Work-Related Human T-lymphotropic Virus 1 and 2 (HTLV-1/2) Infection: A Systematic Review
Abstract
1. Introduction
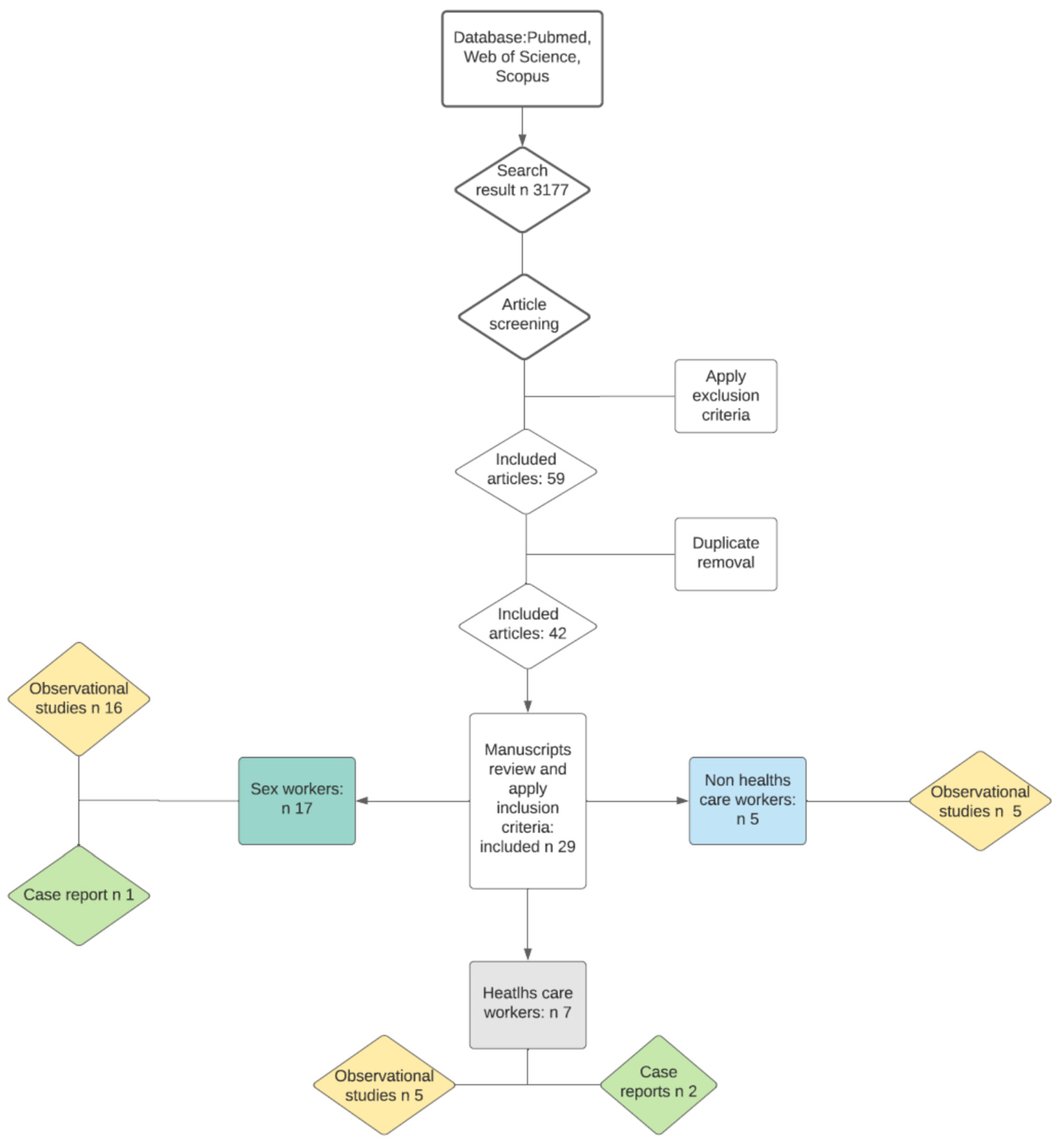
2. Materials and Methods
3. Results
3.1. Health Care Workers
3.2. Non-Health Care Workers
3.3. Sex Workers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Commission on Taxonomy of Viruses. ICTV 9th Report. Virus Taxonomy: 2009 Release. ICTV. 2009. Available online: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/reverse-transcribing-dna-and-rna-viruses-2011/w/rtviruses/161/retroviridae (accessed on 22 June 2021).
- Vrielink, H.; Reesink, H.W. HTLV-I/II prevalence in different geographic locations. Transfus. Med. Rev. 2004, 18, 46–57. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human T-Lymphotropic Virus Type 1; Technical Report; WHO: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/339773 (accessed on 22 June 2021).
- Murphy, E.L.; Cassar, O.; Gessain, A. Estimating the number of HTLV-2 infected persons in the world. Retrovirology 2015, 12 (Suppl. S1), O5. [Google Scholar] [CrossRef]
- Gessain, A.; Rua, R.; Betsem, E.; Turpin, J.; Mahieux, R. HTLV-3/4 and simian foamy retroviruses in humans: Discovery, epidemiology, cross-species transmission and molecular virology. Virology 2013, 435, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Shirdel, A.; Rafatpanah, H.; Akbarin, M.M.; Tarokhian, H.; Rahimi, H.; Bari, A.; Jahantigh, H.R.; Rezaee, S.A. Assessment of HTLV-1 proviral load, LAT, BIM, c-FOS and RAD51 gene expression in adult T cell leukemia/lymphoma. Med. Microbiol. Immunol. 2017, 206, 327–335. [Google Scholar] [CrossRef]
- Derakhshan, R.; Mirhosseini, A.; Ghezeldasht, S.A.; Jahantigh, H.R.; Mohareri, M.; Boostani, R.; Derakhshan, M.; Rezaee, S.A. Abnormal vitamin D and lipid profile in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. Mol. Biol. Rep. 2020, 47, 631–637. [Google Scholar] [CrossRef]
- Nicolás, D.; Ambrosioni, J.; Paredes, R.; Marcos, M.Á.; Manzardo, C.; Moreno, A.; Miró, J.M. Infection with human retroviruses other than HIV-1: HIV-2, HTLV-1, HTLV-2, HTLV-3 and HTLV-4. Expert. Rev. Anti. Infect. Ther. 2015, 13, 947–963. [Google Scholar] [CrossRef]
- Araujo, A.; Hall, W.W. Human T-lymphotropic virus type II and neurological disease. Ann. Neurol. 2004, 56, 10–19. [Google Scholar] [CrossRef]
- Ehrlich, G.D.; Andrews, J.; Sherman, M.P.; Greenberg, S.J.; Poiesz, B.J. DNA sequence analysis of the gene encoding the HTLV-I p21e transmembrane protein reveals inter-and intraisolate genetic heterogeneity. Virology 1992, 186, 619–627. [Google Scholar] [CrossRef]
- Watatani, Y.; Sato, Y.; Miyoshi, H.; Sakamoto, K.; Nishida, K.; Gion, Y.; Nagata, Y.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia 2019, 33, 2867–2883. [Google Scholar] [CrossRef]
- Ullah, I.; Iftikhar, T.; Jadoon, M.A.; Rehman, M.U.; ul-Haq, Q.S.; Akhter, M.N.; Khan, M.Z.; Rehman, A.; Bibi, M.; Urooj, K. Heterogeneous geographic distribution of human Tcell lymphotropic virus I (HTLV-I) and epidemiological, genomical, pathophysiologic, therapeutic knowledge about HTLV. J. Entom. Zool. Stud. 2017, 5, 871–878. [Google Scholar]
- Campos, K.R.; Gonçalves, M.G.; Costa, N.A.; Caterino-de-Araujo, A. Comparative performances of serologic and molecular assays for detecting human T lymphotropic virus type 1 and type 2 (HTLV-1 and HTLV-2) in patients infected with human immunodeficiency virus type 1 (HIV-1). Braz. J. Infect. Dis. 2017, 3, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Brito, V.; Santos, F.; Gonçalves, N.L.S.; Araujo, T.H.A.; Nascimento, D.S.V.; Pereira, F.M.; Boa-Sorte, N.C.A.; Grassi, M.F.R.; Caterino-de-Araujo, A.; Galvão-Castro, B. Performance of Commercially Available Serological Screening Tests for Human T-Cell Lymphotropic Virus Infection in Brazil. J. Clin. Microbiol. 2018, 27, 56. [Google Scholar] [CrossRef] [PubMed]
- Igakura, T.; Stinchcombe, J.C.; Goon, P.K.C.; Taylor, G.P.; Weber, J.N.; Griffiths, G.M.; Tanaka, Y.; Osame, M.; Bangham, C.R.M. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 2003, 299, 1713–1716. [Google Scholar] [CrossRef]
- Gallo, R.C. The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology 2005, 2, 17. [Google Scholar] [CrossRef]
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.R.; Araújo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B.F. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010, 3, 577–589. [Google Scholar] [CrossRef]
- Halbrook, M.; Gadoth, A.; Shankar, A.; Zheng, H.; Campbell, E.M.; Hoff, N.A.; Muyembe, J.J.; Wemakoy, E.O.; Rimoin, A.W.; Switzer, W.M. Human T-cell lymphotropic virus type 1 transmission dynamics in rural villages in the Democratic Republic of the Congo with high nonhuman primate exposure. PLoS Negl. Trop. Dis. 2021, 15, e0008923. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.M.; Taylor, G.P. HTLV-1: The silent impact revealed. Lancet Infect. Dis. 2020, 20, 12–14. [Google Scholar] [CrossRef]
- Overs, C. Sex Workers: Part of the Solution. An Analysis of HIV Prevention Programming to Prevent HIV Transmission during Commercial Sex in Developing Countries; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Hewagama, S.; Krishnaswamy, S.; King, L.; Davis, J.; Baird, R. Human T-Cell Lymphotropic Virus Type 1 exposures following blood-borne virus incidents in Central Australia, 2002–2012. Clin. Infect. Dis. 2014, 59, 85–87. [Google Scholar] [CrossRef]
- Petruccelli, B.P.; Murray, C.K.; Davis, K.W.; McBride, R., Jr.; Peel, S.A.; Michael, N.; Scott, P.T.; Hakre, S. Human T-lymphotropic virus infections in active component service members, US Armed Forces, 2000–2008. MSMR 2014, 21, 2–4. [Google Scholar] [PubMed]
- Stuver, S.O.; Tachibana, N.; Okayama, A.; Romano, F.; Yokota, T.; Mueller, N. Determinants of HTLV-1 seroprevalence in Miyazaki Prefecture, Japan: A cross-sectional study. J. Acquir. Immune Defic. Syndr. 1992, 5, 12–18. [Google Scholar] [PubMed]
- Goubau, P.; Carton, H.; Kazadi, K.; Muya, K.W.; Desmyter, J. HTLV seroepidemiology in a central African population with high incidence of tropical spastic paraparesis. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 577–579. [Google Scholar] [CrossRef]
- Titti, F.; Rezza, G.; Verani, P.; Buttò, S.; Sernicola, L.; Rapicetta, M.; Sarrecchia, B.; Oliva, C.; Rossi, G.B. HIV, HTLV-1, and HBV infections in a cohort of Italian intravenous drug abusers: Analysis of risk factors. J. Acquir. Immune Defic. Syndr. 1988, 1, 405–411. [Google Scholar]
- Menna-Barreto, M. HTLV-II transmission to a health care worker. Am. J. Infect. Control 2006, 34, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Goubau, P.; Carton, H.; Cornet, P.; Vercauteren, G.; Van-Gompel, A.; De-Vooght, H.; Piot, P.; Desmyter, J. Human T-cell lymphotropic virus type 1 infection and tropical spastic paraparesis in belgian expatriates. J. Med. Virol. 1992, 36, 13–15. [Google Scholar] [CrossRef]
- Filippone, C.; Betsem, E.; Tortevoye, P.; Cassar, O.; Bassot, S.; Froment, A.; Fontanet, A.; Gessain, A. A severe bite from a nonhuman primate is a major risk factor for HTLV-1 infection in hunters from Central Africa. Clin. Infect. Dis. 2015, 60, 1667–1676. [Google Scholar] [CrossRef]
- Kazanji, M.; Mouinga-Ondémé, A.; Lekana-Douki-Etenna, S.; Caron, M.; Makuwa, M.; Mahieux, R.; Gessain, A. Origin of HTLV-1 in hunters of nonhuman primates in Central Africa. J. Infect. Dis. 2015, 211, 361–365. [Google Scholar] [CrossRef]
- Zamora-Avila, D.E.; Zapata-Benavides, P.; Cedillo-Rosales, S.; Avalos-Ramírez, R.; Zarate-Ramos, J.J.; Riojas-Valdés, V.; Salinas-Meléndez, J.A.; Rivera-Morales, L.; Trejo-Avila, L. Serological detection of bovine leukemia virus in slaughterhouse workers from San Nicolas de los Garza, Nuevo Len, Mexico. Afr. J. Microb. Res. 2013, 7, 3042–3048. [Google Scholar]
- Norrgren, H.; Andersson, S.; Nauclér, A.; Dias, F.; Johansson, I.; Biberfeld, G. HIV-1, HIV-2, HTLV-I/II and Treponema pallidum infections: Incidence, prevalence, and HIV-2-associated mortality in an occupational cohort in Guinea-Bissau. J. Acquir. Immune Defic. Syndr. 1995, 9, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.L.; Figueroa, J.P.; Gibbs, W.N.; Holding-Cobham, M.; Cranston, B.; Malley, K.; Bodner, A.J.; Alexander, S.S.; Blattner, W.A. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica: I. Demographic determinants. Am. J. Epidemiol. 1991, 133, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.L.; Pereira, M.V.S.; Silva, R.M.D.; Sales, J.B.D.L.; Gardunho, D.C.L.; Monteiro, J.C.; Siravenha, L.Q.; Luz, A.L.B.D.; Fonseca, R.R.D.S.; Oliveira-Filho, A.B.; et al. Molecular epidemiology of HIV-1 and HTLV-1/2 among female SWs in four cities in the State of Para, northern Brazil. Front. Microbiol. 2020, 11, 602664. [Google Scholar] [CrossRef]
- Paulino-Ramirez, R.; Leandro, T.; Ruiz-Matuk, C.; Charow, R.; Budhwani, H.; Routy, J.P. Human T-cell lymphotropic virus 1/2 and human immunodeficiency virus antibodies identification among transactional SWs and drug users in the Dominican Repub. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Frade, P.C.; Raiol, N.C.; da- Costa, L.M.; Pinheiro, L.M.; Silva-Oliveira, G.C.; Pinho, J.R.; Lemos, J.A.; Martins, L.C.; Oliveira-Filho, A.B. Prevalence and genotyping of hepatitis B virus: A cross-sectional study conducted with female sex workers in the Marajó Archipelago, Brazil. Int. J. STD AIDS 2019, 30, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; Heitzinger, K.; Pollett, S.; Calderón, M.; Alarcón, J.; Ton-Thanh, G.N.; Zunt, J.R. The changing epidemiology of humAn T-Cell Lymphotropic Virus Type 1 infection in Peruvian female SWs, 1993–2010. Am. J. Trop. Med. Hyg. 2017, 96, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bautista, C.T.; Pando, M.A.; Reynaga, E.; Marone, R.; Sateren, W.B.; Montano, S.M.; Sanchez, J.L.; Avila, M.M. Sexual practices, drug use behaviors, and prevalence of HIV, syphilis, hepatitis B and C, and HTLV-1/2 in immigrant and non-immigrant female sex workers in Argentina. J. Immigr. Minor. Health 2009, 11, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Forbi, J.; Odetunde, A. Human T-cell lymphotropic virus in a population of pregnant women and commercial SWs in South Western Nigeria. Afr. Health Sci. 2007, 7, 129–132. [Google Scholar] [CrossRef]
- Berini, C.A.; Pando, M.A.; Bautista, C.T.; Eirin, M.E.; Martinez-Peralta, L.; Weissenbacher, M.; Avila, M.M.; Biglione, M.M. HTLV-1/2 among high-risk groups in Argentina: Molecular diagnosis and prevalence of different sexual transmitted infections. J. Med. Virol. 2007, 79, 1914–1920. [Google Scholar] [CrossRef]
- Pando, M.; Berini, C.; Bibini, M.; Fernández, M.; Reinaga, E.; Maulen, S.; Marone, R.; Biglione, M.; Montano, S.M.; Bautista, C.T.; et al. Prevalence of HIV and other sexually transmitted infections among female commercial SWs in Argentina. Am. J. Trop. Med. Hyg. 2006, 74, 233–238. [Google Scholar] [CrossRef]
- Zehender, G.; Colasante, C.; De Maddalena, C.; Bernini, F.; Savasi, V.; Persico, T.; Merli, S.; Ridolfo, A.; Santambrogio, S.; Moroni, M.; et al. High prevalence of human T-lymphotropic virus type 1 (HTLV-1) in immigrant male-to-female transsexual SWs with HIV-1 infection. J. Med. Virol. 2004, 74, 207–215. [Google Scholar] [CrossRef]
- Trujillo, L.; Muñoz, D.; Gotuzzo, E.; Yi, A.; Watts, D.M. Sexual practices and prevalence of HIV, HTLV-I/II, and Treponema pallidum among clandestine female SWs in Lima, Peru. Sex. Transm. Dis. 1999, 26, 115–118. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yu, P.S.; Lin, C.C.; Jen, I. Surveys of HIV-1, HTLV-I, and other sexually transmitted diseases in female SWs in Taipei City, Taiwan, from 1993 to 1996. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998, 18, 299–303. [Google Scholar] [CrossRef]
- Zurita, S.; Costa, C.; Watts, D.; Indacochea, S.; Campos, P.; Sanchez, J.; Gotuzzo, E. Prevalence of human retroviral infection in Quillabamba and Cuzco, Peru: A new endemic area for human T cell lymphotropic virus type 1. Am. J. Trop. Med. Hyg. 1997, 56, 561–565. [Google Scholar] [CrossRef]
- Broutet, N.; De-Queiroz Sousa, A.; Basilio, F.P.; Sá, H.L.; Simon, F.; Dabis, F. Prevalence of HIV-1, HIV-2 and HTLV antibody, in Fortaleza, Ceara, Brazil, 1993–1994. Int. J. STD AIDS 1996, 7, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Bellei, N.C.; Granato, C.F.; Tomyiama, H.; Castelo, A.; Ferreira, O. HTLV infection in a group of prostitutes and their male sexual clients in Brazil: Seroprevalence and risk factors. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 122–125. [Google Scholar] [CrossRef]
- Zapata-Benavides, P.; Lara-Rodríguez, M.A.; Alcocer-González, J.M.; Rodríguez-Padilla, C.; Taméz-Guerra, R.; Trejo-Avila, L.M. Seroprevalence of HTLV-I/II in Different Groups at Risk in Northeast Mexico. Vox Sang. 1996, 70, 181–182. [Google Scholar] [CrossRef]
- Yoshida, S.; Mizuguchi, Y.; Mizue, K.; Sakamoto, H.; Urabe, S. Prevalence of hepatitis B markers, antibodies to adult cell leukemia/lymphoma virus and antibodies to human immune deficiency virus in prostitutes in Fukuoka, Japan. Jpn. J. Med. Sci. Biol. 1987, 40, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Caterino-de-Araujo, A.; Santos-Fortuna, E.; Cavalheiro Magri, M.; Schuelter-Trevisol, F.; Da Silva, M.V. Unpredicted HTLV-1 infection in female sex worker from Imbituba, Santa Catarina, Brazil. Rev. Inst. Med. Trop. S. Paulo 2006, 48, 237–238. [Google Scholar] [CrossRef][Green Version]
- Lewandowski, C.; Ognjan, A.; Rivers, E.; Huitsing, H.; Pohlod, D.; Lee, H.; Saravolatz, L.D. Health care worker exposure to HIV-1 and HTLV I-II in critically ill, resuscitated emergency department patients. Ann. Emerg. Med. 1992, 21, 1353–1359. [Google Scholar] [CrossRef]
- Dutartre, H.; Clavière, M.; Journo, C.; Mahieux, R. Cell-Free versus Cell-to-Cell Infection by Human Immunodeficiency Virus Type 1 and Human T-Lymphotropic Virus Type 1: Exploring the Link among Viral Source, Viral Trafficking, and Viral Replication. J. Virol. 2016, 90, 7607–7617. [Google Scholar] [CrossRef]
- Sobata, R.; Matsumoto, C.; Uchida, S.; Suzuki, Y.; Satake, M.; Tadokoro, K. Estimation of the infectious viral load required for transfusion-transmitted human T-lymphotropic virus type 1 infection (TT-HTLV-1) and of the effectiveness of leukocyte reduction in preventing TT-HTLV-1. Vox Sang. 2015, 109, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rocamonde, B.; Carcone, A.; Mahieux, R.; Dutartre, H. HTLV-1 infection of myeloid cells: From transmission to immune alterations. Retrovirology 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sintasath, D.M.; Wolfe, N.D.; LeBreton, M.; Jia, H.; Garcia, A.D.; Diffo, J.L.D.; Tamoufe, U.; Carr, J.K.; Folks, T.M.; Mpuodi-Ngole, E.; et al. Simian T-lymphotropic virus diversity among nonhuman primates, Cameroon. Emerg. Infect. Dis. 2009, 15, 175. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Heneine, W.; Carr, J.K.; Garcia, A.D.; Shanmugam, V.; Tamoufe, U.; Torimiro, J.N.; Tassy Prosser, A.; LeBreton, M.; Mpuodi-Ngole, E.; et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. USA 2005, 102, 7994–7999. [Google Scholar] [CrossRef] [PubMed]
- Calvignac-Spencer, S.; Adjogoua, E.V.; Akoua-Koffi, C.; Hedemann, C.; Schubert, G.; Ellerbrok, H.; Leendertz, S.A.J.; Pauli, G.; Leendertz, F.H. Origin of human T-lymphotropic virus type 1 in rural Cote d’Ivoire. Emerg. Infect. Dis. 2012, 18, 830. [Google Scholar] [CrossRef]
- Calattini, S.; Betsem, E.; Bassot, S.; Chevalier, S.A.; Tortevoye, P.; Njouom, R.; Mahieux, R.; Froment, A.; Gessain, A. Multiple retroviral infection by HTLV type 1, 2, 3 and simian foamy virus in a family of Pygmies from Cameroon. Virology 2011, 410, 48–55. [Google Scholar] [CrossRef]
- Mossoun, A.; Calvignac-Spencer, S.; Anoh, A.E.; Pauly, M.S.; Driscoll, D.A.; Michel, A.O.; Nazaire, L.G.; Pfister, S.; Sabwe, P.; Thiesen, U.; et al. Bushmeat Hunting and Zoonotic Transmission of Simian T-Lymphotropic Virus 1 in Tropical West and Central Africa. J. Virol. 2017, 91, e02479-16. [Google Scholar] [CrossRef]
- Martin-Latil, S.; Gnädig, N.F.; Mallet, A.; Desdouits, M.; Guivel-Benhassine, F.; Jeannin, P.; Prevost, M.C.; Schwartz, O.; Gessain, A.; Ozden, S.; et al. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood 2012, 120, 572–580. [Google Scholar] [CrossRef]
- Ma, G.; Yasunaga, J.I.; Matsuoka, M. Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Study | Type of Study (Year) | Country | Occupational Study Population | Analytical/Diagnostic Method | Outcome |
|---|---|---|---|---|---|
| Hewagama et al. (2014) [21] | Observational retrospective study (2002–2012) | Central Australia | 53 HCWs monitored after biological accident with HTLV-1/2 infected patient | Serodia particle agglutination assay (2002–2008) and CLIA (2009–2012), confirmed by WB | No HTLV-1/2 seroconversion |
| Petruccelli et al. (2014) [22] | Observational study (2000–2008) | US | Military HCWs vs. combat vs. other military personnel | Diagnostic code for HTLV-1/2, recorded at medical encounter | -HTLV-1/2 rate: HCWs 0.94 vs. combat 0.37 vs. other 0.54 per 100,000 p-yrs. -RR HCWs vs. combat: 2.54 |
| Stuver et al. (1992) [23] | Observational study (1983–1984) | Japan | 7055 individuals screened at an Health Promotion Center | IFA | Higher HTLV-1 prevalence for fishing (RR 3.0), forestry (RR 2.5) and livestock (RR 2.0) workers, but not for HCWs (RR 0.92) |
| Goubau et al. (1990) [24] | Observational study | Congo DR | 42 hospital HCWs vs. 158 patients | ELISA confirmed by IFA and WB | HTLV-1/2 prevalence: no difference between HCWs (14.3%) and patients (13.9%) |
| Titti et al. (1988) [25] | Observational study (1985–1987) | Italy | 39 laboratory HCWs at a methadone maintenance center | ELISA confirmed by WB | No HTLV-1 seropositivity in the laboratory HCWs |
| Barreto (2006) [26] | Case report (1995) | Brazil | 29 year-old Caucasian female laboratory HCW | ELISA confirmed by WB | HTLV-2 seroconversion 18 months after biological accident with HTLV-2 infected patient |
| Goubau et al. (1992) [27] | Case report (1989) | Congo DR | 57 year-old Belgian Caucasian female nurse/midwife | IFA confirmed by WB | Possible occupational HTLV-1 infection (no other risk factors) |
| Study | Type of Study (Year) | Country | Occupational Study Population | Analytical/Diagnostic Method | Outcome |
|---|---|---|---|---|---|
| Filippone et al. (2015) [28] | Observational study (2005–2012) | Cameroon | 269 hunters (254 men, 15 women) bitten by NHPs vs. matched controls with no NHPs bites reported | WB confirmed by PCR | HTLV-1 prevalence: 8.6% in hunters (linked to bite severity) vs. 1.5% in controls |
| Kazanji et al. (2015) [29] | Observational study | Gabon | 78 individuals (mainly hunters) severely bitten by NHPs vs. 85 individuals from the same village with no NHPs bites reported. | ELISA confirmed by WB and PCR (proviral load measure). | HTLV-1 prevalence: 9.0% in NHPs bitten vs. 25.9% in controls. |
| Zamora-Avila et al. (2013) [30] | Observational study | Mexico | 28 slaughterhouse workers | Passive agglutination assay | No cases of HTLV-1 infection |
| Norrgren et al. (1995) [31] | Observational study (1990–1992) | Guinea Bissau | 1377 police officers (1234 men, 143 women), 515 of them performed follow-up (mean time 19.2 months) | ELISA confirmed by WB | -HTLV-1 prevalence: 4.0%, HTLV-2: 0.4%; -follow up: 2 HTLV-1 and 1 HTLV-2 new cases |
| Murphy et al. 1990 [32] | Observational study (1985–1986) | Jamaica | 13,260 subjects (4372 men, 8888 women) applying for food handling licenses | ELISAconfirmed by WB | HTLV-1 prevalence: ORs higher in domestic (1.92), tradesman (1.97), self-employed (2.06), unemployed (2.26), farmer/laborer (2.48), vs. professional/student occupation. |
| Study | Type of Study | Country | Study Population | Method | Outcome |
|---|---|---|---|---|---|
| De Souza et al. (2020) [33] | Observational study (2005–2006) | Brazil | 339 female SWs | ELISA confirmed by WB and PCR | -HTLV-1 prevalence: 1.8% -HTLV-2 prevalence: 0.0% -HTLV-1 infection associated with unprotected sex (OR 9.5) and illicit drug use (OR 7.1). |
| Paulino-Ramirez et al. (2019) [34] | Observational study (2012–2013) | Dominican Republic | 79 transactional SWs (29 males, 50 females) and 119 IDU (70 males, 49 females), some reporting both the conditions. | ELISA confirmed by WB | -HTLV-1/2 prevalence: 27.6% in male and 10% in female. -HTLV infection not associated with sex work |
| Frade et al. (2019) [35] | Observational study (2015–2017) | Brazil | 21 HBV positive female SWs | EIA confirmed by PCR | -No HTLV-1 co-infection -one HTLV-2 co-infection. |
| Stewart et al. (2017) [36] | Observational study (1993–2010) | Perù | 1938 female SWs. | EIA confirmed by WB | -HTLV-1 prevalence: 9.6%; -decreasing trend from 1993 (14.5%) to 2010 (3.1%) -no HTLV-1 cases among the 224 SWs born after 1979. |
| Bautista et al. (2009) [37] | Observational study (2000–2002) | Argentina | 625 immigrants (27%) and non-immigrants (73%) female SWs. | ELISA and particle agglutination assay, confirmed by WB | -HTLV-1 prevalence: non-immigrants 1.3% vs. immigrants 1.8%; -HTLV-2 prevalence: non-immigrants 0.2% vs. immigrants 0.0%. |
| Forbi et al. (2007) [38] | Observational study | Nigeria | 166 female SWs vs. 120 PW vs. 78 female secondary school students | micro-ELISA system | HTLV-1/2 prevalence: 22.9% SWs vs. 16.7% PW vs. 5.1% students. |
| Berini et al. (2007) [39] | Observational study (2000–2003) | Argentina | 613 female SWs vs. 173 IDUs, 682 MSM, 187 TB and 400 STS | ELISA and PAA, confirmed by WB and PCR. | HTLV-1/2 prevalence: 2.0% SWs (1.5% HTLV-1, 0.2% HTLV-2), 19.1% IDUs (4.6% HTLV-1, 15.6% HTLV-2), 2.1% TB (1.6% HTLV-1, 0.5% HTLV-2), 1.0% STIs and 0.4% MSM (all HTLV-1). |
| Pando et al. (2006) [40] | Observational study (2000–2002) | Argentina | 614 female SWs | ELISA and PAA confirmed by WB | -HTLV-1/2 prevalence: 1.6% (HTLV-1 7/10 cases); -HTLV-1 prevalence: higher in Argentinian vs. other countries SWs (OR 28.3). |
| Zehender et al. (2004) [41] | Observational study (1996–2003) | Italy | 52 male-to-female transsexual SWs among 167 HIV-1 positive immigrants vs. 226 PW HIV-1 negative immigrants (controls). | ELISA confirmed by WB and PCR | -HTLV-1 prevalence: 11.5% SWs vs. 0.9% controls. -HTLV-2 prevalence: 6.4% SWs vs. 0.0% controls. |
| Trujillo et al. (1999) [42] | Observational study (1994) | Peru | 158 female SWs | ELISA confirmed by WB | -HTLV-1 prevalence 3.7%; -HTLV-2 prevalence 0.0%. |
| Chen et al. (1998) [43] | Observational study (1993–1996) | Taiwan | 328 massage parlor, 770 karaoke bar and 284 brothel female SWs | ELISA confirmed by WB | -HTLV-1 prevalence: 0.61% massage parlor, 1.30% karaoke bar, 4.23% brothel SWs. -No HTLV-2 infection |
| Zurita et al. (1997) [44] | Observational study | Perù | 51 female SWs vs. 211 healthy PW, 47 suspected STD patients, 48 homosexual/bisexual individuals, 13 promiscuous heterosexual males. | ELISA confirmed by WB | -HTLV-l prevalence: 13.7% SWs, vs. 2.3% PW, 8.5% STD patients, 6.2% homosexual/bisexual individuals, 0.0% promiscuous heterosexual males. -No HTLV-2 infection |
| Broutetet et al. (1996) [45] | Observational study (1993–1994) | Brazil | 496 female and 171 male SWs vs. 814 PW, 494 TB and 395 STD patients, 427 prisoners | ELISA confirmed by WB | -HTLV-1 prevalence: 1.21 female and 0.58% male SWs vs. 0.12% PW, 0.44% TB patients, 0.50% STD patients and 0.47% prisoners. -HTLV-2 prevalence: 0.20% female and 0.0% male SWs vs. 0.12% PW, 0.20% TB patients, 0.0% STD patients, 0.47% prisoners. |
| Bellei et al. (1996) [46] | Observational study (1987–1990) | Brazil | 653 female SWs vs. 153 male sexual clients | EIA confirmed by WB | -HTLV-1 prevalence: 2.8% SWs vs. 2.0% their clients. -No HTLV-2 infection. |
| Zapata-Benavides et al. (1996) [47] | Observational study | Mexico | 75 female SWs vs. 335 leukemia/lymphoma patients, 103 other cancer patients, 387 with multiple blood transfusions, 87 homosexuals, 90 HIV positive, 1 with multiple sclerosis. | PAA confirmed with WB and PCR | -No confirmed HTLV-1/2 infection |
| Yoshida et al. (1987) [48] | Observational study (1986) | Japan | 237 female SWs | Serum screening with PAA | -HTLV-1 prevalence: 5.9% |
| Caterino-De Araujo et al. (2006) [49] | Case Report | Brazil | Female SW | EIA confirmed by WB | Sex work identified as the major via of virus acquisition |
| Study | Country | Study Population | HTLV Prevalence | HIV Prevalence | Other STDs Prevalence |
|---|---|---|---|---|---|
| De Souza et al. (2020) [33] | Brazil | 339 female SWs | -HTLV-1: 1.8% -HTLV-2: 0.0% | -HIV-1: 2.4% | - |
| Stewart et al. (2017) [36] | Perù | 1938 female SWs. | -HTLV-1: 9.6% | -HIV: 0.5% | - |
| Bautista et al. (2009) [37] | Argentina | 625 non-IM (73%) and IM (27%) female SWs. | -HTLV-1: non-IM 1.3% vs. IM 1.8%; -HTLV-2: non-IM 0.2% vs. IM 0.0%. | -HIV-1: non-IM 3.9% vs. IM 1.2% | -HBV: non-IM 12.6% vs. IM 19.4% -HCV: non-IM 5.5% vs. IM 1.2% -TP: non-IM 51.5% vs. IM 30.3% |
| Berini et al. (2007) [39] | Argentina | 613 female SWs | -HTLV-1: 1.5%, -HTLV-2: 0.5% | -HIV: 2.9% | -HBV: 14.2% -HCV: 4.2% -TP: 43.6% |
| Pando et al. (2006) [40] | Argentina | 614 female SWs | -HTLV-1: 1.1%, -HTLV-2: 0.5% | -HIV: 3.2% | -HBV: 14.4% -HCV: 4.3% -TP: 45.7% |
| Trujillo et al. (1999) [42] | Peru | 158 female SWs | -HTLV-1: 3.7%; -HTLV-2: 0.0%. | HIV-1: 0.0% | -TP: 3.0% |
| Chen et al. (1998) [43] | Taiwan | 328 massage parlor, 770 karaoke bar and 284 brothel female SWs | -HTLV-1: 0.6% massage parlors, 1.3% karaoke bars, 4.2% brothel. -HTLV-2: 0.0% all settings | HIV-1: 0.2% karaoke bars, 0.0% other settings. | -TP: 4.1% massage parlors, 3.4% karaoke bars, 34.9% brothels. -HSV-2: 2.4% massage parlors, 7.5% karaoke bars, 3.0% brothels. -Chlamydia: 56.3% massage parlors, 40.6% karaoke bars, 69.9% brothels. |
| Zurita et al. (1997) [44] | Perù | 51 female SWs | -HTLV-l: 13.7% -HTLV-2: 0.0% | -HIV-1: 0.0% | - |
| Broutetet et al. (1996) [45] | Brazil | 496 female and 171 male SWs | -HTLV-1: 1.2 female, 0.6% male -HTLV-2: 0.2% female, 0.0% male | -HIV-1: 1.6% female, 2.9% male -HIV-2: 0.0% | - |
| Bellei et al. (1996) [46] | Brazil | 653 female SWs | -HTLV-1: 2.8% -HTLV-2: 0.0% | -HIV-1: 3.6% -HIV-2: 0.0% | -HBV: 5.1% -HCV: 10.3% |
| Yoshida et al. (1987) [48] | Japan | 237 female SWs | -HTLV-1: 5.9% | -HIV-1: 0.0% | -HBV: 34.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stufano, A.; Jahantigh, H.R.; Cagnazzo, F.; Centrone, F.; Loconsole, D.; Chironna, M.; Lovreglio, P. Work-Related Human T-lymphotropic Virus 1 and 2 (HTLV-1/2) Infection: A Systematic Review. Viruses 2021, 13, 1753. https://doi.org/10.3390/v13091753
Stufano A, Jahantigh HR, Cagnazzo F, Centrone F, Loconsole D, Chironna M, Lovreglio P. Work-Related Human T-lymphotropic Virus 1 and 2 (HTLV-1/2) Infection: A Systematic Review. Viruses. 2021; 13(9):1753. https://doi.org/10.3390/v13091753
Chicago/Turabian StyleStufano, Angela, Hamid Reza Jahantigh, Francesco Cagnazzo, Francesca Centrone, Daniela Loconsole, Maria Chironna, and Piero Lovreglio. 2021. "Work-Related Human T-lymphotropic Virus 1 and 2 (HTLV-1/2) Infection: A Systematic Review" Viruses 13, no. 9: 1753. https://doi.org/10.3390/v13091753
APA StyleStufano, A., Jahantigh, H. R., Cagnazzo, F., Centrone, F., Loconsole, D., Chironna, M., & Lovreglio, P. (2021). Work-Related Human T-lymphotropic Virus 1 and 2 (HTLV-1/2) Infection: A Systematic Review. Viruses, 13(9), 1753. https://doi.org/10.3390/v13091753










