Molecular Biology and Pathological Process of an Infectious Bronchitis Virus with Enteric Tropism in Commercial Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Whole Genome Sequencing
2.3. Experimental Design
2.4. Serology
2.5. RT-qPCR
2.6. Histopathology and Immunohistochemistry
2.7. Statistical Analyses
3. Results
3.1. Whole Genome Analyses
3.2. Maternal Antibodies
3.3. Clinical Signs
3.4. Viral Shedding
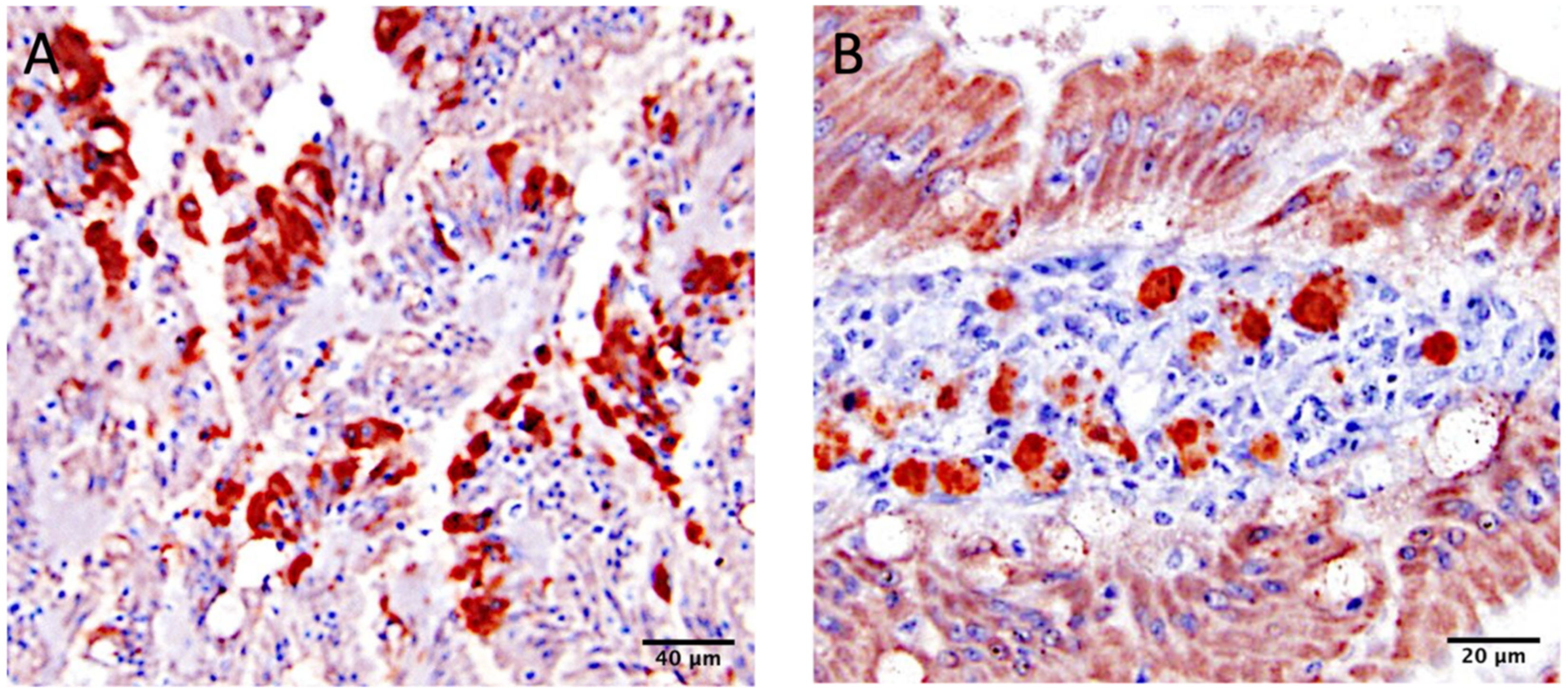
3.5. Histopathology and Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alvarado, I.R.; Villegas, P.; El-Attrache, J.; Jackwood, M.W. Detection of Massachusetts and Arkansas serotypes of infectious bronchitis virus in broilers. Avian Dis. 2006, 50, 292–297. [Google Scholar] [CrossRef]
- Lucio, B.; Fabricant, J. Tissue Tropism of Three Cloacal Isolates and Massachusetts Strain of Infectious Bronchitis Virus. Avian Dis. 1990, 34, 865–870. [Google Scholar] [CrossRef]
- Rimondi, A.; Craig, M.I.; Vagnozzi, A.; König, G.; Delamer, M.; Pereda, A. Molecular characterization of avian infectious bronchitis virus strains from outbreaks in Argentina (2001–2008). Avian Pathol. J. WVPA 2009, 38, 149–153. [Google Scholar] [CrossRef]
- Wentworth, D.E.; Holmes, K.V. Coronavirus binding and entry. In Coronaviruses: Molecular and Cellular Biology; Thiel, V., Ed.; Caister Academic Press: Norfulk, UK, 2007. [Google Scholar]
- Breslin, J.J.; Smith, L.G.; Barnes, H.J.; Guy, J.S. Comparison of Virus Isolation, Immunohistochemistry, and Reverse Transcriptase-Polymerase Chain Reaction Procedures for Detection of Turkey Coronavirus. Avian Dis. 2000, 44, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D. A nomenclature for avian coronavirus isolates and the question of species status. Avian Pathol. J. WVPA 2001, 30, 109–115. [Google Scholar] [CrossRef][Green Version]
- Guy, J.S.; Barnes, H.J.; Smith, L.G.; Breslin, J. Antigenic characterization of a turkey coronavirus identified in poult enteritis- and mortality syndrome-affected turkeys. Avian Dis. 1997, 41, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Loa, C.C.; Wu, C.C. Complete sequences of 3′ end coding region for structural protein genes of turkey coronavirus. Virus Res. 2004, 106, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ambali, A.G.; Jones, R.C. Early pathogenesis in chicks of infection with an enterotropic strain of infectious bronchitis virus. Avian Dis. 1990, 34, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Ambali, A.G. Recent studies on the enterotropic strain of avian infectious bronchitis virus. Vet. Res. Commun. 1992, 16, 153–157. [Google Scholar] [CrossRef] [PubMed]
- El-Houadfi, M.; Jones, R.C.; Cook, J.K.A.; Ambali, A.G. The isolation and Characterisation of six avian infectious bronchitis viruses isolated in morocco. Avian Pathol. 1986, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Chacón, J.L.; Assayag, M.S.; Revolledo, L.; Astolfi-Ferreira, C.S.; Vejarano, M.P.; Jones, R.C.; Piantino Ferreira, A.J. Pathogenicity and molecular characteristics of infectious bronchitis virus (IBV) strains isolated from broilers showing diarrhoea and respiratory disease. Br. Poult. Sci. 2014, 55, 271–283. [Google Scholar] [CrossRef]
- Montgomery, R.D.; Boyle, C.R.; Maslin, W.R.; Magee, D.L. Attempts to reproduce a runting/stunting-type syndrome using infectious agents isolated from affected Mississippi broilers. Avian Dis 1997, 41, 80–92. [Google Scholar] [CrossRef]
- Hauck, R.; Gallardo, R.A.; Woolcock, P.R.; Shivaprasad, H.L. A Coronavirus Associated with Runting Stunting Syndrome in Broiler Chickens. Avian Dis. 2016, 60, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; Stephens, C. Virus isolation and propagation in embryonating eggs. In A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens; Williams, S.M., Dufour-Zavala, L.D.E.S., Jackwood, M.W., Lee, M.D., Lupiani, B., Reed, W.M., Spackman, E., Woolcock, P.R., Eds.; American Association of Avian Pathologists (AAAP): Jacksonville, FL, USA, 2016. [Google Scholar]
- Villegas, P. Titration of biological suspensions. In A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens; Williams, S.M., Dufour-Zavala, L.D.E.S., Jackwood, M.W., Lee, M.D., Lupiani, B., Reed, W.M., Spackman, E., Woolcock, P.R., Eds.; American Association of Avian Pathologists (AAAP): Jacksonville, FL, USA, 2016; pp. 355–360. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; van Santen, V.L.; Li, L.; Lockaby, S.B.; van Santen, E.; Hoerr, F.J. Epidemiological and experimental evidence for immunodeficiency affecting avian infectious bronchitis. Avian Pathol. 2006, 35, 455–464. [Google Scholar] [CrossRef]
- de Quadros, V.L. Das Infektiöse Bronchitis Virus (IBV): Molekularbiologische Untersuchungen zur Diagnostik und zum Vorkommen Sowie zur Pathogenität des Genotyps IBV QX in Spezifisch Pathogenfreien (SPF) Broilern. Ph.D. Dissertation, Freien Universität Berlin, Berlin, Germany, 2012. [Google Scholar]
- da Silva, A.P.; Hauck, R.; Zhou, H.; Gallardo, R.A. Understanding Immune Resistance to Infectious Bronchitis Using Major Histocompatibility Complex Chicken Lines. Avian Dis. 2017, 61, 358–365. [Google Scholar] [CrossRef]
- França, M.; Woolcock, P.R.; Yu, M.; Jackwood, M.W.; Shivaprasad, H.L. Nephritis associated with infectious bronchitis virus Cal99 variant in game chickens. Avian Dis. 2011, 55, 422–428. [Google Scholar] [CrossRef]
- Naqi, S.A. A monoclonal antibody-based immunoperoxidase procedure for rapid detection of infectious bronchitis virus in infected tissues. Avian Dis. 1990, 34, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Karaca, K.; Naqi, S.; Gelb, J., Jr. Production and characterization of monoclonal antibodies to three infectious bronchitis virus serotypes. Avian Dis. 1992, 36, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Goraichuk, I.V.; Davis, J.F.; Kulkarni, A.B.; Afonso, C.L.; Suarez, D.L. Complete Genome Sequence of Avian Coronavirus Strain GA08 (GI-27 Lineage). Microbiol. Resour. Announc. 2020, 9, e00068-20. [Google Scholar] [CrossRef]
- Ismail, M.I.; Tan, S.W.; Hair-Bejo, M.; Omar, A.R. Evaluation of the antigen relatedness and efficacy of a single vaccination with different infectious bronchitis virus strains against a challenge with Malaysian variant and QX-like IBV strains. J. Vet. Sci. 2020, 21, e76. [Google Scholar] [CrossRef]
- Hassan, M.S.H.; Ojkic, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) Variants Isolated in Eastern Canada Show Evidence of Recombination. Viruses 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Casais, R.; Dove, B.; Cavanagh, D.; Britton, P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003, 77, 9084. [Google Scholar] [CrossRef]
- Martin, M.P.; Wakenell, P.S.; Woolcock, P. Evaluation of commercially produced infectious bronchitis virus vaccines against an IBV field isolate obtaned from broilers in California. In Proceedings of the Fiftieth Western Poultry Disease Conference, Sacramento, CA, USA, 24–26 March 2001. [Google Scholar]
- Gallardo, R.A.; Aleuy, O.A.; Pitesky, M.; Senties-Cue, G.; Abdelnabi, A.; Woolcock, P.R.; Hauck, R.; Toro, H. Variability assessment of California infectious bronchitis virus variants. Avian Dis. 2016, 60, 424–429. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Lee, D.H. Different evolutionary trajectories of vaccine-controlled and non-controlled avian infectious bronchitis viruses in commercial poultry. PLoS ONE 2017, 12, e0176709. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Saiada, F.; Gallardo, R.A.; Shivaprasad, H.L.; Corsiglia, C.; Van Santen, V.L. Intestinal tropism of an infectious bronchitis virus isolate not explained by spike protein binding specificity. Avian Dis. 2020, 64, 23–35. [Google Scholar] [CrossRef]
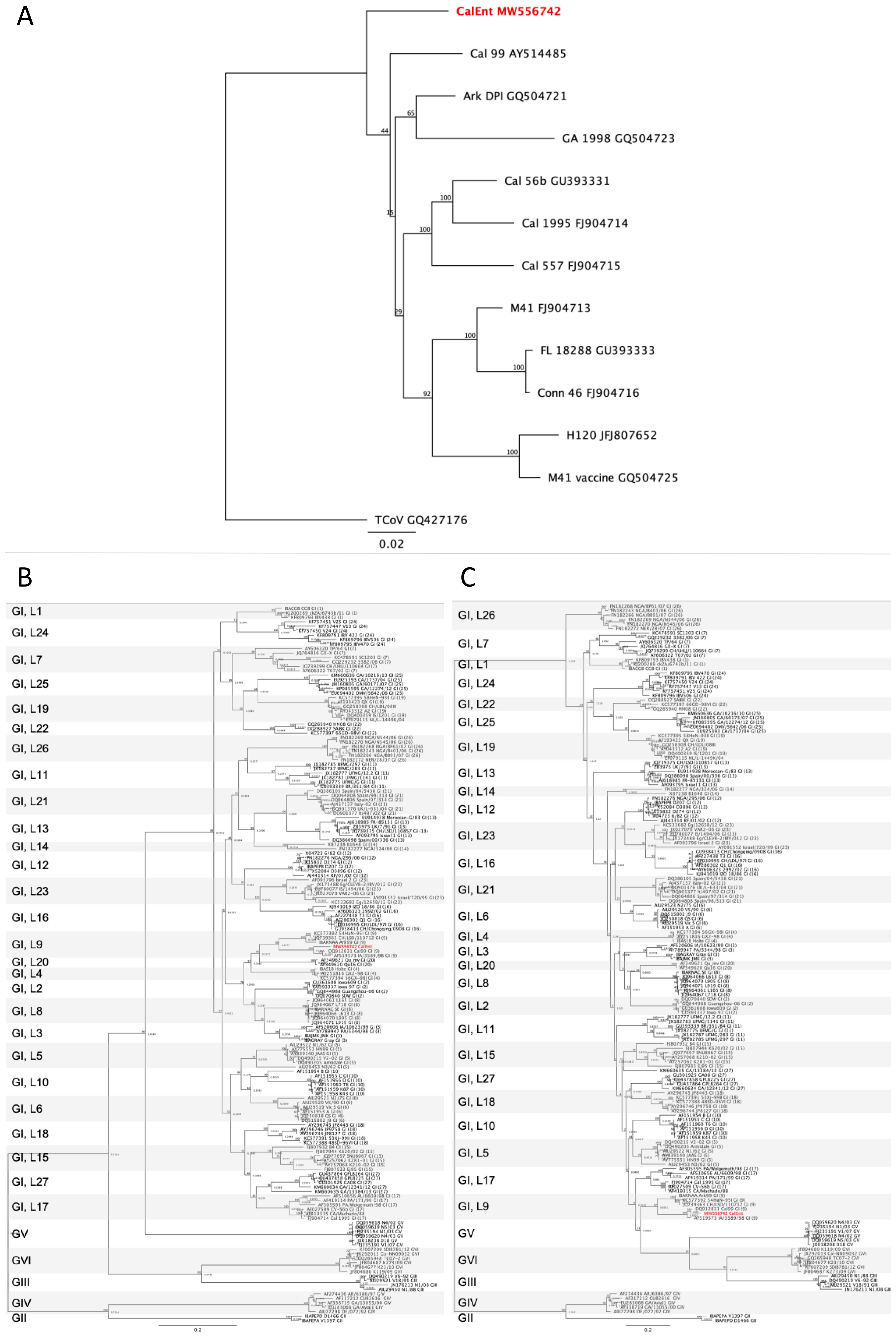
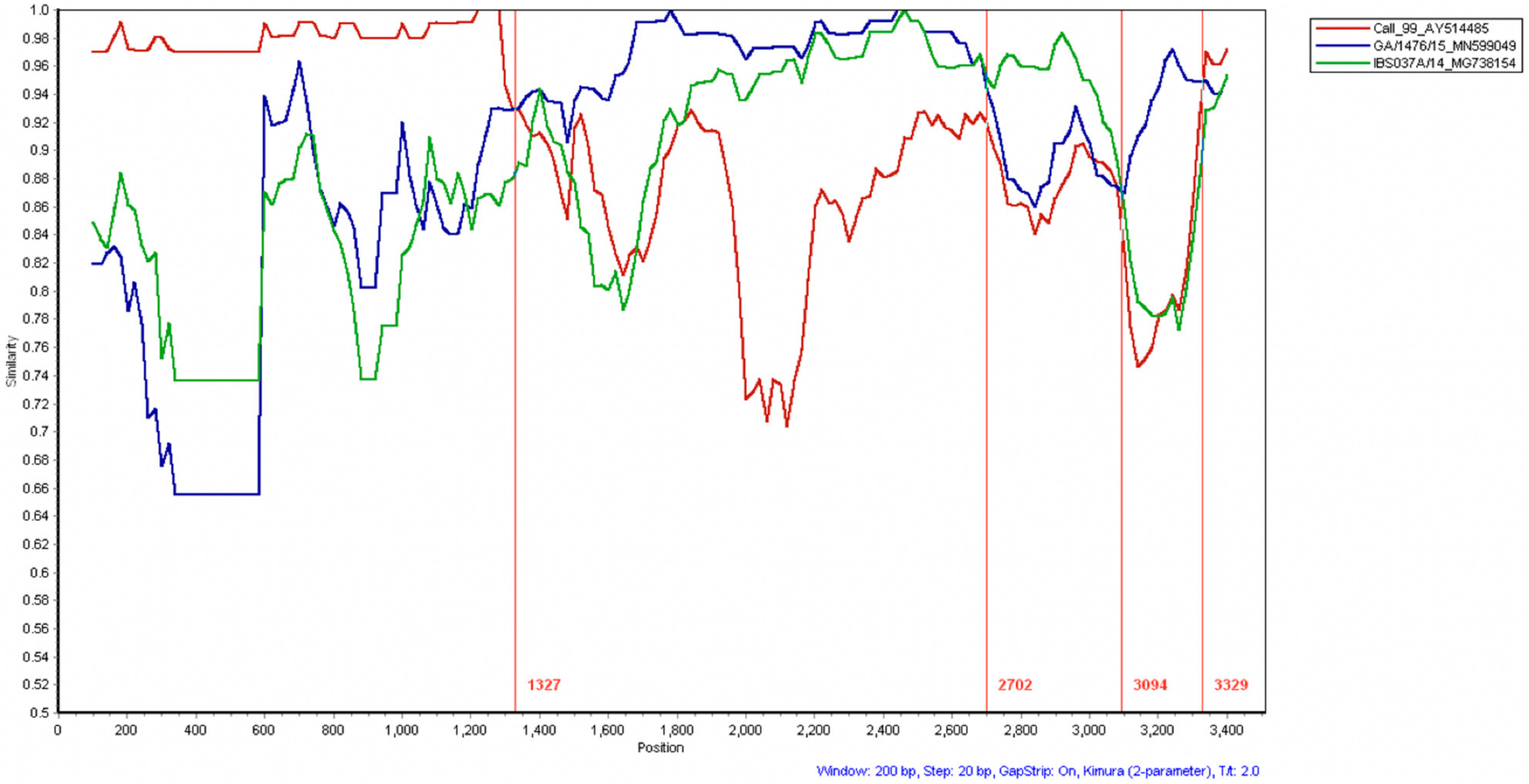
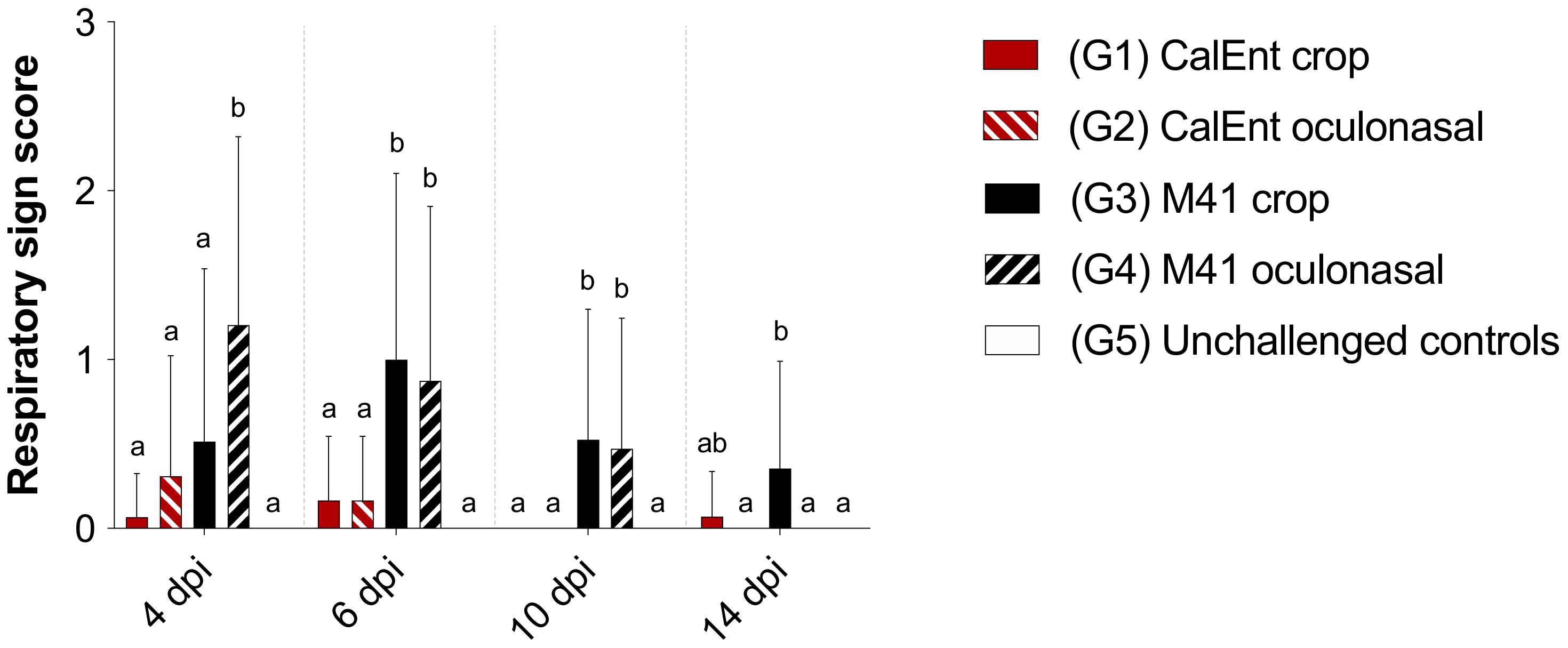
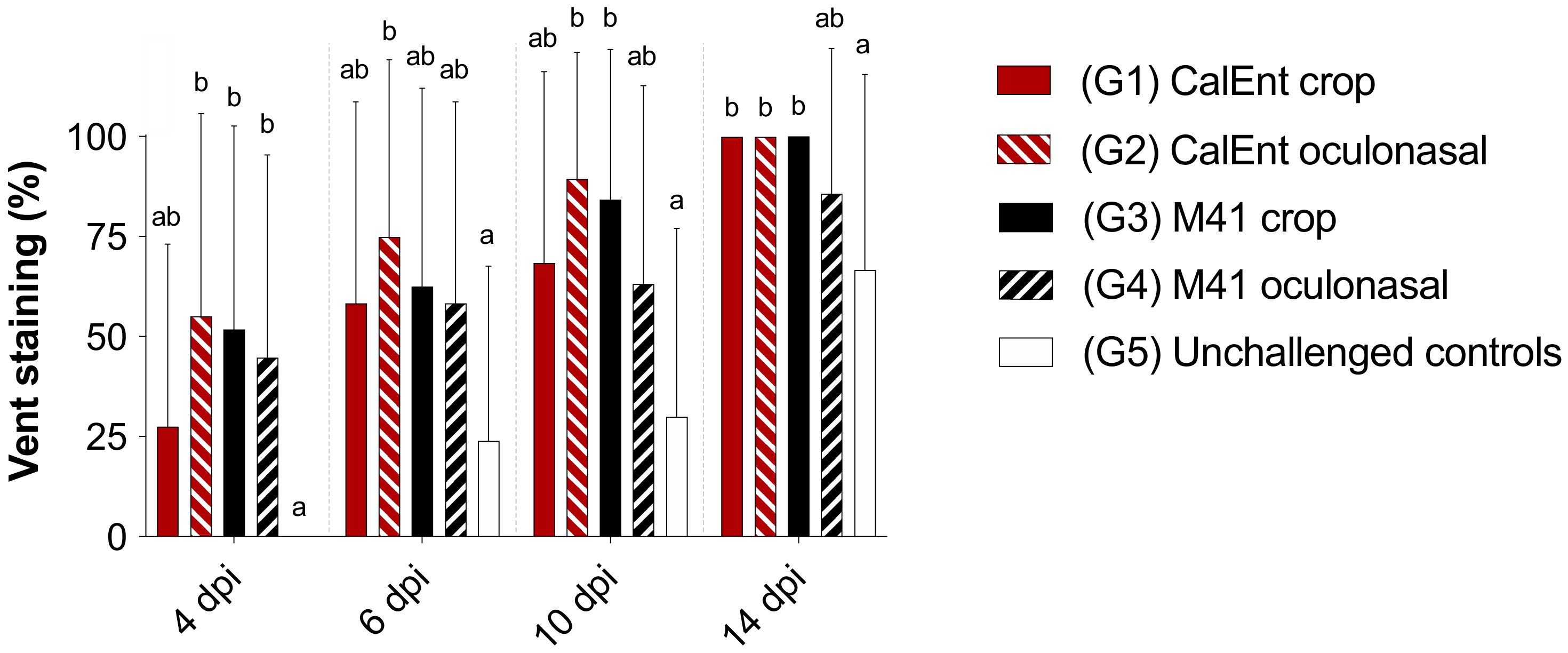

| Group | Virus Strain | Infectious Route | No. of Birds at Challenge 1 |
|---|---|---|---|
| 1 | CalEnt | Crop gavage | 38 |
| 2 | CalEnt | Oculonasal | 39 |
| 3 | M41 | Crop gavage | 39 |
| 4 | M41 | Oculonasal | 40 |
| 5 | Uninfected | --- | 40 |
| Gene | Ark DPI | Cal99 | Cal 557 | Cal 56b | Cal95 | Conn46 | FL18288 | M41 | H120 | M41vac | GA98 | TCoV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spike | 84.6 | 88.4 | 83.6 | 83.2 | 83.4 | 80.47 | 80.2 | 80.8 | 80.8 | 80.8 | 67.2 | 47.5 |
| Envelope | 91.6 | 90.4 | 80.2 | 77.2 | 79.0 | 91.89 | 90.1 | 91.0 | 86.5 | 86.5 | 88.6 | 88.0 |
| 3a | 97.1 | 90.2 | 93.1 | 89.7 | 90.2 | 90.23 | 89.7 | 90.2 | 85.1 | 85.1 | 94.8 | 89.7 |
| Matrix | 95.2 | 95.3 | 91.6 | 89.1 | 89.3 | 95.15 | 95.0 | 95.2 | 90.5 | 90.3 | 89.7 | 94.3 |
| 3b | 96.9 | 96.4 | 91.3 | 93.3 | 91.3 | 97.44 | 96.4 | 97.4 | 83.1 | 82.8 | 94.4 | 95.9 |
| 1a | 94.3 | 93.8 | 94.2 | 95.6 | 94.7 | 93.77 | 93.7 | 95.6 | 93.8 | 93.5 | 95.6 | 94.1 |
| 1ab | 95.1 | 95.2 | 95.1 | 96.3 | 95.3 | 95.34 | 95.3 | 95.8 | 93.8 | 93.9 | 96.0 | 95.0 |
| 5a | 98.0 | 98.0 | 96.7 | 91.4 | 89.9 | 97.98 | 97.5 | 98.0 | 92.4 | 93.7 | 97.5 | 95.0 |
| Nucleocapsid | 98.7 | 95.1 | 94.5 | 98.7 | 94.1 | 99.07 | 98.7 | 99.1 | 93.4 | 95.2 | 99.0 | 95.3 |
| 5b | 98.4 | 98.0 | 97.6 | 98.8 | 98.8 | 99.20 | 99.2 | 98.4 | 97.2 | 97.8 | 99.2 | 96.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.P.; Hauck, R.; Nociti, S.R.C.; Kern, C.; Shivaprasad, H.L.; Zhou, H.; Gallardo, R.A. Molecular Biology and Pathological Process of an Infectious Bronchitis Virus with Enteric Tropism in Commercial Broilers. Viruses 2021, 13, 1477. https://doi.org/10.3390/v13081477
da Silva AP, Hauck R, Nociti SRC, Kern C, Shivaprasad HL, Zhou H, Gallardo RA. Molecular Biology and Pathological Process of an Infectious Bronchitis Virus with Enteric Tropism in Commercial Broilers. Viruses. 2021; 13(8):1477. https://doi.org/10.3390/v13081477
Chicago/Turabian Styleda Silva, Ana P., Ruediger Hauck, Sabrina R. C. Nociti, Colin Kern, H. L. Shivaprasad, Huaijun Zhou, and Rodrigo A. Gallardo. 2021. "Molecular Biology and Pathological Process of an Infectious Bronchitis Virus with Enteric Tropism in Commercial Broilers" Viruses 13, no. 8: 1477. https://doi.org/10.3390/v13081477
APA Styleda Silva, A. P., Hauck, R., Nociti, S. R. C., Kern, C., Shivaprasad, H. L., Zhou, H., & Gallardo, R. A. (2021). Molecular Biology and Pathological Process of an Infectious Bronchitis Virus with Enteric Tropism in Commercial Broilers. Viruses, 13(8), 1477. https://doi.org/10.3390/v13081477







