West Nile and Usutu Viruses’ Surveillance in Birds of the Province of Ferrara, Italy, from 2015 to 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Area
2.2. Sample Collection
2.3. Samples Analyses
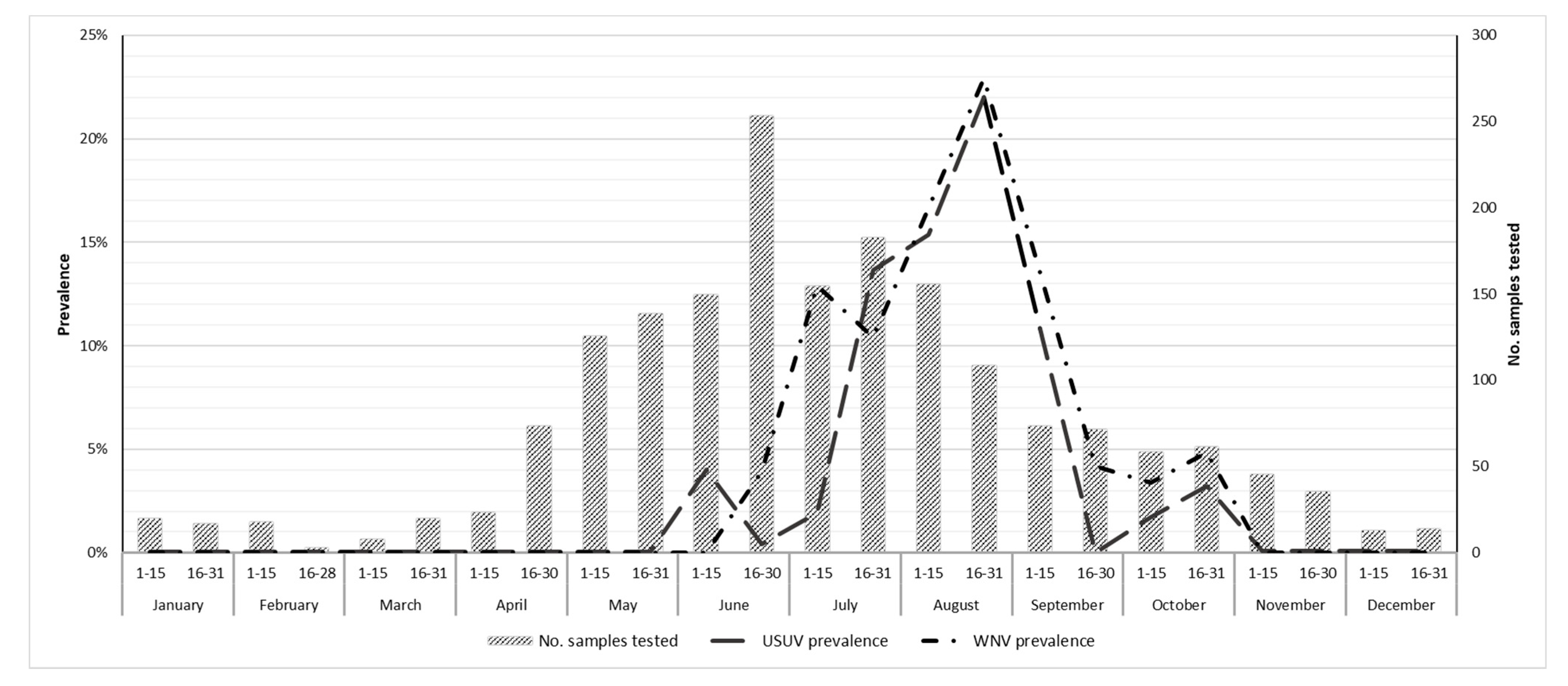
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zannoli, S.; Sambri, V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Domanović, D.; Gossner, C.M.; Lieshout-Krikke, R.; Mayr, W.; Baroti-Toth, K.; Dobrota, A.M.; Escoval, M.A.; Henseler, O.; Jungbauer, C.; Liumbruno, G.; et al. West Nile and Usutu Virus Infections and Challenges to Blood Safety in the European Union. Emerg. Infect. Dis. 2019, 25, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Tamba, M.; Bonilauri, P.; Bellini, R.; Calzolari, M.; Albieri, A.; Sambri, V.; Dottori, M.; Angelini, P. Detection of Usutu Virus within a West Nile Virus Surveillance Program in Northern Italy. Vector Borne Zoonotic Dis. 2011, 11, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzolari, M.; Bonilauri, P.; Bellini, R.; Albieri, A.; Defilippo, F.; Maioli, G.; Galletti, G.; Gelati, A.; Barbieri, I.; Tamba, M.; et al. Evidence of Simultaneous Circulation of West Nile and Usutu Viruses in Mosquitoes Sampled in Emilia-Romagna Region (Italy) in 2009. PLoS ONE 2010, 5, e14324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzolari, M.; Chiapponi, C.; Bonilauri, P.; Lelli, D.; Baioni, L.; Barbieri, I.; Lavazza, A.; Pongolini, S.; Dottori, M.; Moreno, A. Co-Circulation of Two Usutu Virus Strains in Northern Italy between 2009 and 2014. Infect. Genet. Evol. 2017, 51, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Anon. Piano Nazionale Di Prevenzione, Sorveglianza e Risposta Alle Arbovirosi (PNA) 2020–2025. November 2019. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2947_allegato.pdf (accessed on 12 July 2021).
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile Virus Circulation in Emilia-Romagna, Italy: The Integrated Surveillance System 2009. Eurosurveillance 2010. [Google Scholar] [CrossRef]
- Tang, Y.; Anne Hapip, C.; Liu, B.; Fang, C.T. Highly Sensitive TaqMan RT-PCR Assay for Detection and Quantification of Both Lineages of West Nile Virus RNA. J. Clin. Virol. 2006, 36, 177–182. [Google Scholar] [CrossRef]
- Eiden, M.; Vina-Rodriguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two New Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction Assays with Unique Target Sites for the Specific and Sensitive Detection of Lineages 1 and 2 West Nile Virus Strains. J. Vet. Diagn Invest. 2010, 22, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A Novel Quantitative Multiplex Real-Time RT-PCR for the Simultaneous Detection and Differentiation of West Nile Virus Lineages 1 and 2, and of Usutu Virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A Rapid and Specific Real-Time RT-PCR Assay to Identify Usutu Virus in Human Plasma, Serum, and Cerebrospinal Fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef]
- Scaramozzino, N.; Crance, J.M.; Jouan, A.; DeBriel, D.A.; Stoll, F.; Garin, D. Comparison of Flavivirus Universal Primer Pairs and Development of a Rapid, Highly Sensitive Heminested Reverse Transcription-PCR Assay for Detection of Flaviviruses Targeted to a Conserved Region of the NS5 Gene Sequences. J. Clin. Microbiol. 2001, 39, 1922–1927. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.S.; Kerst, A.J.; Nasci, R.S.; Godsey, M.S.; Mitchell, C.J.; Savage, H.M.; Komar, N.; Panella, N.A.; Allen, B.C.; Volpe, K.E.; et al. Rapid Detection of West Nile Virus from Human Clinical Specimens, Field-Collected Mosquitoes, and Avian Samples by a TaqMan Reverse Transcriptase-PCR Assay. J. Clin. Microbiol. 2000, 38, 4066–4071. [Google Scholar] [CrossRef] [Green Version]
- Manarolla, G.; Bakonyi, T.; Gallazzi, D.; Crosta, L.; Weissenböck, H.; Dorrestein, G.M.; Nowotny, N. Usutu Virus in Wild Birds in Northern Italy. Vet. Microbiol. 2010, 141, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Moreno, A.; Brocchi, E.; Sozzi, E.; Capucci, L.; Canelli, E.; Barbieri, I.; Zeller, H.; Cordioli, P. West Nile Virus: Characterization and Diagnostic Applications of Monoclonal Antibodies. Virol. J. 2012, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veo, C.; Della Ventura, C.; Moreno, A.; Rovida, F.; Percivalle, E.; Canziani, S.; Torri, D.; Calzolari, M.; Baldanti, F.; Galli, M.; et al. Evolutionary Dynamics of the Lineage 2 West Nile Virus That Caused the Largest European Epidemic: Italy 2011–2018. Viruses 2019, 11, 814. [Google Scholar] [CrossRef] [Green Version]
- Bellini, R.; Calzolari, M.; Mattivi, A.; Tamba, M.; Angelini, P.; Bonilauri, P.; Albieri, A.; Cagarelli, R.; Carrieri, M.; Dottori, M.; et al. The Experience of West Nile Virus Integrated Surveillance System in the Emilia-Romagna Region: Five Years of Implementation, Italy, 2009 to 2013. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, N.; Young, G.; Ndaluka, C.; Bielefeldt-Ohmann, H.; Komar, N.; Bowen, R. Persistent West Nile Virus Infection in the House Sparrow (Passer domesticus). Arch. Virol. 2009, 154, 783–789. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenböck, H. Limited Pathogenicity of Usutu Virus for the Domestic Goose (Anser anser f. domestica) Following Experimental Inoculation. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 171–175. [Google Scholar] [CrossRef]
- Busquets, N.; Bertran, K.; Costa, T.P.; Rivas, R.; de la Fuente, J.G.; Villalba, R.; Solanes, D.; Bensaid, A.; Majó, N.; Pagès, N. Experimental West Nile Virus Infection in Gyr-Saker Hybrid Falcons. Vector Borne Zoonotic Dis. 2012, 12, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Angenvoort, J.; Fischer, D.; Fast, C.; Eiden, M.; Rodriguez, A.V.; Revilla-Fernández, S.; Nowotny, N.; de la Fuente, J.G.; Lierz, M.; et al. Pathogenesis of West Nile Virus Lineage 1 and 2 in Experimentally Infected Large Falcons. Vet. Microbiol. 2013, 161, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenböck, H. Limited Pathogenicity of Usutu Virus for the Domestic Chicken (Gallus domesticus). Avian Pathol. 2005, 34, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamino, V.; Escribano-Romero, E.; Blázquez, A.-B.; Gutiérrez-Guzmán, A.-V.; Martín-Acebes, M.-Á.; Saiz, J.-C.; Höfle, U. Experimental North American West Nile Virus Infection in the Red-Legged Partridge (Alectoris rufa). Vet. Pathol. 2016, 53, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzarti, E.; Rivas, J.; Sarlet, M.; Franssen, M.; Desmecht, D.; Schmidt-Chanasit, J.; Savini, G.; Lorusso, A.; Van Laere, A.-S.; Garigliany, M.-M. Experimental Usutu Virus Infection in Domestic Canaries Serinus Canaria. Viruses 2020, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, E.K.; Lund, M.; Shearn Bochsler, V. West Nile Virus Infection in American Singer Canaries: An Experimental Model in a Highly Susceptible Avian Species. Vet. Pathol. 2018, 55, 531–538. [Google Scholar] [CrossRef]
- Del Amo, J.; Llorente, F.; Figuerola, J.; Soriguer, R.C.; Moreno, A.M.; Cordioli, P.; Weissenböck, H.; Jiménez-Clavero, M.A. Experimental Infection of House Sparrows (Passer domesticus) with West Nile Virus Isolates of Euro-Mediterranean and North American Origins. Vet. Res. 2014, 45, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez de Oya, N.; Camacho, M.-C.; Blázquez, A.-B.; Lima-Barbero, J.-F.; Saiz, J.-C.; Höfle, U.; Escribano-Romero, E. High Susceptibility of Magpie (Pica pica) to Experimental Infection with Lineage 1 and 2 West Nile Virus. PLoS Negl. Trop. Dis. 2018, 12, e0006394. [Google Scholar] [CrossRef]
- Lim, S.M.; Brault, A.C.; van Amerongen, G.; Bosco-Lauth, A.M.; Romo, H.; Sewbalaksing, V.D.; Bowen, R.A.; Osterhaus, A.D.M.E.; Koraka, P.; Martina, B.E.E. Susceptibility of Carrion Crows to Experimental Infection with Lineage 1 and 2 West Nile Viruses. Emerg. Infect. Dis. 2015, 21, 1357–1365. [Google Scholar] [CrossRef]
- Dridi, M.; Vangeluwe, D.; Lecollinet, S.; van den Berg, T.; Lambrecht, B. Experimental Infection of Carrion Crows (Corvus corone) with Two European West Nile Virus (WNV) Strains. Vet. Microbiol. 2013, 165, 160–166. [Google Scholar] [CrossRef]
- Lim, S.M.; Brault, A.C.; van Amerongen, G.; Sewbalaksing, V.D.; Osterhaus, A.D.M.E.; Martina, B.E.E.; Koraka, P. Susceptibility of European Jackdaws (Corvus monedula) to Experimental Infection with Lineage 1 and 2 West Nile Viruses. J. Gen. Virol. 2014, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Hahn, D.C.; Gould, D.H.; Bowen, R.A. Experimental West Nile Virus Infection in Eastern Screech Owls (Megascops asio). Avian Dis. 2006, 50, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Pierro, A.M.; Cavrini, F.; Rossini, G.; Landini, M.P.; Sambri, V. False-Positive Transcription-Mediated Amplification Assay Detection of West Nile Virus in Blood from a Patient with Viremia Caused by an Usutu Virus Infection. J. Clin. Microbiol. 2010, 48, 3338–3339. [Google Scholar] [CrossRef] [Green Version]
- Benzarti, E.; Sarlet, M.; Franssen, M.; Cadar, D.; Schmidt-Chanasit, J.; Rivas, J.F.; Linden, A.; Desmecht, D.; Garigliany, M. Usutu Virus Epizootic in Belgium in 2017 and 2018: Evidence of Virus Endemization and Ongoing Introduction Events. Vector Borne Zoonotic Dis. 2020, 20, 43–50. [Google Scholar] [CrossRef]
- Michel, F.; Sieg, M.; Fischer, D.; Keller, M.; Eiden, M.; Reuschel, M.; Schmidt, V.; Schwehn, R.; Rinder, M.; Urbaniak, S.; et al. Evidence for West Nile Virus and Usutu Virus Infections in Wild and Resident Birds in Germany, 2017 and 2018. Viruses 2019, 11, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Order | Common Name | Scientific Name | Migration Pattern | No. Tested Birds | No. USUV Positive (%) | No. WNV Positive (%) | No. Co-Infected (%) |
|---|---|---|---|---|---|---|---|
| Accipitriformes | Common Buzzard | Buteo buteo | R,P,S | 36 | 0 (0%) | 0 (0%) | 0 (0%) |
| Eurasian Sparrowhawk | Accipiter nisus | L | 7 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Western Marsh Harrier | Circus aeruginosus | L | 3 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Subtotal | 47 | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Anseriformes | Mallard | Anas platyrhynchos | R,P,S | 7 | 1 (14.3%) | 0 (0%) | 0 (0%) |
| Other 4 species * | 7 | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Subtotal | 14 | 1 (7.1%) | 0 (0%) | 0 (0%) | |||
| Apodiformes | Common Swift | Apus apus | L | 145 | 7 (4.8%) | 7 (4.8%) | 3 (2.1%) |
| Charadriiformes | European Herring Gull | Larus argentatus | R,P,S,L | 46 | 3 (6.5%) | 3 (6.5%) | 1 (2.2%) |
| Common Tern | Sterna hirundo | L | 1 | 0 (0%) | 1 (100%) | 0 (0%) | |
| Other 4 species * | 11 | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Subtotal | 58 | 3 (5.2%) | 3 (5.2%) | 1 (1.7%) | |||
| Ciconiiformes | Cattle Egret | Bubulcus ibis | L | 7 | 1 (14.3%) | 0 (0%) | 0 (0%) |
| Purple Heron | Ardea purpurea | L | 5 | 1 (20%) | 0 (0%) | 0 (0%) | |
| Other 5 species * | 20 | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Subtotal | 32 | 2 (6.2%) | 0 (0%) | 0 (0%) | |||
| Columbiformes | Collared Dove | Streptopelia decaocto | R | 257 | 11 (4.3%) | 18 (7%) | 3 (1.2%) |
| Common Wood Pigeon | Columba palumbus | R,P,S | 88 | 17 (19.3%) | 17 (19.3%) | 7 (7.9%) | |
| Subtotal | 345 | 27 (7.8%) | 35 (10.1%) | 10 (2.9%) | |||
| Coraciiformes | European Bee-eater | Merops apiaster | L | 7 | 0 (0%) | 0 (0%) | 0 (0%) |
| Cuculiformes | Cukoo | Cuculus canorus | L | 4 | 0 (0%) | 0 (0%) | 0 (0%) |
| Falconiformes | Common Kestrel | Falco tinnunculus | R,P,S | 146 | 3 (2.1% | 9 (6.2%) | 2 (1.4%) |
| Eurasian Hobby | Falco subbuteo | L | 3 | 0 (0%) | 1 (33.3%) | 0 (0%) | |
| Red-footed Falcon | Falco vespertinus | M | 1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Subtotal | 150 | 3 (2.0%) | 10 (6.7%) | 2 (1.3%) | |||
| Galliformes | Ring-necked Pheasant | Phasianus colchicus | R | 16 | 1 (6.3%) | 2 (12.5%) | 1 (6.3%) |
| Gruiformes | Common Moorhen | Gallinula chloropus | R | 25 | 1 (4%) | 0 (0%) | 0 (0%) |
| Water-cheeked Rail | Rallus aquaticus | M | 1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Subtotal | 26 | 1 (3.8%) | 0 (0%) | 0 (0%) | |||
| Passeriformes | European Blackbird | Turdus merula | R,P | 184 | 24 (13%) | 8 (4.3%) | 5 (2.7%) |
| Eurasian Magpie | Pica pica | R | 166 | 4 (2.4%) | 2 (1.2%) | 0 (0%) | |
| Eurasian Jay | Garrulus glandarius | R,P | 81 | 2 (2.5%) | 3 (3.7%) | 0 (0%) | |
| Common Starling | Sturnus vulgaris | R,P,S | 64 | 1 (1.6%) | 1 (1.6%) | 0 (0%) | |
| Barn Swallow | Hirundo rustica | L | 43 | 2 (4.7%) | 5 (11.6%) | 0 (0%) | |
| Great Tit | Parus major | R,S | 40 | 2 (5%) | 2 (5%) | 2 (5%) | |
| Hooded Crow | Corvus cornix | R | 40 | 0 (0%) | 3 (7.5%) | 0 (0%) | |
| House Sparrow | Passer domesticus | R | 34 | 1 (2.9%) | 5 (14.7%) | 0 (0%) | |
| Common House Martin | Delichon urbicum | L | 15 | 1 (6.7%) | 3 (20%) | 1 (6.7%) | |
| European Goldfinch | Carduelis carduelis | L | 14 | 2 (14.3%) | 1 (7.1%) | 0 (0%) | |
| European Greenfinch | Carduelis chloris | L | 12 | 0 (0%) | 4 (33.3%) | 0 (0%) | |
| Eurasian Reed Warbler | Acrocephalus scirpaceus | L | 6 | 1 (16.7%) | 0 (0%) | 0 (0%) | |
| Eurasian Golden Oriole | Oriolus oriolus | L | 4 | 1 (25%) | 0 (0%) | 0 (0%) | |
| Blue Tit | Cyanistes caeruleus | L | 3 | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | |
| European Serin | Serinus serinus | L | 3 | 1 (33.3%) | 0 (0%) | 0 (0%) | |
| European Pied Flycatcher | Ficedula hypoleuca | L | 2 | 0 (0%) | 1 (50%) | 0 (0%) | |
| Common Redstart | Phoenicurus phoenicurus | L | 1 | 0 (0%) | 1 (100%) | 0 (0%) | |
| Other 22 species * | 82 | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Subtotal | 794 | 43 (5.4%) | 40 (5.0%) | 9 (1.1%) | |||
| Pelecaniformes | Great Cormorant | Phalacrocorax carbo | L | 5 | 0 (0%) | 0 (0%) | 0 (0%) |
| Phoenicopteriformes | Flamingo | Phoenicopterus roseus | L | 3 | 0 (0%) | 0 (0%) | 0 (0%) |
| Piciformes | European Green Woodpecker | Picus viridis | R,P | 48 | 0 (0%) | 1 (2.1%) | 0 (0%) |
| Great Spotted Woodpecker | Dendrocopos Major | R,M | 2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Eurasian Wryneck | Jynx torquilla | R,M | 1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Subtotal | 51 | 0 (0%) | 1 (2.0%) | 0 (0%) | |||
| Podicipediformes | Great Crested Grebe | Podiceps cristatus | L | 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| Strigiformes | Barn Owl | Tyto alba | R | 11 | 0 (0%) | 1 (9.1%) | 0 (0%) |
| Little Owl | Athene noctua | R | 84 | 3 (3.6%) | 13 (15.5%) | 1 (1.2%) | |
| Long-eared Owl | Asio otus | R,P,S | 35 | 0 (0%) | 2 (5.7%) | 0 (0%) | |
| Eurasian scops Owl | Otus scops | L | 14 | 1 (7.1%) | 3 (21.4%) | 1 (7.1%) | |
| Tawny Owl | Strix aluco | R | 1 | 1 (100%) | 0 (0%) | 0 (0%) | |
| Subtotal | 145 | 5 (3.4%) | 20 (13.8) | 2 (1.4%) | |||
| Total | 1842 | 94 (4.5%) | 118 (6.5%) | 28 (1.5%) |
| Order | Common Name | Scientific Name | Virus Used | Infection Route | Dose | Died/Infected (%) | Mean No. Days to Death (Range) | Maximum Viral Persistence in Organs | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Accipitriformes | American Kestrel | Falco sparverius | WNV 1 | MB, OS | Various * | 0/5 (0%) | - | 11 d.p.i. | [20] |
| Anseriformes | Domestic Goose | Anser anser domesticus | USUV 1 | IM | 5 × 104 TCID50 | 5/11 (45%) | 4.6 (2–16) | 16 d.p.i. | [21] |
| Charadriformes | Killdeer | Charadrius vociferus | WNV 1 | MB, OS | 5–15 mosquitoes * | 0/2 (0%) | - | 10 d.p.i. | [20] |
| Columbiformes | Mourning Dove | Zenaida macroura | WNV 1 | MB, OS | Various * | 0/6 (0%) | - | 11 d.p.i. | [20] |
| Falconiformes | Gyr-Saker hibrid falcon | Falco rusticolus x Falco cherrug | WNV 1 | SC | 104 TCID50 | 0/6 (0%) | - | 14 d.p.i. | [22] |
| Gyrfalcon and hibrid falcon | Falco rusticolus | WNV 1 | SC | 500 TCID50, 104 TCID50, 106 TCID50 | 4/12 (30%) | 9.75 (8–12) | 21 d.p.i. | [23] | |
| Galliformes | Domestic Chicken | Gallus domesticus | USUV 1 | IV | 103 TCID50 | 0/18 (0%) | - | 7 d.p.i. | [24] |
| Japanese Quail | Coturnix japonica | WNV 1 | MB, OS | Various * | 0/6 (0%) | - | 14 d.p.i. | [20] | |
| Red-legged Partridge | Alectoris rufa | WNV 1 | SC | 107 PFU | 3/13 (23%) | 5.7 (2–8) | 10 d.p.i. | [25] | |
| Passeriformes | Domestic Canary | Serinus canaria domestica | USUV 2 | IP | 103 TCID50; 106 TCID50 | 3/10 (30%) | 7 (5–9) | NR | [26] |
| Domestic Canary | Serinus canaria domestica | WNV | SC | 105 PFU,102 PFU,10 PFU | 0/23 (0%) | - | 5 d.p.i. | [27] | |
| House Sparrow | Passer domesticus | WNV 1 | MB, OS | Various * | 3/15 (20%) | 4.7 (3–6) | 10 d.p.i. | [20] | |
| House Sparrow | Passer domesticus | WNV 1 | SC | 105 PFU | 5/24 (21%) | 5.6 (4–7) | 21 d.p.i. | [28] | |
| House Sparrow | Passer domesticus | WNV 1 | SC | 1000–4000 PFU | 19/150 (13%) | (8–354) | 65 d.p.i. | [19] | |
| Red-winged Blackbird | Agelaius phoeniceus | WNV 1 | MB, OS | 5–15 inf. Mosquitoes * | 0/4 (0%) | - | 11 d.p.i. | [20] | |
| Common Grackle | Quiscalus quiscula | WNV 1 | MB, OS | Various * | 2/12 (17%) | 4.5 (4–5) | 10 d.p.i. | [20] | |
| Fish Crow | Corvus ossifragus | WNV 1 | MB, OS | Various * | 5/20 (25%) | 9.6 (6–13) | 9 d.p.i. | [20] | |
| Blue Jay | Cyanocitta cristata | WNV 1 | MB, OS | Various * | 3/6 (50%) | 4.7 (4–5) | 9 d.p.i. | [20] | |
| Eurasian Magpie | Pica pica | WNV 1,2 | SC | 5000 PFU | 13/19 (68%) | 6.6 (6–8) | 17 d.p.i. | [29] | |
| Carrion Crows | Corvus corone | WNV 1,2 | NR | 2000 TCID50 | 22/30 (73%) | 7.3 (6–9) | 14 d.p.i. | [30] | |
| Carrion Crows | Corvus corone | WNV 1 | SC | 1500 PFU | 8/12 (67%) | 6.9 (5–10) | 10 d.p.i. | [31] | |
| European Jackdaws | Corvus monedula | WNV 1,2 | NR | 2000 TCID50 | 11/26 (42%) | 7.3 (5–9) | 14 d.p.i. | [32] | |
| Psittaciformes | Budgerigar | Melopsittacus undulatus | WNV 1 | MB, OS | Various * | 0/6 (0%) | - | 13 d.p.i. | [20] |
| Strigiformes | Eastern Screech Owl | Megascops asio | WNV 1 | SC, OS | 1000–2000 PFU, 1 inf. mouse | 2/9 (22%) | 8.5 (8–9) | 14 d.p.i. | [33] |
| Birds | Equids | Human Beings | ||||
|---|---|---|---|---|---|---|
| Year | Total WNV Positive Birds | Date of Death of the First Positive Bird | Total Cases of WNV Disease | Date of Onset of Signs of the First Case | Total Cases of WNV Neurological Disease | Date of Onset of Signs of The First Case |
| 2015 | 14 | 20 July 2015 | 0 | 1 | 13 August 2015 | |
| 2016 | 6 | 6 August 2016 | 0 | 2 | 18 July 2016 | |
| 2017 | 18 | 17 August 2017 | 0 | 2 | 4 August 2017 | |
| 2018 | 45 | 4 June 2018 | 0 | 14 | 18 July 2018 | |
| 2019 | 11 | 22 July 2019 | 0 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauriano, A.; Rossi, A.; Galletti, G.; Casadei, G.; Santi, A.; Rubini, S.; Carra, E.; Lelli, D.; Calzolari, M.; Tamba, M. West Nile and Usutu Viruses’ Surveillance in Birds of the Province of Ferrara, Italy, from 2015 to 2019. Viruses 2021, 13, 1367. https://doi.org/10.3390/v13071367
Lauriano A, Rossi A, Galletti G, Casadei G, Santi A, Rubini S, Carra E, Lelli D, Calzolari M, Tamba M. West Nile and Usutu Viruses’ Surveillance in Birds of the Province of Ferrara, Italy, from 2015 to 2019. Viruses. 2021; 13(7):1367. https://doi.org/10.3390/v13071367
Chicago/Turabian StyleLauriano, Alessandra, Arianna Rossi, Giorgio Galletti, Gabriele Casadei, Annalisa Santi, Silva Rubini, Elena Carra, Davide Lelli, Mattia Calzolari, and Marco Tamba. 2021. "West Nile and Usutu Viruses’ Surveillance in Birds of the Province of Ferrara, Italy, from 2015 to 2019" Viruses 13, no. 7: 1367. https://doi.org/10.3390/v13071367
APA StyleLauriano, A., Rossi, A., Galletti, G., Casadei, G., Santi, A., Rubini, S., Carra, E., Lelli, D., Calzolari, M., & Tamba, M. (2021). West Nile and Usutu Viruses’ Surveillance in Birds of the Province of Ferrara, Italy, from 2015 to 2019. Viruses, 13(7), 1367. https://doi.org/10.3390/v13071367









