-Induced Viral Clearance in Hepatitis B Virus Transgenic Mice. J. Virol. 2008, 82, 6585–6590. [Google Scholar] [CrossRef] [Green Version]
- Turelli, P.; Mangeat, B.; Jost, S.; Vianin, S.; Trono, D. Inhibition of hepatitis B virus replication by APOBEC3G. Science 2004, 303, 1829. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Cai, X.; Huang, Y.; Zhou, X.; Tu, Z.; Hu, J.; Tavis, J.E.; Tang, N.; Huang, A.; et al. APOBEC3B edits HBV DNA and inhibits HBV replication during reverse transcription. Antivir. Res. 2018, 149, 16–25. [Google Scholar] [CrossRef]
- Kanagaraj, A.; Sakamoto, N.; Que, L.; Li, Y.; Mohiuddin, M.; Koura, M.; Wakae, K.; Kurachi, M.; Muramatsu, M.; Kitamura, K. Different antiviral activities of natural APOBEC3C, APOBEC3G, and APOBEC3H variants against hepatitis B virus. Biochem. Biophys. Res. Commun. 2019, 518, 26–31. [Google Scholar] [CrossRef]
- Peretti, A.; Geoghegan, E.M.; Pastrana, D.V.; Smola, S.; Feld, P.; Sauter, M.; Lohse, S.; Ramesh, M.; Lim, E.S.; Wang, D.; et al. Characterization of BK Polyomaviruses from Kidney Transplant Recipients Suggests a Role for APOBEC3 in Driving In-Host Virus Evolution. Cell Host Microbe 2018, 23, 628–635.E7. [Google Scholar] [CrossRef] [Green Version]
- Verhalen, B.; Starrett, G.J.; Harris, R.S.; Jiang, M. Functional Upregulation of the DNA Cytosine Deaminase APOBEC3B by Polyomaviruses. J. Virol. 2016, 90, 6379–6386. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Ma, G.; Nosaka, K.; Tanabe, J.; Satou, Y.; Koito, A.; Wain-Hobson, S.; Vartanian, J.P.; Matsuoka, M. APOBEC3G Generates Nonsense Mutations in HTLV-1 Proviral Genomes In Vivo. J. Virol. 2010, 84, 7278–7287. [Google Scholar] [CrossRef] [Green Version]
- Sasada, A.; Takaori-Kondo, A.; Shirakawa, K.; Kobayashi, M.; Abudu, A.; Hishizawa, M.; Imada, K.; Tanaka, Y.; Uchiyama, T. APOBEC3G targets human T-cell leukemia virus type 1. Retrovirology 2005, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Argyris, P.P.; Wilkinson, P.E.; Jarvis, M.C.; Magliocca, K.R.; Patel, M.R.; Vogel, R.I.; Gopalakrishnan, R.; Koutlas, I.G.; Harris, R.S. Endogenous APOBEC3B overexpression characterizes HPV-positive and HPV-negative oral epithelial dysplasias and head and neck cancers. Mod. Pathol. 2021, 34, 280–290. [Google Scholar] [CrossRef]
- Zhu, B.; Xiao, Y.; Yeager, M.; Clifford, G.; Wentzensen, N.; Cullen, M.; Boland, J.F.; Bass, S.; Steinberg, M.K.; Raine-Bennett, T.; et al. Mutations in the HPV16 genome induced by APOBEC3 are associated with viral clearance. Nat. Commun. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.; Xu, T.; Guo, K.; Griffin, L.M.; Westrich, J.A.; Lee, D.; Lambert, P.F.; Santiago, M.L.; Pyeon, D. APOBEC3A Functions as a Restriction Factor of Human Papillomavirus. J. Virol. 2014, 89, 688–702. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Moraes, S.; Shaban, N.; Fanunza, E.; Bierle, C.; Southern, P.; Bresnahan, W.; Rice, S.; Harris, R. APOBECs and Herpesviruses. Viruses 2021, 13, 390. [Google Scholar] [CrossRef]
- Cheng, A.Z.; Moraes, S.N.; Attarian, C.; Yockteng-Melgar, J.; Jarvis, M.C.; Biolatti, M.; Galitska, G.; Dell’Oste, V.; Frappier, L.; Bierle, C.J.; et al. A Conserved Mechanism of APOBEC3 Relocalization by Herpesviral Ribonucleotide Reductase Large Subunits. J. Virol. 2019, 93, 64. [Google Scholar] [CrossRef]
- Suspène, R.; Aynaud, M.-M.; Koch, S.; Pasdeloup, D.; Labetoulle, M.; Gaertner, B.; Vartanian, J.-P.; Meyerhans, A.; Wain-Hobson, S. Genetic Editing of Herpes Simplex Virus 1 and Epstein-Barr Herpesvirus Genomes by Human APOBEC3 Cytidine Deaminases in Culture and In Vivo. J. Virol. 2011, 85, 7594–7602. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.Z.; Yockteng-Melgar, J.; Jarvis, M.; Malik-Soni, N.; Borozan, I.; Carpenter, M.A.; McCann, J.L.; Ebrahimi, D.; Shaban, N.M.; Marcon, E.; et al. Epstein–Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity. Nat. Microbiol. 2019, 4, 78–88. [Google Scholar] [CrossRef]
- Martinez, T.; Shapiro, M.; Bhaduri-McIntosh, S.; MacCarthy, T. Evolutionary effects of the AID/APOBEC family of mutagenic enzymes on human gamma-herpesviruses. Virus Evol. 2019, 5, vey040. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Stavrou, S.; Blouch, K.; Tattersall, P.; Ross, S.R. In Vivo Examination of Mouse APOBEC3- and Human APOBEC3A- and APOBEC3G-Mediated Restriction of Parvovirus and Herpesvirus Infection in Mouse Models. J. Virol. 2016, 90, 8005–8012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.A.; Holland, T.C.; Bhagwat, A.S. Human Herpes Simplex Virus-1 depletes APOBEC3A from nuclei. Virology 2019, 537, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, K.R.; McCauley, M.J.; Wang, W.; Qualley, M.F.; Wu, T.; Kitamura, S.; Geertsema, H.; Chan, D.S.B.; Hertz, A.; Iwatani, Y.; et al. Oligomerization transforms human APOBEC3G from an efficient enzyme to a slowly dissociating nucleic acid-binding protein. Nat. Chem. 2013, 6, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.; Huo, R.; Feng, Y.; Rouzina, I.; Chelico, L.; Williams, M.C. Dimerization regulates both deaminase-dependent and deaminase-independent HIV-1 restriction by APOBEC3G. Nat. Commun. 2017, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Cen, S.; Niu, M.; Saadatmand, J.; Kleiman, L. Inhibition of tRNA3Lys-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J. Virol. 2006, 80, 11710–11722. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Cen, S.; Niu, M.; Yang, Y.; Gorelick, R.J.; Kleiman, L. The Interaction of APOBEC3G with Human Immunodeficiency Virus Type 1 Nucleocapsid Inhibits tRNA 3 Lys Annealing to Viral RNA. J. Virol. 2007, 81, 11322–11331. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, S.; Emerman, M.; Malik, H.S. Ancient Adaptive Evolution of the Primate Antiviral DNA-Editing Enzyme APOBEC3G. PLoS Biol. 2004, 2, e275. [Google Scholar] [CrossRef]
- Ito, J.; Gifford, R.J.; Sato, K. Retroviruses drive the rapid evolution of mammalianAPOBEC3genes. Proc. Natl. Acad. Sci. USA 2020, 117, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Uriu, K.; Kosugi, Y.; Suzuki, N.; Ito, J.; Sato, K. Elucidation of the Complicated Scenario of Primate APOBEC3 Gene Evolution. J. Virol. 2021, 95, 12. [Google Scholar] [CrossRef]
- Oh Ainle, M.; Kerns, J.A.; Malik, H.S.; Emerman, M. Adaptive Evolution and Antiviral Activity of the Conserved Mammalian Cytidine Deaminase APOBEC3H. J. Virol. 2006, 80, 3853–3862. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, M.; Bleiber, G.; Martinez, R.; Kaessmann, H.; Telenti, A. Patterns of evolution of host proteins involved in retroviral pathogenesis. Retrovirology 2006, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Bleiber, G.; Duggal, P.; Nelson, G.; May, M.; Mangeat, B.; Alobwede, I.; Trono, D.; Vlahov, D.; Donfield, S.; et al. APOBEC3G Genetic Variants and Their Influence on the Progression to AIDS. J. Virol. 2004, 78, 11070–11076. [Google Scholar] [CrossRef] [Green Version]
- Matume, N.D.; Tebit, D.; Gray, L.R.; Turner, S.D.; Rekosh, D.; Bessong, P.O.; Hammarskjöld, M.-L. Characterization of APOBEC3 variation in a population of HIV-1 infected individuals in northern South Africa. BMC Med. Genet. 2019, 20, 21. [Google Scholar] [CrossRef]
- Li, M.M.H.; Emerman, M. Polymorphism in Human APOBEC3H Affects a Phenotype Dominant for Subcellular Localization and Antiviral Activity. J. Virol. 2011, 85, 8197–8207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, A.; Wang, T.; Zhao, K.; Xiong, Y.; Yu, X.-F. A Single Amino Acid Difference in Human APOBEC3H Variants Determines HIV-1 Vif Sensitivity. J. Virol. 2009, 84, 1902–1911. [Google Scholar] [CrossRef] [Green Version]
- Harari, A.; Ooms, M.; Mulder, L.C.F.; Simon, V. Polymorphisms and Splice Variants Influence the Antiretroviral Activity of Human APOBEC3H. J. Virol. 2009, 83, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, K.G.; Wissing, S.; Lobritz, M.A.; Santiago, M.L.; Greene, W.C. Identification of Two APOBEC3F Splice Variants Displaying HIV-1 Antiviral Activity and Contrasting Sensitivity to Vif*. J. Biol. Chem. 2010, 285, 29326–29335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Chen, Q.; Xiao, X.; Ito, F.; Wolfe, A.; Chen, X.S. Biochemical Characterization of APOBEC3H Variants: Implications for Their HIV-1 Restriction Activity and mC Modification. J. Mol. Biol. 2016, 428, 4626–4638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doehle, B.P.; Schafer, A.; Cullen, B.R. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology 2005, 339, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogerd, H.P.; Wiegand, H.L.; Doehle, B.P.; Cullen, B.R. The intrinsic antiretroviral factor APOBEC3B contains two enzymatically active cytidine deaminase domains. Virology 2007, 364, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, G.; Durand, S.; Fargier, G.; Nguyen, X.-N.; Cordeil, S.; Bouaziz, S.; Muriaux, D.; Darlix, J.-L.; Cimarelli, A. APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells. PLoS Pathog. 2011, 7, e1002221. [Google Scholar] [CrossRef] [Green Version]
- Suspène, R.; Guétard, D.; Henry, M.; Sommer, P.; Wain-Hobson, S.; Vartanian, J.-P. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 8321–8326. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.; Westrich, J.A.; Van Doorslaer, K.; Pyeon, D. Roles of APOBEC3A and APOBEC3B in Human Papillomavirus Infection and Disease Progression. Viruses 2017, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Narvaiza, I.; Linfesty, D.C.; Greener, B.N.; Hakata, Y.; Pintel, D.J.; Logue, E.; Landau, N.R.; Weitzman, M.D. Deaminase-Independent Inhibition of Parvoviruses by the APOBEC3A Cytidine Deaminase. PLoS Pathog. 2009, 5, e1000439. [Google Scholar] [CrossRef] [Green Version]
- Bogerd, H.P.; Wiegand, H.L.; Hulme, A.E.; Garcia-Perez, J.L.; O’Shea, K.S.; Moran, J.V.; Cullen, B.R. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. USA 2006, 103, 8780–8785. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lilley, C.E.; Yu, Q.; Lee, D.V.; Chou, J.; Narvaiza, I.; Landau, N.R.; Weitzman, M.D. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr. Biol. 2006, 16, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Esnault, C.; Priet, S.; Ribet, D.; Heidmann, O.; Heidmann, T. Restriction by APOBEC3 proteins of endogenous retroviruses with an extracellular life cycle: Ex vivo effects and in vivo “traces” on the murine IAPE and human HERV-K elements. Retrovirology 2008, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Refsland, E.W.; Stenglein, M.D.; Shindo, K.; Albin, J.S.; Brown, W.L.; Harris, R.S. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: Implications for HIV-1 restriction. Nucleic Acids Res. 2010, 38, 4274–4284. [Google Scholar] [CrossRef] [Green Version]
- A Roberts, S.; Lawrence, M.S.; Klimczak, L.J.; A Grimm, S.; Fargo, D.; Stojanov, P.; Kiezun, A.; Kryukov, G.; Carter, S.L.; Saksena, G.; et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 2013, 45, 970–976. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Ng, A.W.T.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Klonowska, K.; Kluzniak, W.; Rusak, B.; Jakubowska, A.; Ratajska, M.; Krawczynska, N.; Vasilevska, D.; Czubak, K.; Wojciechowska, M.; Cybulski, C.; et al. The 30 kb deletion in the APOBEC3 cluster decreases APOBEC3A and APOBEC3B expression and creates a transcriptionally active hybrid gene but does not associate with breast cancer in the European population. Oncotarget 2017, 8, 76357–76374. [Google Scholar] [CrossRef] [Green Version]
- Kidd, J.; Newman, T.L.; Tuzun, E.; Kaul, R.; E Eichler, E. Population Stratification of a Common APOBEC Gene Deletion Polymorphism. PLoS Genet. 2007, 3, e63. [Google Scholar] [CrossRef]
- Prasetyo, A.A.; Sariyatun, R.; Reviono; Sari, Y.; Hudiyono; Haryati, S.; Adnan, Z.A.; Hartono; Kageyama, S. The APOBEC3B deletion polymorphism is associated with prevalence of hepatitis B virus, hepatitis C virus, Torque Teno virus, and Toxoplasma gondii co-infection among HIV-infected individuals. J. Clin. Virol. 2015, 70, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ochi, H.; Maekawa, T.; Hatakeyama, T.; Tsuge, M.; Kitamura, S.; Kimura, T.; Miki, D.; Mitsui, F.; Hiraga, N.; et al. Effects of structural variations of APOBEC3A and APOBEC3B genes in chronic hepatitis B virus infection. Hepatol. Res. 2009, 39, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Ezzikouri, S.; Kitab, B.; Rebbani, K.; Marchio, A.; Wain-Hobson, S.; Dejean, A.; Vartanian, J.-P.; Pineau, P.; Benjelloun, S. Polymorphic APOBEC3 modulates chronic hepatitis B in Moroccan population. J. Viral Hepat. 2013, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Marathe, S.D.; Nain, S.; Nema, V.; Ghate, M.V.; Gangakhedkar, R.R. APOBEC3B deletion impacts on susceptibility to acquire HIV-1 and its advancement among individuals in western India. APMIS 2016, 124, 881–887. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Johnson, R.; Phair, J.; Kirk, G.D.; Yu, X.F.; Donfield, S.; Buchbinder, S.; Goedert, J.J.; Winkler, C.A. APOBEC3B deletion and risk of HIV-1 acquisition. J. Infect. Dis. 2009, 200, 1054–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, T.; E Nakayama, E.; Kaur, G.; Terunuma, H.; Mimaya, J.-I.; Ohtani, H.; Mehra, N.; Shioda, T.; Kimura, A. Impact of novel TRIM5α variants, Gly110Arg and G176del, on the anti-HIV-1 activity and the susceptibility to HIV-1 infection. AIDS 2009, 23, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Itaya, S.; Nakajima, T.; Kaur, G.; Terunuma, H.; Ohtani, H.; Mehra, N.; Kimura, A. No evidence of an association between the APOBEC3B deletion polymorphism and susceptibility to HIV infection and AIDS in Japanese and Indian populations. J. Infect. Dis. 2010, 202, 815–816. [Google Scholar] [CrossRef] [Green Version]
- Imahashi, M.; Izumi, T.; Watanabe, D.; Imamura, J.; Matsuoka, K.; Ode, H.; Masaoka, T.; Sato, K.; Kaneko, N.; Ichikawa, S.; et al. Lack of association between intact/deletion polymorphisms of the APOBEC3B gene and HIV-1 risk. PLoS ONE 2014, 9, e92861. [Google Scholar] [CrossRef]
- Koning, F.A.; Newman, E.N.; Kim, E.Y.; Kunstman, K.J.; Wolinsky, S.M.; Malim, M.H. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J. Virol. 2009, 83, 9474–9485. [Google Scholar] [CrossRef] [Green Version]
- Muckenfuss, H.; Hamdorf, M.; Held, U.; Perković, M.; Löwer, J.; Cichutek, K.; Flory, E.; Schumann, G.G.; Münk, C. APOBEC3 Proteins Inhibit Human LINE-1 Retrotransposition. J. Biol. Chem. 2006, 281, 22161–22172. [Google Scholar] [CrossRef] [Green Version]
- Horn, A.V.; Klawitter, S.; Held, U.; Berger, A.; Vasudevan, A.A.J.; Bock, A.; Hofmann, H.; Hanschmann, K.-M.O.; Trösemeier, J.-H.; Flory, E.; et al. Human LINE-1 restriction by APOBEC3C is deaminase independent and mediated by an ORF1p interaction that affects LINE reverse transcriptase activity. Nucleic Acids Res. 2014, 42, 396–416. [Google Scholar] [CrossRef] [Green Version]
- Wittkopp, C.J.; Adolph, M.B.; Wu, L.I.; Chelico, L.; Emerman, M. A Single Nucleotide Polymorphism in Human APOBEC3C Enhances Restriction of Lentiviruses. PLoS Pathog. 2016, 12, e1005865. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Ikeda, T.; Moghadasi, S.A.; Martin, A.S.; Brown, W.L.; Harris, R.S. Natural APOBEC3C variants can elicit differential HIV-1 restriction activity. Retrovirology 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refsland, E.W.; Hultquist, J.F.; Harris, R.S. Endogenous Origins of HIV-1 G-to-A Hypermutation and Restriction in the Nonpermissive T Cell Line CEM2n. PLoS Pathog. 2012, 8, e1002800. [Google Scholar] [CrossRef] [PubMed]
- Chaipan, C.; Smith, J.L.; Hu, W.-S.; Pathak, V.K. APOBEC3G Restricts HIV-1 to a Greater Extent than APOBEC3F and APOBEC3DE in Human Primary CD4+ T Cells and Macrophages. J. Virol. 2012, 87, 444–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillick, K.; Pollpeter, D.; Phalora, P.; Kim, E.-Y.; Wolinsky, S.; Malim, M.H. Suppression of HIV-1 Infection by APOBEC3 Proteins in Primary Human CD4+ T Cells Is Associated with Inhibition of Processive Reverse Transcription as Well as Excessive Cytidine Deamination. J. Virol. 2013, 87, 1508–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggal, N.K.; Fu, W.; Akey, J.M.; Emerman, M. Identification and antiviral activity of common polymorphisms in the APOBEC3 locus in human populations. Virology 2013, 443, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, R.K.; Koning, F.A.; Bishop, K.N.; Malim, M.H. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation—Comparisons with APOBEC3G. J. Biol. Chem. 2007, 282, 2587–2595. [Google Scholar] [CrossRef] [Green Version]
- Krisko, J.F.; Begum, N.; Baker, C.E.; Foster, J.L.; Garcia, J.V. APOBEC3G and APOBEC3F Act in Concert to Extinguish HIV-1 Replication. J. Virol. 2016, 90, 4681–4695. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, S.R.; Hache, G.; Stenglein, M.D.; Fahrenkrug, S.C.; Andresdottir, V.; Harris, R.S. Evolutionarily conserved and non-conserved retrovirus restriction activities of artiodactyl APOBEC3F proteins. Nucleic Acids Res. 2006, 34, 5683–5694. [Google Scholar] [CrossRef]
- Ara, A.; Love, R.P.; Follack, T.B.; Ahmed, K.A.; Adolph, M.B.; Chelico, L. Mechanism of Enhanced HIV Restriction by Virion Coencapsidated Cytidine Deaminases APOBEC3F and APOBEC3G. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbisa, J.L.; Bu, W.; Pathak, V.K. APOBEC3F and APOBEC3G Inhibit HIV-1 DNA Integration by Different Mechanisms. J. Virol. 2010, 84, 5250–5259. [Google Scholar] [CrossRef] [Green Version]
- Adolph, M.B.; Ara, A.; Chelico, L. APOBEC3 Host Restriction Factors of HIV-1 Can Change the Template Switching Frequency of Reverse Transcriptase. J. Mol. Biol. 2019, 431, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, N.; Love, R.P.; Gibson, R.; Arts, E.J.; Poon, A.F.; Chelico, L. Role of co-expressed APOBEC3F and APOBEC3G in inducing HIV-1 drug resistance. Heliyon 2019, 5, e01498. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Penugonda, S.; Thorball, C.W.; Bartha, I.; Goedert, J.J.; Donfield, S.; Buchbinder, S.; Binns-Roemer, E.; Kirk, G.D.; Zhang, W.; et al. Role of APOBEC3F Gene Variation in HIV-1 Disease Progression and Pneumocystis Pneumonia. PLoS Genet. 2016, 12, e1005921. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, N.; Follack, T.B.; Love, R.P.; Stewart, K.; Sanche, S.; Chelico, L. Polymorphisms of the cytidine deaminase APOBEC3F have different HIV-1 restriction efficiencies. Virology 2019, 527, 21–31. [Google Scholar] [CrossRef]
- Malim, M.H. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, C.; Hiraga, N.; Mori, N.; Tsuge, M.; Imamura, M.; Takahashi, S.; Fujimoto, Y.; Ochi, H.; Abe, H.; Maekawa, T.; et al. Dual effect of APOBEC3G on Hepatitis B virus. J. Gen. Virol. 2007, 88, 432–440. [Google Scholar] [CrossRef]
- Lei, Y.C.; Hao, Y.H.; Zhang, Z.M.; Tian, Y.J.; Wang, B.J.; Yang, Y.; Zhao, X.P.; Lu, M.J.; Gong, F.L.; Yang, D.L. Inhibition of hepatitis B virus replication by APOBEC3G in vitro and in vivo. World J. Gastroenterol. 2006, 12, 4492–4497. [Google Scholar] [CrossRef]
- Hulme, A.E.; Bogerd, H.P.; Cullen, B.R.; Moran, J.V. Selective inhibition of Alu retrotransposition by APOBEC3G. Gene 2007, 390, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.; Ooms, M.; Letko, M.; Garrett, N.; Simon, V.; Ndung’U, T. Functional characterization of Vif proteins from HIV-1 infected patients with different APOBEC3G haplotypes. AIDS 2016, 30, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.K.; Wang, Y.; Gray, K.P.; Farhad, M.; Brummel, S.; Fenton, T.; Trout, R.; Spector, S.A. Genetic Variants in the Host Restriction Factor APOBEC3G are Associated With HIV-1–Related Disease Progression and Central Nervous System Impairment in Children. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 62, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Do, H.; Vasilescu, A.; Diop, G.; Hirtzig, T.; Heath, S.C.; Coulonges, C.; Rappaport, J.; Therwath, A.; Lathrop, M.; Matsuda, F.; et al. Exhaustive Genotyping of theCEM15 (APOBEC3G)Gene and Absence of Association with AIDS Progression in a French Cohort. J. Infect. Dis. 2005, 191, 159–163. [Google Scholar] [CrossRef]
- Valcke, H.S.; Bernard, N.F.; Bruneau, J.; Alary, M.; Tsoukas, C.M.; Roger, M. APOBEC3G genetic variants and their association with risk of HIV infection in highly exposed Caucasians. AIDS 2006, 20, 1984–1986. [Google Scholar] [CrossRef]
- De Maio, F.A.; Rocco, C.A.; Aulicino, P.C.; Bologna, R.; Mangano, A.; Sen, L. Effect of HIV-1 Vif variability on progression to pediatric AIDS and its association with APOBEC3G and CUL5 polymorphisms. Infect. Genet. Evol. 2011, 11, 1256–1262. [Google Scholar] [CrossRef]
- De Maio, F.A.; Rocco, C.A.; Aulicino, P.C.; Bologna, R.; Mangano, A.; Sen, L. APOBEC3-Mediated Editing in HIV Type 1 from Pediatric Patients and Its Association withAPOBEC3G/CUL5Polymorphisms and Vif Variability. AIDS Res. Hum. Retrovir. 2012, 28, 619–627. [Google Scholar] [CrossRef]
- Singh, H.; Marathe, S.; Nain, S.; Nema, V.; Angadi, M.; Bapat, S.; Pawar, J.; Ghate, M.; Sahay, S.; Gangakhedkar, R.R. Coding region variant 186H/R in Exon 4 of APOBEC3G among individuals of Western India. APMIS 2016, 124, 401–405. [Google Scholar] [CrossRef]
- Rathore, A.; Chatterjee, A.; Yamamoto, N.; Dhole, T.N.T.N. Absence of H186R Polymorphism in Exon 4 of the APOBEC3G Gene among North Indian Individuals. Genet. Test. 2008, 12, 453–456. [Google Scholar] [CrossRef]
- Reddy, K.; A Winkler, C.; Werner, L.; Mlisana, K.; Karim, S.A.; Ndung’U, T. APOBEC3G expression is dysregulated in primary HIV-1 infection and polymorphic variants influence CD4+ T-cell counts and plasma viral load. AIDS 2010, 24, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Compaore, T.R.; Soubeiga, S.T.; Ouattara, A.K.; Obiri-Yeboah, D.; Tchelougou, D.; Maiga, M.; Assih, M.; Bisseye, C.; Bakouan, D.; Compaore, I.P.; et al. APOBEC3G Variants and Protection against HIV-1 Infection in Burkina Faso. PLoS ONE 2016, 11, e0146386. [Google Scholar] [CrossRef] [Green Version]
- Compaore, T.R.; Diarra, B.; Assih, M.; Obiri-Yeboah, D.; Soubeiga, S.T.; Ouattara, A.K.; Tchelougou, D.; Bisseye, C.; Bakouan, D.R.; Compaore, I.P.; et al. HBV/HIV co-infection and APOBEC3G polymorphisms in a population from Burkina Faso. BMC Infect. Dis. 2016, 16, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizinoto, M.C.; Leal, É.; Diaz, R.S.; Janini, L.M. Loci Polymorphisms of the APOBEC3G Gene in HIV Type 1-Infected Brazilians. AIDS Res. Hum. Retrovir. 2011, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Gangakhedkar, R. Occurrence of APOBEC3G variations in West Indian HIV patients. Microb. Pathog. 2018, 121, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Mhandire, K.; Duri, K.; Mhandire, D.; Musarurwa, C.; Stray-Pedersen, B.; Dandara, C. Evaluating the contribution of APOBEC3G haplotypes, on influencing HIV infection in a Zimbabwean paediatric population. South. Afr. Med. J. 2016, 106, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Pace, C.; Keller, J.; Nolan, D.; James, I.; Gaudieri, S.; Moore, C.; Mallal, S. Population Level Analysis of Human Immunodeficiency Virus Type 1 Hypermutation and Its Relationship with APOBEC3G and vif Genetic Variation. J. Virol. 2007, 81, 8843–8845. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Abudu, A.; Son, S.; Dang, Y.; Venta, P.J.; Zheng, Y.-H. Analysis of Human APOBEC3H Haplotypes and Anti-Human Immunodeficiency Virus Type 1 Activity. J. Virol. 2011, 85, 3142–3152. [Google Scholar] [CrossRef] [Green Version]
- Oh Ainle, M.; Kerns, J.A.; Li, M.M.; Malik, H.S.; Emerman, M. Antiretroelement Activity of APOBEC3H Was Lost Twice in Recent Human Evolution. Cell Host Microbe 2008, 4, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Chesarino, N.M.; Emerman, M. Polymorphisms in Human APOBEC3H Differentially Regulate Ubiquitination and Antiviral Activity. Viruses 2020, 12, 378. [Google Scholar] [CrossRef] [Green Version]
- Ooms, M.; Majdak, S.; Seibert, C.W.; Harari, A.; Simon, V. The Localization of APOBEC3H Variants in HIV-1 Virions Determines Their Antiviral Activity. J. Virol. 2010, 84, 7961–7969. [Google Scholar] [CrossRef] [Green Version]
- Gourraud, P.-A.; Karaouni, A.; Woo, J.; Schmidt, T.; Oksenberg, J.; Hecht, F.; Liegler, T.; Barbour, J. APOBEC3H haplotypes and HIV-1 pro-viral vif DNA sequence diversity in early untreated human immunodeficiency virus–1 infection. Hum. Immunol. 2011, 72, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Binka, M.; Ooms, M.; Steward, M.; Simon, V. The Activity Spectrum of Vif from Multiple HIV-1 Subtypes against APOBEC3G, APOBEC3F, and APOBEC3H. J. Virol. 2011, 86, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Ooms, M.; Letko, M.; Binka, M.; Simon, V. The Resistance of Human APOBEC3H to HIV-1 NL4-3 Molecular Clone Is Determined by a Single Amino Acid in Vif. PLoS ONE 2013, 8, e57744. [Google Scholar] [CrossRef]
- Benito, J.M.; Hillung, J.; Restrepo, C.; Cuevas, J.M.; Leon, A.; Ruiz-Mateos, E.; Palacios-Muñoz, R.; Górgolas, M.; Sanjuán, R.; Rallón, N. Role of APOBEC3H in the Viral Control of HIV Elite Controller Patients. Int. J. Med. Sci. 2018, 15, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, D.; Iwatani, Y.; Ohtani, H.; Naruse, T.K.; Terunuma, H.; Sugiura, W.; Kimura, A. APOBEC3H polymorphisms associated with the susceptibility to HIV-1 infection and AIDS progression in Japanese. Immunogenetics 2015, 67, 253–257. [Google Scholar] [CrossRef]
- Naruse, T.K.; Sakurai, D.; Ohtani, H.; Sharma, G.; Sharma, S.K.; Vajpayee, M.; Mehra, N.K.; Kaur, G.; Kimura, A. APOBEC3H polymorphisms and susceptibility to HIV-1 infection in an Indian population. J. Hum. Genet. 2015, 61, 263–265. [Google Scholar] [CrossRef]


| Description | Allelic Frequency | Studied Populations and References | Effect | |||
|---|---|---|---|---|---|---|
| African | European | Asian | ||||
| APOBEC3A/B | ||||||
| rs12628403 (surrogate for ~30 kb APOBEC3B deletion) | ~30 kb deletion (Δ) of all APOBEC3B exons and introns except for exon 8; In-frame fusion to APOBEC3A. | n = 3394 Δ = 0.03 | n = 20,404 Δ = 0.08 | n = 116 Δ = 0.4 | Indonesian PMID: 26305823 | HIV-1 co-infections: Δ > reference |
| Moroccan PMID: 24010642 | %Δ: HBV patients ~ healthy donors Progression to liver diseases: Δ > reference | |||||
| Western Indian PMID: 27522954 | Risk of HIV-1 acquisition: Δ > reference | |||||
| European Americans and African Americans PMID: 19698078 | HIV-1 acquisition, progression to AIDS, and viral set point: Δ/Δ > reference | |||||
| Japanese and Indian PMID: 20684727 | %Δ: HIV-1 patients ~ healthy donors | |||||
| Japanese PMID: 19788695 | %Δ: HBV patients ~ healthy donors Hypermutation context: Δ ~ reference | |||||
| Japanese PMID: 24667791 | %Δ: HIV-1 patients ~ healthy donors HIV-1 co-infections: Δ ~ reference HIV-1 progression: Δ ~ reference | |||||
| APOBEC3C | ||||||
| rs112120857 | Missense (G > C / G > T) S188I | n = 3574 G = 0.94 C = 0.00 T = 0.06 | n = 37,290 G = 0.99962 C = 0.00003 T = 0.00035 | n = 168 G = 1.00 C = 0.00 T = 0.00 | PMIDs: 27732658, 30558640 | In vitro anti-HIV-1 activity: I > S |
| APOBEC3D | ||||||
| rs201709403 | Missense (A > G) T238A | n = 2898 A = 1.00 G = 0.00 | n = 9690 A = 1.00 G = 0.00 | n = 112 A = 1.00 G = 0.00 | Northern South African PMID: 30660178 | %G (238A): HIV-1 patients > 1000 Genomes Africans |
| rs75858538 | Missense (C > T) R97C | n = 5146 C = 0.98 T = 0.02 | n = 180,258 C = 0.9999 T = 0.0001 | n = 6364 C = 0.9998 T = 0.0002 | Northern South African PMID: 30660178 | %T (97C): HIV-1 patients > 1000 Genomes Africans |
| PMID: 23755966 | Protein expression: R ~ C Anti-HIV-1 activity: R > C Sensitivity to HIV-1 Vif: R ~ C Anti-Alu activity: R ~ C | |||||
| rs61748819 | Missense (G > A) R248K | n = 3574 G = 0.91 A = 0.09 | n = 32,834 G = 0.9997 A = 0.00034 | n = 168 G = 1.00 A = 0.000 | PMID: 23755966 | Protein expression: R > K Anti-HIV-1 activity: R > K Sensitivity to HIV-1 Vif: R ~ K Anti-Alu activity: R > K |
| APOBEC3F | ||||||
| rs2020390 | Missense (G > T) A108S | n = 3512 G = 0.60 T = 0.40 | n = 37,024 G = 0.49 T = 0.51 | n = 168 G = 0.29 T = 0.71 | Northern South African PMID: 30660178 | %T (108S): HIV-1 patients > 1000 Genomes African populations |
| rs12157816 | Missense (A > G) Y307C | n = 3574 A = 0.97 G = 0.03 | n = 37,264 A = 0.9881 G = 0.0119 | n = 168 A = 1.0 G = 0.0 | Northern South African PMID: 30660178 | %G (307C): HIV-1 patients > 1000 Genomes African populations |
| rs2076101 | Missense (G > A) V231I | n = 8220 G = 0.73 A = 0.27 | n = 256,418 G = 0.48 A = 0.52 | n = 3812 G = 0.3 A = 0.7 | European American and African American PMID: 26942578 | Set-point viral load: I > V Progression to AIDS: I > V Pneumocystis pneumonia: I > V |
| PMID: 30448640 | HIV-1 restriction: V > I Protection against HIV-1 Vif: V > I Level of viral encapsidation: V > I | |||||
| APOBEC3FΔ2 | Isoform lacking exon 2 | PMID: 20624919 | Sensitivity to Vif: main isoform > APOBEC3FΔ2 Viral packaging: main isoform > APOBEC3FΔ2 Antiviral activity: main isoform > APOBEC3FΔ2 Deamination: main isoform > APOBEC3FΔ2 | |||
| APOBEC3FΔ2-4 | Isoform lacking exons 2, 3 and 4 | PMID: 20624919 | Sensitivity to Vif: APOBEC3FΔ2–4 > main isoform Viral packaging: main isoform > APOBEC3FΔ2–4 Antiviral activity: Main isoform > APOBEC3FΔ2–4 deamination: main isoform > APOBEC3FΔ2–4 | |||
| APOBEC3G | ||||||
| rs5757463 | -571G/C upstream (G > C) | n = 106 G = 0.29 C = 0.71 | n = 6564 G = 0.06 C = 0.94 | n = 4 G = 0.0 C = 1.0 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: G ~ C |
| Brazilian PMID: 20874421 | CD4 T-cell count: CC > CG and GG | |||||
| West Indian PMID: 29864532 | %CG: HIV patients > healthy donors %(GC + CC): HIV patients > healthy donors | |||||
| Zimbabwean PMID: 27245545 | Susceptibility to HIV-1 infection: G ~ C | |||||
| rs34550797 | -199G/A upstream (G > A) | n = 2816 G = 0.995 A = 0.005 | n = 7618 G = 0.9992 A = 0.0008 | n = 108 G = 1.0 A = 0.0 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: G ~ A |
| rs5750743 | -90C/G upstream/5′UTR (C > G) | n = 276 C = 1.00 G = 0.00 | n = 5330 C = 0.54 G = 0.46 | n = 8 C = 1.0 G = 0.0 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: C ~ G |
| West Indian PMID: 29864532 | %CG: HIV-1 patients > healthy donors %GG: healthy donors > HIV patients | |||||
| Zimbabwean PMID: 27245545 | Susceptibility to HIV-1 infection: C ~ G | |||||
| rs5757465 | Synonymous (T > C) F119F | n = 11,214 T = 0.90 C = 0.10 | n = 248,594 T = 0.56 C = 0.44 | n = 3908 T = 0.76 C = 0.24 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: T ~ C |
| Diverse populations PMID: 23138837 | HIV-1 disease progression: T > C CNS impairment: T > C | |||||
| rs3736685 | 197193T/C intron (T > C) | n = 7982 T = 0.63 C = 0.37 | n = 223,498 T = 0.97 C = 0.03 | n = 3870 T = 0.92 C = 0.08 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: T ~ C |
| Zimbabwean PMID: 27245545 | Susceptibility to HIV-1 infection: T ~ C | |||||
| rs2294367 | 199376G/C intron (C > G) | n = 2036 C = 0.99 G = 0.01 | n = 8254 C = 0.57 G = 0.43 | n = 16 C = 0.75 G = 0.25 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: C ~ G Progression to AIDS: G > C |
| Zimbabwean PMID: 27245545 | Susceptibility to HIV-1 infection: C ~ G | |||||
| rs8177832 | Missense (A > G) H186R | n = 11,678 A = 0.66 G = 0.34 | n = 252,598 A = 0.97 G = 0.03 | n = 6820 A = 0.92 G = 0.08 | African Americans and European Americans PMID: 15452227 | Susceptibility to HIV-1 infection: H ~ R Rate of CD4 T cell loss: R > H Progression to AIDS: R > H |
| PMID: 27064995 | Antiviral activity: H > R Counteraction by Vif: H ~ R | |||||
| Diverse populations PMID: 23138837 | HIV-1 disease progression: R > H CNS impairment: R > H | |||||
| South Africans PMID: 19996938 | Viral load: R > H CD4 T cell count: H > R | |||||
| French PMID: 15609224 | Progression to AIDS: H ~ R | |||||
| Caucasians PMID: 16988524 | Progression to AIDS: H ~ R | |||||
| Argentinian PMID: 22145963 | Progression to AIDS: H ~ R Viral G-to-A mutation: H ~ R | |||||
| Argentinian PMID: 21571098 | HIV-1 transmission: H ~ R Progression to AIDS: H ~ R Viral G-to-A mutation: H ~ R | |||||
| Zimbabwean PMID: 27245545 | Susceptibility to HIV-1 infection: H ~ R | |||||
| Morocco PMID: 24010642 | Risk of HBV acquisition: H ~ R | |||||
| Burkina Fasoian PMID: 26741797 | Protection against HIV-1 infection: GGT [rs6001417, rs8177832 (H186R), rs35228531] > Other haplotypes Risk of HIV-1 infection: GGC and CGC > Other haplotypes | |||||
| Burkina Fasoian PMID: 27449138 | HIV-1/HBV co-infection rate: other haplotypes > GGT [rs6001417, rs8177832 (H186R), rs35228531] | |||||
| Western Indian PMID: 26853443 | Risk of HIV-1 acquisition: H ~ R Progression to AIDS: H ~ R | |||||
| North Indian PMID: 18652534 | Absence of 186R variant | |||||
| rs17496018 | C40693T Intron (C > T) | n = 4296 C = 0.94 T = 0.06 | n = 26,952 C = 0.93 T = 0.07 | n = 128 C = 0.97 T = 0.03 | Caucasians PMID: 16988524 | Risk of HIV-1 infection: T > C |
| Argentinian PMID 22145963 | Progression to AIDS: H ~ R Viral G > A mutation: H ~ R | |||||
| Argentinian PMID: 21571098 | HIV-1 transmission: H ~ R Progression to AIDS: H ~ R Viral G-to-A mutation: H ~ R C > T polymorphism was associated with Vif variation. | |||||
| rs17496046 | Missense (C > G) Q275E | n = 4374 C = 0.87 G = 0.13 | n = 96,948 C = 0.93 G = 0.07 | n = 3326 C = 0.97 G = 0.03 | Caucasians PMID: 16988524 | Risk of HIV-1 infection: C ~ G |
| Northern South African PMID: 30660178 | % G (275E): HIV-1 patients > 1000 Genomes Africans | |||||
| rs6001417 | Intron (C > G) | n = 2960 C = 0.63 G = 0.37 | n = 16,474 C = 0.97 G = 0.03 | n = 112 C = 0.94 G = 0.06 | Burkina Fasoian PMID: 26741797 | Protection against HIV-1 infection: GGT [rs6001417, rs8177832 (H186R), rs35228531] > Other haplotypes Risk of HIV-1 infection: GGC and CGC > Other haplotypes |
| Burkina Fasoian PMID: 27449138 | HIV-1/HBV co-infection rate: other haplotypes > GGT [rs6001417, rs8177832 (H186R), rs35228531] | |||||
| rs35228531 | Downstream (C > T) | n = 1564 C = 0.994 T = 0.006 | n = 9814 C = 1.00 T = 0.00 | n = 112 C = 1.00 T = 0.00 | South African PMID: 19996938 | Viral load: T > C CD4 T-cell count: C > T |
| Burkina Fasoian PMID: 26741797 | Protection against HIV-1 infection: GGT [rs6001417, rs8177832 (H186R), rs35228531] > Other haplotypes Risk of HIV-1 infection: GGC and CGC > Other haplotypes | |||||
| Burkina Fasoian PMID: 27449138 | HIV-1/HBV co-infection rate: C > T HIV-1/HBV co-infection rate: Other haplotypes > GGT [rs6001417, rs8177832 (H186R), rs35228531] | |||||
| rs5757467 | Intron (C > T) | n = 2946 C = 0.31 T = 0.69 | n = 15,414 C = 0.36 T = 0.64 | n = 112 C = 0.21 T = 0.79 | West Australian PMID: 16940537 | HIV-1 hypermutation: C > T |
| APOBEC3H | ||||||
| rs139292 (Previously rs140936762) | Indel (CAA/Δ) N15Δ | n = 3506 Δ = 0.32 | n = 20,264 Δ = 0.34 | n = 168 Δ = 0.23 | PMID: 32235597 | Antiviral activity: Reference > Δ |
| Northern South African PMID: 30660178 | %Δ: HIV-1 patients >1000 Genomes Africans | |||||
| Japanese PMID: 25721876 | Susceptibility to HIV-1 infection: Δ > reference | |||||
| Indian PMID: 26559750 | Susceptibility to HIV-1 infection: Δ > reference | |||||
| rs139293 | Missense (G > T) R18L | n = 3158 G = 0.96 T = 0.04 | n = 36,146 G = 0.75 T = 0.25 | n = 166 G = 0.92 T = 0.08 | Northern South Africa PMID: 30660178 | %T(18L): HIV-1 patients > 1000 Genomes Africans |
| rs139297 | Missense (G > C) G105R | n = 1308 G = 0.66 C = 0.34 | n = 35,022 G = 0.56 C = 0.44 | n = 148 G = 0.89 C = 0.11 | PMID: 32235597 | Ubiquitination: G (HapI) > R (HapII) |
| PMID: 20519396 | Antiviral activity: R (HapII) > G (HapI) HIV-1 encapsidation: R (HapII) ~ G (HapI) Protein expression: R (HapII) > G (HapI) Interaction with Gag: R (HapII) with nucleocapsid; G (HapI) with C-terminal of matrix and N-terminal of capsid | |||||
| Japanese PMID: 25721876 | Susceptibility to HIV-1 infection: G (HapI) > R (HapII) Progression to AIDS: G (HapI) > R (HapII) | |||||
| Indian PMID: 26559750 | %15Δ-105R: HIV-1 patients > healthy donors | |||||
| Diverse populations PMID: 21167246 | Rate of HIV-1 GA-to-AA mutation: R (HapII) > G (HapI) | |||||
| rs139299 | Missense (G > C) K121D | n = 3574 G = 0.24 C = 0.76 | n = 37,158 G = 0.52 C = 0.48 | n = 168 G = 0.72 C = 0.28 | Diverse populations PMID: 21167246 | Rate of HIV-1 GA-to-AA mutation: D (HapII) > K (HapI) |
| rs139298 | Missense (A > G) K121D | n = 11,188 A = 0.21 G = 0.79 | n = 252,052 A = 0.53 G = 0.47 | n = 6744 A = 0.69 G = 0.31 | Diverse populations PMID: 21167246 | Rate of HIV-1 GA-to-AA mutation: D (HapII) > K (HapI) |
| rs139302 | Missense (G > C) E178D | n = 3882 G = 0.26 C = 0.74 | n = 97,472 G = 0.54 C = 0.46 | n = 3326 G = 0.67 C = 0.33 | PMID: 21167246 | Rate of HIV-1 GA-to-AA mutation: D (HapII) > E (HapI) |
| Northern South African PMID: 30660178 | %C (178D): HIV-1 patients > 1000 Genomes Africans | |||||
| PMID: 27534815 | Cytosine deamination: E (HapV) > D (other haplotypes) Methyl cytosine deamination: D (HapII) >> E (other haplotypes) DNA binding: all haplotypes are the same. | |||||
| rs149229175 | Indel (CTC/Δ) intron | n = 3410 Δ = 0.32 | n = 18,142 Δ = 0.13 | n = 164 Δ = 0.08 | Diverse populations PMID: 30297863 | %SV200: Δ > CTC |
| SV200, SV183, SV182, and SV154 | Splice variants with different C-terminals | Diverse populations PMID: 18945781 | Viral restriction: SV200 (HapII) > other variants | |||
| Diverse populations PMID: 30297863 | Antiviral activity: HapII-SV200 > SV182/183 Viral encapsidation: HapII-SV182/183 > SV200 Protease processing: only HapII SV200 L1 fragment in transcript: only HapII SV200 | |||||
| PMID: 27534815 | Cytosine and methyl cytosine deamination: HapI-SV182/183 > SV200 >> SV154 | |||||
| PMID: 31400856 | HBV restriction: HapII SV183 > other variants | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghpour, S.; Khodaee, S.; Rahnama, M.; Rahimi, H.; Ebrahimi, D. Human APOBEC3 Variations and Viral Infection. Viruses 2021, 13, 1366. https://doi.org/10.3390/v13071366
Sadeghpour S, Khodaee S, Rahnama M, Rahimi H, Ebrahimi D. Human APOBEC3 Variations and Viral Infection. Viruses. 2021; 13(7):1366. https://doi.org/10.3390/v13071366
Chicago/Turabian StyleSadeghpour, Shiva, Saeideh Khodaee, Mostafa Rahnama, Hamzeh Rahimi, and Diako Ebrahimi. 2021. "Human APOBEC3 Variations and Viral Infection" Viruses 13, no. 7: 1366. https://doi.org/10.3390/v13071366
APA StyleSadeghpour, S., Khodaee, S., Rahnama, M., Rahimi, H., & Ebrahimi, D. (2021). Human APOBEC3 Variations and Viral Infection. Viruses, 13(7), 1366. https://doi.org/10.3390/v13071366






