Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses
Abstract
:1. Introduction
2. The Origins and Early Steps of Natural Source-Based Antiviral Drug Development
3. Naturally Occurring Sulfated Polysaccharides-Based Antivirals
3.1. Seaweed-Derived Compounds
3.1.1. Fucoidans
3.1.2. Galactans
3.1.3. Ulvan
3.1.4. Alginic Acids
3.2. Animal-Derived Compounds
3.2.1. Heparin
3.2.2. Chondroitin Sulfate
4. Sulfated Polysaccharides Generated by Chemical Sulfation Reaction
5. Synthesis of New Molecules Possessing Diverse Structures by a Single-Step Process Will Be a Useful Addition to the Arsenal of Antivirals
6. Low Molecular Weight Heparin Mimetics
7. Relationship between Structures of Sulfated Polysaccharide and Their Antiviral Activities
8. Antiviral Mode of Action (MoA) of Sulfated Polysaccharides
8.1. Sulfated Polysaccharides’ Role in Infections with Human Immunodeficiency Virus Type 1
8.2. The Putative MoA of Sulfated Polysaccharides in Infections with Herpes Simplex Viruses
8.3. The Putative MoA of Sulfated Polysaccharides in Infections with SARS-CoV-2
8.4. Additional Aspects of MoA That are Independent of Virus Entry
9. Polysaccharide-Based Compounds Possess an Intrinsic Potential of Broad-Spectrum Antiviral Activity
10. Polysaccharide-Based Antiviral Agents in Pre-Clinical and Clinical Studies
10.1. Carrageenan
10.2. Fucoidans
10.3. Lectins
10.4. Spirulan
10.5. Alginic Acids
10.6. Modified Polysaccharides
11. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Choi, J.H.; Croyle, M.A. Emerging Targets and Novel Approaches to Ebola Virus Prophylaxis and Treatment. BioDrugs 2013, 27, 565–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniruzzaman, M.; Martinez-Gutierrez, C.A.; Weinheimer, A.R.; Aylward, F.O. Dynamic genome evolution and complex virocell metabolism of globally-distributed giant viruses. Nat. Commun. 2020, 11, 1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson, C.S.; Chibale, K.; Goss, R.J.M.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral drug discovery: Preparing for the next pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Christou, L. The global burden of bacterial and viral zoonotic infections. Clin. Microbiol. Infect. 2011, 17, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasansuklab, A.; Theerasri, A.; Rangsinth, P.; Sillapachaiyaporn, C.; Chuchawankul, S.; Tencomnao, T. Anti-COVID-19 drug candidates: A review on potential biological activities of natural products in the management of new coronavirus infection. J. Tradit. Complement. Med. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Ray, B.; Schütz, M.; Mukherjee, S.; Jana, S.; Ray, S.; Marschall, M. Exploiting the Amazing Diversity of Natural Source-Derived Polysaccharides: Modern Procedures of Isolation, Engineering, and Optimization of Antiviral Activities. Polymers 2021, 13, 136. [Google Scholar] [CrossRef]
- Ramallo, I.A.; Salazar, M.O.; Mendez, L.; Furlan, R.L.E. Chemically Engineered Extracts: Source of Bioactive Compounds. Acc. Chem. Res. 2011, 44, 241–250. [Google Scholar] [CrossRef]
- Ren, X.; Xie, X.; Chen, B.; Liu, L.; Jiang, C.; Qian, Q. Marine Natural Products: A Potential Source of Anti-hepatocellular Carcinoma Drugs. J. Med. Chem. 2021, 64, 7879–7899. [Google Scholar] [CrossRef]
- Snelgrove, P. An Ocean of Discovery: Biodiversity Beyond the Census of Marine Life. Planta Med. 2016, 82, 790–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; de Bruyn Kops, C.; Kirchmair, J. Data Resources for the Computer-Guided Discovery of Bioactive Natural Products. J. Chem. Inf. Model. 2017, 57, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral Polymers: Past Approaches and Future Possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, synthesis, and biomedical applications of synthetic sulfated polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, R.; Hegazy, H.; Achconzoarya, B. A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method. Energies 2021, 14, 3092. [Google Scholar] [CrossRef]
- Germershaus, O.; Lühmann, T.; Rybak, J.-C.; Ritzer, J.; Meinel, L. Application of natural and semi-synthetic polymers for the delivery of sensitive drugs. Int. Mater. Rev. 2015, 60, 101–131. [Google Scholar] [CrossRef]
- Gogineni, V.; Schinazi, R.F.; Hamann, M.T. Role of Marine Natural Products in the Genesis of Antiviral Agents. Chem. Rev. 2015, 115, 9655–9706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2020, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Yu, G.; He, Y.; Xu, C.; Zhang, L.; Wang, W. Marine glycan–based antiviral agents in clinical or preclinical trials. Rev. Med. Virol. 2019, 29, e2043. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Liang, C.-H.; Wu, H.-T.; Pang, H.-Y.; Chen, C.; Wang, G.-H.; Chan, L.-P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Ghosh, P.; Biswas, G.R. Absorption, distribution, metabolism and elimination (ADME) and toxicity profile of marine sulfated polysaccharides used in bionanotechnology. Afr. J. Pharm. Pharmacol. 2018, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure–activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated Polysaccharides Extracted from Sea Algae as Potential Antiviral Drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasongkla, N.; Chen, B.; Macaraeg, N.; Fox, M.E.; Fréchet, J.M.J.; Szoka, F.C. Dependence of Pharmacokinetics and Biodistribution on Polymer Architecture: Effect of Cyclic versus Linear Polymers. J. Am. Chem. Soc. 2009, 131, 3842–3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Vicent, M.J. Polymer therapeutics-prospects for 21st century: The end of the beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef]
- Vicent, M.J.; Ringsdorf, H.; Duncan, R. Polymer therapeutics: Clinical applications and challenges for development. Adv. Drug Deliv. Rev. 2009, 61, 1117–1120. [Google Scholar] [CrossRef]
- Bernardi, A.; Jiménez-Barbero, J.; Casnati, A.; De Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.-K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Conzelmann, C.; Müller, J.A.; Perkhofer, L.; Sparrer, K.M.; Zelikin, A.N.; Münch, J.; Kleger, A. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin. Med. 2020, 20, e218–e221. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Arnold, K.; Pawlinski, R.; Key, N.S. Using heparin molecules to manage COVID-2019. Res. Pract. Thromb. Haemost. 2020, 4, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Mycroft-West, C.; Su, D.; Elli, S.; Li, Y.; Guimond, S.; Miller, G.; Turnbull, J.; Yates, E.; Guerrini, M.; Fernig, D.; et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Pujol, C.A.; Damonte, E.B.; Ray, B. Additionally sulfated xylomannan sulfates from Scinaia hatei and their antiviral activities. Carbohydr. Polym. 2015, 131, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Mukherjee, S.; Ribelato, E.V.; Darido, M.L.; Faccin-Galhardi, L.C.; Ray, B.; Ray, S. The heparin-mimicking arabinogalactan sulfates from Anogeissus latifolia gum: Production, structures, and anti-herpes simplex virus activity. Int. J. Biol. Macromol. 2021, 183, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulfated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pujol, C.A.; Jana, S.; Damonte, E.B.; Ray, B.; Ray, S. Chemically sulfated arabinoxylans from Plantago ovata seed husk: Synthesis, characterization and antiviral activity. Carbohydr. Polym. 2021, 256, 117555. [Google Scholar] [CrossRef]
- Ray, B.; Hutterer, C.; Bandyopadhyay, S.S.; Ghosh, K.; Chatterjee, U.R.; Ray, S.; Zeitträger, I.; Wagner, S.; Marschall, M. Chemically engineered sulfated glucans from rice bran exert strong antiviral activity at the stage of viral entry. J. Nat. Prod. 2013, 76, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef]
- Desomer, P.; Declercq, E.; Billiau, A.; Schonne, E.; Claesen, M. Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J. Virol. 1968, 2, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Desomer, P.; Declercq, E.; Billiau, A.; Schonne, E.; Claesen, M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J. Virol. 1968, 2, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, H.S.; Goebel, W.F.; Horsfall, F.L. Inhibition of Mumps Virus Multiplication by a Polysaccharide. Exp. Biol. Med. 1947, 66, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H. Inhibition by certain polysaccharides of hemagglutination and of multiplication of influenza virus. J. Exp. Med. 1947, 86, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.S. The inhibitory effect of polysaccharide on mumps virus multiplication. J. Exp. Med. 1948, 87, 385–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, P.; Dutcher, J.D.; Adams, E.V.; Sherman, J.H. Protective Effect of Seaweed Extracts for Chicken Embryos Infected with Influenza B or Mumps Virus. Exp. Biol. Med. 1958, 99, 590–593. [Google Scholar] [CrossRef]
- Ehresmann, D.W.; Deig, E.F.; Hatch, M.T.; DiSalvo, L.H.; Vedros, N.A. Antiviral substances from california marine algae. J. Phycol. 1977, 13, 37–40. [Google Scholar] [CrossRef]
- Richards, J.T.; Kern, E.R.; Glasgow, L.A.; Overall, J.C.; Deign, E.F.; Hatch, M.T. Antiviral activity of extracts from marine algae. Antimicrob. Agents Chemother. 1978, 14, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, H.; Kido, Y.; Kobayashi, N.; Motoki, Y.; Neushul, M.; Yamamoto, N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob. Agents Chemother. 1987, 31, 1524–1528. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, H.; Kido, Y.; Kobayashi, N.; Motoki, Y.; Neushul, M.; Yamamoto, N. Antiretroviral activity in a marine red alga: Reverse transcriptase inhibition by an aqueous extract of Schizymenia pacifica. J. Cancer Res. Clin. Oncol. 1987, 113, 413–416. [Google Scholar] [CrossRef]
- Liebhaber, H.; Takemoto, K.K. Alteration of plaque morphology of EMC virus with polycations. Virology 1961, 14, 502–504. [Google Scholar] [CrossRef]
- Takemoto, K.K.; Liebhaber, H. Virus-polysaccharide interactions. Virology 1961, 14, 456–462. [Google Scholar] [CrossRef]
- Takemoto, K.K.; Fabisch, P. Inhibition of Herpes Virus by Natural and Synthetic Acid Polysaccharides. Exp. Biol. Med. 1964, 116, 140–144. [Google Scholar] [CrossRef]
- Vaheri, A. Heparin and related polyioninc substances as viral inhibitors. Acta Pathol. Microbiol. Scand. 1964, 60, 1–98. [Google Scholar]
- Schulze, I.T. Reversible inhibition of type 2 dengue virus by agar polysaccharide. Virology 1964, 22, 79–90. [Google Scholar] [CrossRef]
- Kleinschmidt, W.J.; Cline, J.C.; Murphy, E.B. Interferon production induced by statolon. Proc. Natl. Acad. Sci. USA 1964, 52, 741–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merigan, T.C.; Regelson, W. Interferon Induction in Man by a Synthetic Polyanion of Defined Composition. N. Engl. J. Med. 1967, 277, 1283–1287. [Google Scholar] [CrossRef]
- Merigan, T.C.; Finkelstein, M.S. Interferon-stimulating and in vivo antiviral effects of various synthetic anionic polymers. Virology 1968, 35, 363–374. [Google Scholar] [CrossRef]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Luescher-Mattli, M. Algae, a possible source for new drugs in the treatment of HIV and other viral diseases. Curr. Med. Chem. Anti-Infect. Agents 2003, 2, 219–225. [Google Scholar] [CrossRef]
- Pujol, C.A.; Carlucci, M.J.; Matulewicz, M.C.; Damonte, E.B. Natural sulfated polysaccharides for the prevention and control of viral infections. In Bioactive Heterocycles V; Springer: Berlin/Heidelberg, Germany, 2007; pp. 259–281. [Google Scholar]
- Ito, M.; Baba, M.; Sato, A.; Pauwels, R.; De Clercq, E.; Shigeta, S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir. Res. 1987, 7, 361–367. [Google Scholar] [CrossRef]
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar] [CrossRef]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmakar, P.; Pujol, C.A.; Damonte, E.B.; Ghosh, T.; Ray, B. Polysaccharides from Padina tetrastromatica: Structural features, chemical modification and antiviral activity. Carbohydr. Polym. 2010, 80, 513–520. [Google Scholar] [CrossRef]
- Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry 2010, 71, 235–242. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel Antiviral Fucoidan from Sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Navid, M.H.; Bandyopadhyay, S.S.; Schnitzler, P.; Ray, B. Sulfated polysaccharides from Laminaria angustata: Structural features and in vitro antiviral activities. Carbohydr. Polym. 2012, 87, 123–130. [Google Scholar] [CrossRef]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural Features and Antiviral Activity of Sulfated Fucans from the Brown Seaweed Cystoseira Indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Xie, E.-Y.; Du, J.; Deng, F.; Dong, C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In vitro and In vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef] [Green Version]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Kwon, P.S.; Oh, H.; Kwon, S.-J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Ghosh, T.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Polysaccharides from Gracilaria Corticata: Sulfation, Chemical Characterization and Anti-HSV Activities. Int. J. Biol. Macromol. 2008, 43, 346–351. [Google Scholar] [CrossRef]
- Duarte, M.E.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Pujol, C.; Estevez, J.; Carlucci, M.; Ciancia, M.; Cerezo, A.; Damonte, E. Novel dl-Galactan Hybrids from the Red Seaweed Gymnogongrus Torulosus are Potent Inhibitors of Herpes Simplex Virus and Dengue Virus. Antivir. Chem. Chemother. 2002, 13, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrin, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Desf-Tischer, P.; Talarico, L.; Noseda, M.; Pitabguimaraes, S.; Damonte, E.; Duarte, M. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006, 63, 459–465. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.E.; Alarcon, B.; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, P.; Yu, G.-Y.; Li, C.-X.; Hao, C.; Qi, X.; Zhang, L.-J.; Guan, H.-S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulfated derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Beer, M.; Fazekas, T.; Unger, H.; Prieschl-Grassauer, E.; et al. Iota-Carrageenan Is a Potent Inhibitor of Influenza A Virus Infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.I.; Kim, C.W.; Lee, H.R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Pérez Recalde, M.; Carlucci, M.J.; Noseda, M.D. Matulewicz, M.C. Chemical modifications of algal mannans and xylomannans: Effects on antiviral activity. Phytochemistry 2012, 73, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ghosh, T.; Ray, B. Xylans from Scinaia hatei: Structural features, sulfation and anti-HSV activity. Int. J. Biol. Macromol. 2010, 46, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Pujol, C.A.; Damonte, E.B.; Sinha, S.; Ray, B. Sulfated Xylomannans from the Red Seaweed Sebdenia polydactyla: Structural Features, Chemical Modification and Antiviral Activity. Antivir. Chem. Chemother. 2009, 19, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Mandal, P.; Pujol, C.A.; Carlucci, M.J.; Chattopadhyay, K.; Damonte, E.B.; Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry 2008, 69, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Takeshita, A.; Hayashi, K.; Hayashi, T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011, 86, 995–999. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Hao, C.; Yunjia, Y.; Qin, L.; He, M.; Mao, W. Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr. Polym. 2018, 200, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.N.; Yusof, R.; Rothan, H.A. Antiviral and virucidal activities of sulfated polysaccharides against Japanese encephalitis virus. Trop. Biomed. 2020, 37, 713–721. [Google Scholar] [CrossRef]
- Morán-Santibañez, K.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Robledo, D.; Freile-Pelegrín, Y.; Peña-Hernández, M.A.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. Synergistic Effects of Sulfated Polysaccharides from Mexican Seaweeds against Measles Virus. BioMed Res. Int. 2016, 2016, 8502123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.Z.; Zhang, M.F.; Wang, X.; Fu, X.J.; Guan, H.S.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseno, J.; Cruz-Suarez, L.; Sassi, J.-F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L. Sulfated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95, 3. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Hippensteel, J.A.; LaRiviere, W.B.; Colbert, J.F.; Langouët-Astrié, C.J.; Schmidt, E.P. Heparin as a Therapy for COVID-19: Current Evidence and Future Possibilities. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L211–L217. [Google Scholar] [CrossRef]
- Baba, M.; De Clercq, E.; Schols, D.; Pauwels, R.; Snoeck, R.; Van Boeckel, C.; Kraaijeveld, N.; Hobbelen, P.; Ottenheijm, H.; Den Hollander, F. Novel Sulfated Polysaccharides: Dissociation of Anti-Human Immunodeficiency Virus Activity from Antithrombin Activity. J. Infect. Dis. 1990, 161, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wu, M.-Y.; Zheng, C.-B.; Zhu, L.; Zhao, J.-H.; Zheng, Y.-T. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydr. Res. 2013, 380, 64–69. [Google Scholar] [CrossRef]
- Galus, A.; Mallet, J.M.; Lembo, D.; Cagno, V.; Djabourov, M.; Lortat-Jacob, H.; Bouchemal, K. Hexagonal-shaped chondroitin sulfate self-assemblies have exalted anti-HSV-2 activity. Carbohydr. Polym. 2016, 136, 113–120. [Google Scholar] [CrossRef]
- Kato, D.; Era, S.; Watanabe, I.; Arihara, M.; Sugiura, N.; Kimata, K.; Suzuki, Y.; Morita, K.; Hidari, K.I.; Suzuki, T. Antiviral activity of chondroitin sulfate E targeting dengue virus envelope protein. Antivir. Res. 2010, 88, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, V.A.; Navarro, D.A.; Ponce, N.M.A.; Stortz, C.A. Seaweed Polysaccharides: Structure and Applications. In Industrial Applications of Renewable Biomass Products Past, Present and Future; D’Accorso, N.B., Goyanes, S.N., Eds.; Springer: Cham, Switzerland, 2017; pp. 75–116. [Google Scholar] [CrossRef]
- Painter, T.J. Algal polysaccharides. In The Polysaccharides, 1st ed.; Aspinall, G.O., Ed.; Elsevier: New York, NY, USA, 1983; Volume 2, pp. 195–285. [Google Scholar]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 115–217. [Google Scholar] [CrossRef]
- Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Mar. Drugs 2021, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [Green Version]
- Conchie, J.; Percival, E.G.V. Fucoidan part II. The hydrolysis of a methylated fucoidin prepared from Fucus vesiculosus. J. Chem. Soc. 1950, 827–832. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for sulfated fucan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [CrossRef]
- Chevelot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationship with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Chevelot, L.; Molly, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in sulfated fucans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Daniel, R.; Berteau, O.; Jozefonvicz, J.; Goasdoue, N. Degradation of algal (Ascophyllum nodosum) fucoidan by an enzymatic activity contained in digestive glands of the marine mollusc Pecten maximus. Carbohydr. Res. 1999, 322, 291–297. [Google Scholar] [CrossRef]
- Karmakar, P.; Ghosh, T.; Sinha, S.; Saha, S.; Mandal, P.; Ghosal, P.K.; Ray, B. Polysaccharides from the brown seaweed Padina tetrastromatica: Characterization of a sulfated fucan. Carbohydr. Polym. 2009, 78, 416–421. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana JV Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Trinchero, J.; Ponce, N.M.A.; Córdoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomon, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res. 2009, 23, 707–712. [Google Scholar] [CrossRef]
- Feldman, S.C.; Reynaldi, S.; Stortz, C.A.; Cerezo, A.S.; Damonte, E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 1999, 6, 335–340. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic Activities of Sulfated Polysaccharides from Green Algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Preeprame, S.; Hayashi, K.; Lee, J.-B.; Sankawa, U.; Hayashi, T. A Novel Antivirally Active Fucan Sulfate Derived from an Edible Brown Alga, Sargassum horneri. Chem. Pharm. Bull. 2001, 49, 484–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Wang, W.; Zhao, X.; Zhang, J.; Ewart, S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean Univ. China 2012, 11, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Makarenkova, I.D.; Deriabin, P.G.; L’vov, D.K.; Zviagintseva, T.N.; Besednova, N.N. Antiviral activity of sulfated polysaccharide from the brown algae Laminaria japonica against avian influenza A (H5N1) virus infection in the cultured cells. Vopr. Virusol. 2010, 55, 41–45. [Google Scholar]
- Richards, C.; Williams, N.A.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y. Oral Fucoidan Attenuates Lung Pathology and Clinical Signs in a Severe Influenza a Mouse Model. Mar. Drugs 2020, 18, 246. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, X.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.; Chen, A. Studies on Antiviral and Immuno-Regulation Activity of Low Molecular Weight Fucoidan from Laminaria japonica. J. Ocean Univ. China 2018, 17, 705–711. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.D.; Tai, W.J.; Yu, G. Inhibition of Influenza A Virus Infection by Fucoidan Targeting Viral Neuraminidase and Cellular EGFR Pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Mar. Drugs 2015, 14, 4. [Google Scholar] [CrossRef]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; McCauley, J.W.; Flick-Smith, H. Interactions of bovine viral diarrhoea virus glycoprotein Erns with cell surface glycosaminoglycans. J. Gen. Virol. 2000, 81, 451–459. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Mitra, D.; McCandless, M.G.; Sharma, P.; Tandon, R.; Zhang, F.; Linhardt, R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.R.; Wang, Q.L.; Liu, Z.Q.; Dong, X.P.; Wen, C.R.; Ai, C.Q.; Zhang, Y.J.; Wang, Z.F.; Zhu, B.W. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.-K.; Kim, K.; Kim, I.; Chun, S.H.; Oh, T.H.; Kim, J.-U.; Kim, J.; Jung, W.H.; Moon, H.; Ku, B.; et al. Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera In vitro. Mar. Drugs 2021, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, C.Y.; Lee, D.B.; Seok, J.H.; Kim, K.H.; Chung, M.S. Inhibitory Effects of Laminaria japonica Fucoidans Against Noroviruses. Viruses 2020, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.R.; Cauduro, J.P.; Noseda, D.G.; Noseda, M.D.; Gonçalves, A.G.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S. The structure of the agaran sulfate from Acanthophora spicifera (Rhodomelaceae, Ceramiales) and its antiviral activity. Relation between structure and antiviral activity in agarans. Carbohydr. Res. 2004, 339, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, K.; Mateu, C.G.; Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ray, B. Galactan sulfate of Grateloupia indica: Isolation, structural features and antiviral activity. Phytochemistry 2007, 68, 1428–1435. [Google Scholar] [CrossRef]
- Malagoli, B.G.; Cardozo, F.T.G.S.; Gomes, J.H.S.; Ferraz, V.P.; Simões, C.M.O.; Braga, F.C. Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnion muelleri. Int. J. Biol. Macromol. 2014, 66, 332–337. [Google Scholar] [CrossRef]
- Mendes, G.S.; Duarte, M.E.R.; Colodi, F.G.; Noseda, M.D.; Ferreira, L.G.; Berté, S.D.; Cavalcanti, J.F.; Santos, N.; Romanos, M.T.V. Structure and anti-metapneumovirus activity of sulfated galactans from the red seaweed Cryptonemia seminervis. Carbohydr. Polym. 2014, 101, 313–323. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Hayashi, T. Isolation of Sulfated Galactan from Codium fragile and Its Antiviral Effect. Biol. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Rudtanatip, T.; Asuvapongpatana, S.; Withyachumnarnkul, B.; Wongprasert, K. Sulfated galactans isolated from the red seaweed Gracilaria fisheri target the envelope proteins of white spot syndrome virus and protect against viral infection in shrimp haemocytes. J. Gen. Virol. 2014, 95, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Inhibitory action of natural carrageenans on herpes simplex virus infection of mouse astrocytes. Chemotherapy 1999, 45, 429–436. [Google Scholar] [CrossRef]
- Gomaa, H.H.; Elshoubaky, G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 34–42. [Google Scholar]
- Kolender, A.A.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M.C. Sulfation of kappa-carrageenan and antiviral activity. An. Asoc. Quim. Argent. 1998, 86, 304–311. [Google Scholar]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- Girond, S.; Crance, J.M.; Van Cuyck-Gandre, H.; Renaudet, J.; Deloince, R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res. Virol. 1991, 142, 261–270. [Google Scholar] [CrossRef]
- Piccini, L.E.; Carro, A.C.; Quintana, V.M.; Damonte, E.B. Antibody-independent and dependent infection of human myeloid cells with dengue virus is inhibited by carrageenan. Virus Res. 2020, 290, 198150. [Google Scholar] [CrossRef]
- Talarico, L.B.; Noseda, M.D.; Ducatti, D.R.; Duarte, M.E.; Damonte, E.B. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J. Gen. Virol. 2011, 92, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef] [Green Version]
- Reunov, A.; Nagorskaya, V.; Lapshina, L.; Yermak, I.; Barabanova, A. Effect of κ/ß-Carrageenan from red alga Tichocarpus crinitus (Tichocarpaceae) on infection of detached tobacco leaves with tobacco mosaic virus. J. Plant Dis. Prot. 2004, 111, 165–172. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.-M.; Unger, H.; Grassauer, A.; et al. The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model. PLoS ONE 2015, 10, e0128794. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Guo, Q.; Xu, W.; Li, Z.; Zhao, T. Specific Inhibitory Effect of κ-Carrageenan Polysaccharide on Swine Pandemic 2009 H1N1 Influenza Virus. PLoS ONE 2015, 10, e0126577. [Google Scholar] [CrossRef]
- Diogo, J.V.; Novo, S.G.; González, M.J.; Ciancia, M.; Bratanich, A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Vet. Sci. 2015, 98, 142–144. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, Z.; Yu, P.; Zhang, X.; Dong, W.; Wang, X.; Chen, Y.; Liu, X. Inhibitory effect of iota-carrageenan on porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2019, 24, 261–270. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, D.; Zhou, M.; Xiao, W.; Zhang, Y.; Li, M.; Sui, B.; Wang, W.; Guan, H.; Chen, H.; et al. λ-Carrageenan P32 Is a Potent Inhibitor of Rabies Virus Infection. PLoS ONE 2015, 10, e0140586. [Google Scholar] [CrossRef]
- Gasbarri, M.; V’kovski, P.; Torriani, G.; Thiel, V.; Stellacci, F.; Tapparel, C.; Cagno, V. SARS-CoV-2 Inhibition by Sulfonated Compounds. Microorganisms 2020, 8, 1894. [Google Scholar] [CrossRef]
- Bansal, S.; Jonsson, C.B.; Taylor, S.L.; Figueroa, J.M.; Dugour, A.V.; Palacios, C.; Vega, J.C. Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Chahla, R.E.; Ruiz, L.M.; Ortega, E.S.; Moracelo, F.M.; Barreiro, F.; George, A.; Mancilla, C.; D’Amato, S.P.; Barrenechea, G.; Goroso, D.G.; et al. A randomized trial-intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents. medRxiv 2021. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Lombardo, M.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.P.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M.; et al. Efficacy of a nasal spray containing Iota-Carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data. Pharmacol. Res. Perspect. 2021, 9, e00810. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses in mice and severe acute respiratory syndrome coronavirus 2 in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef] [PubMed]
- Schütz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Scolaro, L.A.; Noseda, M.D.; Cerezo, A.S.; Damonte, E.B. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Maguire, R.A.; Zacharopoulos, V.R.; Phillips, D.M. Carrageenan-Based nonoxynol-9 spermicides for prevention of sexually transmitted infections. Sex. Transm. Dis. 1998, 25, 494–500. [Google Scholar] [CrossRef]
- Hamasuna, R.; Eizuru, Y.; Shishime, Y.; Minamishima, Y. Protective Effect of Carrageenan against Murine Cytomegalovirus Infection in Mice. Antivir. Chem. Chemother. 1993, 4, 353–360. [Google Scholar] [CrossRef]
- Lynch, S.A.; Breslin, R.; Bookelaar, B.; Rudtanatip, T.; Wongprasert, K.; Culloty, S.C. Immunomodulatory and Antiviral Effects of Macroalgae Sulfated Polysaccharides: Case Studies Extend Knowledge on Their Importance in Enhancing Shellfish Health, and the Control of a Global Viral Pathogen Ostreid Herpesvirus-1 microVar. Polysaccharides 2021, 2, 14. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulfated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Lahaye, M.; Ray, B. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta)—NMR analysis of ulvan oligosaccharides. Carbohydr. Res. 1996, 283, 161–173. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva “rigida”(Ulvales, Chlorophyta). Extraction and chemical composition. Carbohydr. Res. 1995, 274, 251–261. [Google Scholar] [CrossRef]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr. Res. 1995, 274, 313–318. [Google Scholar] [CrossRef]
- Ray, B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr. Polym. 2006, 66, 408–416. [Google Scholar] [CrossRef]
- Ivanova, V.; Rouseva, R.; Kolarova, M.; Serkedjieva, J.; Rachev, R.; Manolova, N. Isolation of a polysaccharide with antiviral effect from Ulva lactuca. Prep. Biochem. 1994, 24, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W.; Zhi’en, L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chan, Y.L.; Li, T.L.; Wu, C.J. Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar. Biotechnol. 2012, 14, 468–478. [Google Scholar] [CrossRef]
- Mendes, G.S.; Soares, A.R.; Martins, F.O.; Albuquerque, M.C.; Costa, S.S.; Yoneshigue-Valentin, Y.; Gestinari, L.M.; Santos, N.; Romanos, M.T. Antiviral activity of green marine alga Ulva fasciata on the replication of human metapneumovirus. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, X.; Song, L.; Liu, S.; Yu, H.; Wang, X.; Qin, Y.; Li, P. Antiviral Activity against Avian Leucosis Virus Subgroup J of Degraded Polysaccharides from Ulva pertusa. BioMed Res. Int. 2018, 2018, 9415965. [Google Scholar] [CrossRef] [Green Version]
- Reisky, L.; Préchoux, A.; Zühlke, M.K.; Bäumgen, M.; Robb, C.S.; Gerlach, N.; Roret, T.; Stanetty, C.; Larocque, R.; Michel, G.; et al. A marine bacterial enzymatic cascade degrades the algal polysaccharide ulvan. Nat. Chem. Biol. 2019, 15, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Grasdalen, H.B.; Larsen, B.; Smidsrod, O. 13C-NMR studies of monomieric composition and sequence in alginate. Carbohydr. Res. 1981, 89, 179–191. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Smidsrod, O. A study of the constitution of alginic acid by partial acid hydrolysis. Acta Chem. Scand. 1966, 20, 183–190. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Smidsrod, O. Uronic acid sequence in alginate from different sources. Carbohydr.Res. 1974, 32, 217–225. [Google Scholar] [CrossRef]
- McHugh, D.J. (Ed.) Production, properties and uses of alginates. In Production and Utilization of Products from Commercial Seaweeds; FAO: Rome, Italy, 1987; pp. 58–115. [Google Scholar]
- Xin, X.L.; Geng, M.Y.; Guan, H.S.; Li, Z.L. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitro. Chin. J. Mar. Drugs 2000, 19, 15–18. [Google Scholar]
- Jiang, B.; Xu, X.; Li, L.; Yuan, W. Study on—911k anti-HBV effect in HepG2. 2.15 cells culture. Prev. Med. 2003, 30, 517–518. [Google Scholar]
- Peng, Y.; Xie, E.; Zheng, K.; Fredimoses, M.; Yang, X.; Zhou, X.; Wang, Y.; Yang, B.; Lin, X.; Liu, J.; et al. Nutritional and chemical composition and antiviral activity of cultivated seaweed Sargassum naozhouense Tseng et Lu. Mar. Drugs 2013, 11, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Son, E.-W.; Rhee, D.-K.; Pyo, S. Antiviral and tumoricidal activities of alginate-stimulated macrophages are mediated by different mechanisms. Arch. Pharmacal. Res. 2003, 26, 960–966. [Google Scholar] [CrossRef]
- Wang, S.-X.; Zhang, X.-S.; Guan, H.-S.; Wang, W. Potential anti-HPV and related cancer agents from marine resources: An overview. Mar. Drugs 2014, 12, 2019–2035. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Wang, W.; Zhang, X.; Zhao, X.; Yu, G. Anti-HBV activity and mechanism of marine-derived polyguluronate sulfate (PGS) in vitro. Carbohydr. Polym. 2016, 143, 139–148. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, R.; Li, M.; Sun, H.; Su, M.; Ding, Y.; Chen, X.; Du, Z.; Jin, C.; Huang, C.; et al. Structural characterization of cocktail-like targeting polysaccharides from Ecklonia kurome Okam and their anti-SARS-CoV-2 activities invitro. bioRxiv 2021. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Meiyu, G.; Fuchuan, L.; Xianliang, X.; Jing, L.; Zuowei, Y.; Huashi, G. The Potential Molecular Targets of Marine Sulfated Polymannuroguluronate Interfering with HIV-1 Entry: Interaction between SPMG and HIV-1 Rgp120 and CD4 Molecule. Antivir. Res. 2003, 59, 127–135. [Google Scholar] [CrossRef]
- Hui, B.; Li, J.; Mei, Y.G. Sulfated Polymannuroguluronate, a Novel Anti-Acquired Immune Deficiency Syndrome Drug Candidate, Decreased Vulnerability of PC12 Cells to Human Immunodeficiency Virus Tat Protein through Attenuating Calcium Overload. J. Neurosci. Res. 2008, 86, 1169–1177. [Google Scholar] [CrossRef]
- Ueno, M.; Nogawa, M.; Siddiqui, R.; Watashi, K.; Wakita, T.; Kato, N.; Ikeda, M.; Okimura, T.; Isaka, S.; Oda, T.; et al. Acidic Polysaccharides Isolated from Marine Algae Inhibit the Early Step of Viral Infection. Int. J. Biol. Macromol. 2019, 124, 282–290. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and Antioxidant Properties of Active Alginate Edible Films Containing Phenolic Extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Falcó, I.; Flores-Meraz, P.L.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. Antiviral Activity of Alginate-Oleic Acid Based Coatings Incorporating Green Tea Extract on Strawberries and Raspberries. Food Hydrocoll. 2019, 87, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.M.; Dufresne, M.; Helle, F.; Hoffmann, T.W.; Francois, C.; Brochot, E.; Paullier, P.; Legallais, C.; Duverlie, G.; Castelain, S. Alginate Hydrogel Protects Encapsulated Hepatic HuH-7 Cells against Hepatitis C Virus and Other Viral Infections. PLoS ONE 2014, 9, e109969. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, V.; Seganti, L.; Marchetti, M.; Sinibaldi, L.; Orsi, N.; Nicoletti, R. Effect of Natural and Semisynthetic Polymers on Rabies Virus Infection in CER Cells. Res. Virol. 1993, 144, 151–158. [Google Scholar] [CrossRef]
- Mastromarino, P.; Petruzziello, R.; Macchia, S.; Rieti, S.; Nicoletti, R.; Orsi, N. Antiviral Activity of Natural and Semisynthetic Polysaccharides on the Early Steps of Rubella Virus Infection. J. Antimicrob. Chemother. 1997, 39, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Han, G.; Li, X.; Wu, Y.; Zhang, Y.; Xia, Y.; Yue, C.; Wu, D. Cytotoxicity and Antiviral Activity of Calcium Alginate Fibers and Zinc Alginate Fibers. Adv. Mater. Res. 2010, 152–153, 1475–1478. [Google Scholar] [CrossRef]
- Remichkova, M.; Galabova, D.; Roeva, I.; Karpenko, E.; Shulga, A.; Galabov, A.S. Anti-Herpesvirus Activities of Pseudomonas sp. S-17 Rhamnolipid and Its Complex with Alginate. Z. Naturforsch. C J. Biosci. 2008, 63, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.; Tobias, R.S.; Ayliffe, G.A.J.; Browne, R.M. An in Vitro Study of the Antiviral Properties of an Alginate Impression Material Impregnated with Disinfectant. J. Dent. 1989, 17, 137–139. [Google Scholar] [CrossRef]
- Pardee, K.I.; Ellis, P.; Bouthillier, M.; Towers, G.H.N.; French, C.J. Plant Virus Inhibitors from Marine Algae. Can. J. Bot. 2004, 82, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Lv, X.; He, L.; Shi, H.; Liao, S.; Liu, C.; Huang, Q.; Li, X.; He, X.; Chen, H.; et al. Dual-Action Pesticide Carrier That Continuously Induces Plant Resistance, Enhances Plant Anti-Tobacco Mosaic Virus Activity, and Promotes Plant Growth. J. Agric. Food Chem. 2019, 67, 10000–10009. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y. Antiviral Activity of Alginate against Infection by Tobacco Mosaic Virus. Carbohydr. Polym. 1999, 38, 183–186. [Google Scholar] [CrossRef]
- Terasawa, M.; Hayashi, K.; Lee, J.-B.; Nishiura, K.; Matsuda, K.; Hayashi, T.; Kawahara, T. Anti-Influenza A Virus Activity of Rhamnan Sulfate from Green Algae Monostroma nitidum in Mice with Normal and Compromised Immunity. Mar. Drugs 2020, 18, 254. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef]
- Meneghetti, M.C.Z.; Hughes, A.J.; Rudd, T.R.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [Green Version]
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2015, 68, 76–141. [Google Scholar] [CrossRef]
- Vicenzi, E.; Canducci, F.; Pinna, D.; Mancini, N.; Carletti, S.; Lazzarin, A.; Bordignon, C.; Poli, G.; Clementi, M. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg. Infect. Dis. 2004, 10, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Bermejo-Jambrina, M.; Eder, J.; Kaptein, T.M.; van Hamme, J.L.; Helgers, L.C.; Vlaming, K.E.; Brouwer, P.J.M.; Vlaar, A.P.J.; van Baarle, F.E.H.P.; Spaargaren, M.; et al. SARS-CoV-2 infection and transmission depends on heparan sulfates and is blocked by low molecular weight heparins. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gupta, Y.; Maciorowski, D.; Zak, S.E.; Kulkarni, C.V.; Herbert, A.S.; Durvasula, R.; Fareed, J.; Dye, J.M.; Kempaiah, P. Heparin: A simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy. Int. J. Biol. Macromol. 2021, 183, 203–212. [Google Scholar] [CrossRef]
- Kalra, R.S.; Kandimalla, R. Engaging the spikes: Heparan sulfate facilitates SARS-CoV-2 spike protein binding to ACE2 and potentiates viral infection. Signal Transduct. Target. Ther. 2021, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Liu, L.; Chopra, P.; Li, X.; Bouwman, K.M.; Tompkins, S.M.; Wolfert, M.A.; de Vries, R.P.; Boons, G.J. Heparan sulfate proteoglycans as attachment factor for SARS-CoV-2. ACS Cent. Sci. 2021, 7, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.J.; Green, L.R.; Monk, P.N. Unfractionated heparin potently inhibits the binding of SARS-CoV-2 spike protein to a human cell line. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tree, J.A.; Turnbull, J.E.; Buttigieg, K.R.; Elmore, M.J.; Coombes, N.; Hogwood, J.; Mycroft-West, C.J.; Lima, M.A.; Skidmore, M.A.; Karlsson, R.; et al. Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. Br. J. Pharmacol. 2021, 178, 626–635. [Google Scholar] [CrossRef]
- Kim, S.Y.; Koetzner, C.A.; Payne, A.F.; Nierode, G.J.; Yu, Y.; Wang, R.; Barr, E.; Dordick, J.S.; Kramer, L.D.; Zhang, F.; et al. Glycosaminoglycan compositional analysis of relevant tissues in Zika virus pathogenesis and in vitro evaluation of heparin as an antiviral against Zika virus infection. Biochemistry 2019, 58, 1155–1166. [Google Scholar] [CrossRef]
- Pourianfar, H.R.; Poh, C.L.; Fecondo, J.; Grollo, L. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against Enterovirus 71. Virus Res. 2012, 169, 22–29. [Google Scholar] [CrossRef]
- Goodfellow, I.G.; Sioofy, A.B.; Powell, R.M.; Evans, D.J. Echoviruses Bind Heparan Sulfate at the Cell Surface. J. Virol. 2001, 75, 4918–4921. [Google Scholar] [CrossRef] [Green Version]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef]
- Young, E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar] [CrossRef]
- Lindahl, U.; Li, J.P. Heparin—An old drug with multiple potential targets in COVID-19 therapy. J. Thromb. Haemost. 2020, 18, 2422–2424. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Su, D.; Li, Y.; Guimond, S.E.; Rudd, T.R.; Elli, S.; Miller, G.; Nunes, Q.M.; Procter, P.; Bisio, A.; et al. SARS-CoV-2 Spike S1 Receptor Binding Domain undergoes Conformational Change upon Interaction with Low Molecular Weight Heparins. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Wang, H.; Yang, C.; Cai, F.; Zeng, F.; Cheng, F.; Liu, Y.; Zhou, T.; Deng, B.; et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: A retrospective clinical study. Clin. Transl. Sci. 2020, 13, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Bray, H.G.; Gregory, J.E.; Stacey, M. Chemistry of tissues. I. Chondroitin Cartilage. Biochem. J. 1944, 38, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Djerbal, L.; Lortat-Jacob, H.; Kwok, J. Chondroitin sulfates and their binding molecules in the central nervous system. Glycoconj. J. 2017, 34, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Roseman, S. Reflections on glycobiology. J. Biol. Chem. 2001, 276, 41527–41542. [Google Scholar] [CrossRef] [Green Version]
- Silbert, J.E.; Sugumaran, G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life 2002, 54, 177–186. [Google Scholar] [CrossRef]
- Marchetti, M.; Trybala, E.; Superti, F.; Johansson, M.; Bergstorm, T. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 2004, 318, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergefall, K.; Trybala, E.; Johansson, M.; Uyama, T.; Naito, S.; Yamada, S.; Kitagawa, H.; Sugahara, K.; Bergstrom, T. Chondroitin Sulfate characterised by the E-disaccharide unit is a potent inhibitor of Herpes Simplex Virus infectivity and provides the virus binding sites on gro2C cells. J. Biol. Chem. 2005, 280, 32193–32199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wander, R.; Xu, Y.; Liu, J. Enzyme-Based Methods to Synthesize Homogeneous Glycosaminoglycan Oligosaccharides; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Yates, E.A.; Skidmore, M.A. Marine glycosaminoglycan-like carbohydrates as potential drug candidates for infectious disease. Biochem. Soc. Trans. 2018, 46, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Xiao, C.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 activity and structure-activity-relationship study of a fucosylated glycosaminoglycan from an echinoderm by targeting the conserved CD4 induced epitope. Biochim. Biophys. Acta 2013, 1830, 4681–4691. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Pauwels, R.; Balzarini, J.; Arnout, J.; Desmyter, J.; Declercq, E. Mechanism of Inhibitory Effect of Dextran Sulfate and Heparin on Replication of Human Immunodeficiency Virus In vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 6132–6136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yu, G.; Li, Y.; Shen, L.; Qian, Y.; Yang, J.; Wang, F. Inhibitory effects of sulfated lentinan with different degree of sulfation against tobacco mosaic virus (TMV) in tobacco seedlings. Pestic. Biochem. Phys. 2015, 122, 38–43. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.-Y.; Xia, X.-M.; Li, P.; Wang, K.-Y. Inhibitory effect of sulfated lentinan and lentinan against tobacco mosaic virus (TMV) in tobacco seedlings. Int. J. Biol. Macromol. 2013, 61, 264–269. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Y.; Wang, D.; Ma, X.; Zhao, X.; Zhao, B.; Zhao, B.; Wang, J.; Liu, P. Sulfated modification can enhance the adjuvanticity of lentinan and improve the immune effect of ND vaccine. Vaccine 2009, 27, 660–665. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Z.; Ma, X.; Hu, Y.; Huang, X.; Fan, Y.; Yang, S.; Guo, L. Effects of sulfated lentinan on cellular infectivity of avian infectious bronchitis virus. Carbohydr. Polym. 2010, 79, 461–465. [Google Scholar] [CrossRef]
- Gao, Y.; Fukuda, A.; Katsuraya, K.; Kaneko, Y.; Mimura, T.; Nakashima, H.; Uryu, T. Synthesis of Regioselective Substituted Curdlan Sulfates with Medium Molecular Weights and Their Specific Anti-HIV-1 Activities. Macromolecules 1997, 30, 3224–3228. [Google Scholar] [CrossRef]
- Ichiyama, K.; Gopala Reddy, S.B.; Zhang, L.F.; Chin, W.X.; Muschin, T.; Heinig, L.; Suzuki, Y.; Nanjundappa, H.; Yoshinaka, Y.; Ryo, A.; et al. Sulfated polysaccharide, curdlan sulfate, efficiently prevents entry/fusion and restricts antibody-dependent enhancement of dengue virus infection in vitro: A possible candidate for clinical application. PLoS Negl. Trop. Dis. 2013, 7, e2188. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Tan, H.; Xu, D.; Yin, F.; Cheng, Y.; Zhang, X.; Liu, Y.; Wang, F. Effect and mechanisms of curdlan sulfate on inhibiting HBV infection and acting as an HB vaccine adjuvant. Carbohydr. Polym. 2014, 110, 446–455. [Google Scholar] [CrossRef]
- Faccin-Galhardi, L.C.; Ray, S.; Lopes, N.; Ali, I.; Espada, S.F.; Dos Santos, J.P.; Ray, B.; Linhares, R.E.C.; Nozawa, C. Assessment of antiherpetic activity of nonsulfated and sulfated polysaccharides from Azadirachta indica. Int. J. Biol. Macromol. 2019, 137, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Galhardi, L.C.F.; Yamamoto, K.A.; Linhares, R.E.C.; Bandyopadhyay, S.S.; Sinha, S.; Nozawa, C.; Ray, B. Water-extracted polysaccharides from Azadirachta indica leaves: Structural features, chemical modification and anti-bovine herpesvirus type 1 (BoHV-1) activity. Int. J. Biol. Macromol. 2010, 47, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Faccin-Galhardi, L.C.; Yamamoto, K.I.; Ray, S.; Ray, B.; Linhares, R.E.C.; Nozawa, C. The in vitro antiviral property of Azadirachta indica polysaccharides for poliovirus. J. Ethnopharmacol. 2012, 142, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Muschin, T.; Budragchaa, D.; Kanamoto, T.; Nakashima, H.; Ichiyama, K.; Yamamoto, N.; Shuqin, H.; Yoshida, T. Chemically sulfated natural galactomannans with specific antiviral and anticoagulant activities. Int. J. Biol. Macromol. 2016, 89, 415–420. [Google Scholar] [CrossRef]
- de Godoi, A.M.; Faccin-Galhardi, L.C.; Lopes, N.; Rechenchoski, D.Z.; de Almeida, R.R.; Ricardo, N.M.; Nozawa, C.; Linhares, R.E. Antiviral Activity of Sulfated Polysaccharide of Adenanthera pavonina against Poliovirus in HEp-2 Cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 712634. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.M.; de Morais, S.M.; da Silva, A.R.; Barroso, N.D.; Pontes Filho, T.R.; Araújo, F.M.; Vieira, Í.G.; Lima, D.M.; Guedes, M.I. Antiviral and Antioxidant Activities of Sulfated Galactomannans from Plants of Caatinga Biome. Evid.-Based Complement. Altern. Med. 2015, 2015, 591214. [Google Scholar] [CrossRef]
- Chrestani, F.; Sierakowski, M.R.; de Andrade Uchoa, D.E.; Nozawa, C.; Sassaki, G.L.; Gorin, P.A.J.; Ono, L. In vitro antiherpetic and antirotaviral activities of a sulfate prepared from Mimosa scabrella galactomannan. Int. J. Biol. Macromol. 2009, 45, 453–457. [Google Scholar] [CrossRef]
- Ono, L.; Wollinger, W.; Rocco, I.M.; Coimbra, T.L.; Gorin, P.A.; Sierakowski, M.-R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antivir. Res. 2003, 60, 201–208. [Google Scholar] [CrossRef]
- Budragchaa, D.; Bai, S.; Kanamoto, T.; Nakashima, H.; Han, S.; Yoshida, T. Synthetic galactomannans with potent anti-HIV activity. Carbohydr. Polym. 2015, 130, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Muschin, T.; Kanamoto, T.; Nakashima, H.; Yoshida, T. Sulfation and biological activities of konjac glucomannan. Carbohydr Polym. 2013, 94, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, Z.H.; Chen, J. Research on Anti-Coxsackievirus B Activity of Konjac Oligo-Glucomannan Sulfate in Hela Cell Line. Adv. Mater. Res. 2013, 662, 417–423. [Google Scholar] [CrossRef]
- Cardozo, F.T.G.S.; Larsen, I.V.; Carballo, E.V.; Jose, G.; Stern, R.A.; Brummel, R.C.; Camelini, C.M.; Rossi, M.J.; Simões, O.C.M.; Brandt, C.R. In vivo Anti-Herpes Simplex Virus Activity of a Sulfated Derivative of Agaricus brasiliensis Mycelial Polysaccharide. Antimicrob. Agents Chemother. 2013, 57, 2541–2549. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, F.T.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R.; de Mendonça, M.M.; Simões, C.M. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, Y.; Wang, D.; Qin, T.; Liu, C.; Liu, X.; Sheng, X.; Chang, S.; Fan, Y.; Guo, L.; et al. The optimization of sulfation modification conditions for ophiopogonpolysaccharide based on antiviral activity. Int. J. Biol. Macromol. 2012, 51, 657–662. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Matsuda, M.; Shigeta, S.; Okutani, K. Revelation of Antiviral Activities by Artificial Sulfation of a Glycosaminoglycan from a Marine Pseudomonas. Mar. Biotechnol. 1999, 1, 102–106. [Google Scholar] [CrossRef]
- Ghosh, T.; Auerochs, S.; Saha, S.; Ray, B.; Marschall, M. Anti-Cytomegalovirus Activity of Sulfated Glucans Generated from a Commercial Preparation of Rice Bran. Antivir. Chem. Chemother. 2010, 21, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Ghosh, K.; Hahn, F.; Wangen, C.; Strojan, H.; Müller, R.; Anand, N.; Ali, I.; Bera, K.; Ray, B. Chemically sulfated polysaccharides from natural sources: Assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int. J. Biol. Macromol. 2019, 136, 521–530. [Google Scholar] [CrossRef]
- Wang, S.C.; Bligh, S.W.; Zhu, C.L.; Shi, S.S.; Wang, Z.T.; Hu, Z.B.; Crowder, J.; Branford-White, C.; Vella, C. Sulfated beta-glucan derived from oat bran with potent anti-HIV activity. J. Agric. Food Chem. 2008, 56, 2624–2629. [Google Scholar] [CrossRef]
- Qiu, H.; Tang, W.; Tong, X.; Ding, K.; Zuo, J. Structure elucidation and sulfated derivatives preparation of two alpha-d-glucans from Gastrodia elata Bl. and their anti-dengue virus bioactivities. Carbohydr. Res. 2007, 342, 2230–2236. [Google Scholar] [CrossRef]
- Tong, X.K.; Qiu, H.; Zhang, X.; Shi, L.P.; Wang, G.F.; Ji, F.H.; Ding, H.Y.; Tang, W.; Ding, K.; Zuo, J.P. WSS45, a sulfated alpha-D-glucan, strongly interferes with Dengue 2 virus infection in vitro. Acta Pharmacol. Sin. 2010, 31, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, F.T.; Camelini, C.M.; Leal, P.C.; Kratz, J.M.; Nunes, R.J.; Mendonça, M.M.; Simões, C.M. Antiherpetic mechanism of a sulfated derivative of Agaricus brasiliensis fruiting bodies polysaccharide. Intervirology 2014, 57, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Sacchelli, B.A.L.; Faccin-Galhardi, L.C.; Ito, V.Y.; Lopes, J.L.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Orsato, A. Botryosphaeran and sulfonated derivatives as novel antiviral agents for herpes simplex and dengue fever. Int. J. Biol. Macromol. 2019, 138, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Flexner, C.; Barditch-Crovo, P.A.; Kornhauser, D.M.; Farzadegan, H.; Nerhood, L.J.; Chaisson, R.E.; Bell, K.M.; Lorentsen, K.J.; Hendrix, C.W.; Petty, B.G. Pharmacokinetics, Toxicity, and Activity of Intravenous Dextran Sulfate in Human Immunodeficiency Virus Infection. Antimicrob. Agents Chemother. 1991, 35, 2544–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Damme, L.; Govinden, R.; Mirembe, F.M.; Guédou, F.; Solomon, S.; Becker, M.L.; Pradeep, B.S.; Krishnan, A.K.; Alary, M.; Pande, B.; et al. Lack of Effectiveness of Cellulose Sulfate Gel for the Prevention of Vaginal HIV Transmission. N. Engl. J. Med. 2008, 359, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Wijgert, J.H.H.M.; Shattock, R.J. Vaginal Microbicides: Moving Ahead After an Unexpected Setback. AIDS 2007, 21, 2369. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Takayama, K.; Honma, K.; Gonda, T.; Matsuzaki, K.; Hatanaka, K.; Uryu, T.; Yoshida, O.; Nakashima, H.; Yamamoto, N.; et al. Synthesis, Structure and Antiviral Activity of Sulfates of Cellulose and its Branched Derivatives. Carbohydr. Polym. 1990, 14, 53–63. [Google Scholar] [CrossRef]
- Yang, T.; Jia, M.; Zhou, S.; Pan, F.; Mei, Q. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int. J. Biol. Macromol. 2012, 50, 768–772. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Chen, K.; Gao, Y.; Gao, S.; Liu, X.; Li, J. Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Int. J. Biol. Macromol. 2013, 52, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, Y.; Zhao, X.; Lu, Y.; Wang, J.; Zhang, F.; Sun, J. Sulfated modification can enhance the adjuvant activity of astragalus polysaccharide for ND vaccine. Carbohydr. Polym. 2008, 73, 303–308. [Google Scholar] [CrossRef]
- López, S.N.; Ramallo, I.A.; Sierra, M.G.; Zacchino, S.A.; Furlan, R.L. Chemically engineered extracts as an alternative source of bioactive natural product-like compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 441–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendricks, G.L.; Velazquez, L.; Pham, S.; Qaisar, N.; Delaney, J.C.; Viswanathan, K.; Albers, L.; Comolli, J.C.; Shriver, Z.; Knipe, D.M.; et al. Heparin Octasaccharide Decoy Liposomes Inhibit Replication of Multiple Viruses. Antivir. Res. 2015, 116, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Said, J.S.; Trybala, E.; Görander, S.; Ekblad, M.; Liljeqvist, J.Å.; Jennische, E.; Lange, S.; Bergström, T. The Cholestanol-Conjugated Sulfated Oligosaccharide PG545 Disrupts the Lipid Envelope of Herpes Simplex Virus Particles. Antimicrob. Agents Chemother. 2016, 60, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Said, J.S.; Trybala, E.; Andersson, E.; Johnstone, K.; Liu, L.; Wimmer, N.; Ferro, V.; Bergström, T. Lipophile-conjugated sulfated oligosaccharides as novel microbicides against HIV-1. Antivir. Res. 2010, 86, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Bergström, T.; Andrighetti-Fröhner, C.R.; Bendrioua, L.; Ferro, V.; Trybala, E. Potent anti-respiratory syncytial virus activity of a cholestanol-sulfated tetrasaccharide conjugate. Antivir. Res. 2012, 93, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Supramaniam, A.; Liu, X.; Ferro, V.; Herrero, L.J. Prophylactic Antiheparanase Activity by PG545 Is Antiviral In vitro and Protects against Ross River Virus Disease in Mice. Antimicrob. Agents Chemother. 2018, 62, 4. [Google Scholar] [CrossRef] [Green Version]
- Modhiran, N.; Gandhi, N.S.; Wimmer, N.; Cheung, S.; Stacey, K.; Young, P.R.; Ferro, V.; Watterson, D. Dual targeting of dengue virus virions and NS1 protein with the heparan sulfate mimic PG545. Antivir. Res. 2019, 168, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, M.; Adamiak, B.; Bergstrom, T.; Johnstone, K.D.; Karoli, T.; Liu, L.; Ferro, V.; Trybala, E. A highly lipophilic sulfated tetrasaccharide glycoside related to muparfostat (PI-88) exhibits virucidal activity against herpes simplex virus. Antivir. Res. 2010, 86, 196–203. [Google Scholar] [CrossRef]
- Guimond, S.E.; Mycroft-West, C.J.; Gandhi, N.S.; Tree, J.A.; Buttigieg, K.R.; Coombes, N.; Nystrom, K.; Said, J.; Setoh, Y.X.; Amarilla, A.; et al. Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike-ACE2 interaction. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Kim, S.-K. Production of chitooligosaccharides using ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Polym. 2000, 41, 7. [Google Scholar] [CrossRef]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.E.; Cho, H.; Jung, H.G.; Lee, W.; Seo, H.Y.; Lee, S.H.; Ahn, D.G.; Kim, S.J.; Yu, J.W.; et al. Antiviral efficacy of orally delivered neoagarohexaose, a nonconventional TLR4 agonist, against norovirus infection in mice. Biomaterials 2020, 263, 120391. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Choi, J.W.; Jang, W.J.; Kang, Y.-S.; Lee, C.W.; Synytsya, A.; Park, Y.I. Low-molecular weight mannogalactofucans prevent herpes simplex virus type 1 infection via activation of Toll-like receptor 2. Int. J. Biol. Macromol. 2017, 103, 286–293. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Errea, M.I.; Matulewicz, M.C.; Damonte, E.B. Antiviral activity of natural sulfated galactans on herpes virus multiplication in cell culture. Planta Med. 1997, 63, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Ji, J.; Wang, L.; Wu, H.; Luan, H.-M. Bio-function summary of marine oligosaccharides. Int. J. Biol. 2011, 3, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Miao, B.C.; Geng, M.Y.; Li, J.; Li, F.; Chen, H.; Guan, H.S.; Ding, J. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem. Pharmacol. 2004, 68, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular Basis of the Therapeutical Potential of Clove (Syzygium aromaticum L.) and Clues to Its Anti-COVID-19 Utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Salih, A.E.M.; Thissera, B.; Yaseen, M.; Hassane, A.S.I.; El-Seedi, H.R.; Sayed, A.M.; Rateb, M.E. Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS CoV-2. Mar. Drugs 2021, 19, 406. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, J.; Salmivirta, M.; Sturiale, L.; Gimenez-Gallego, G.; Lindahl, U. Sequence analysis of heparan sulfate epitopes with graded affinities for fibroblast growth factors 1 and 2. J. Biol. Chem. 2001, 276, 30744–30752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfield, B.W.; Leduc, Y.; Esford, L.; Visalli, R.J.; Brandt, C.R.; Tufaro, F. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology 1995, 208, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, R.; Balasubramaniam, A.; Tiwari, V.; Zhang, F.; Bridges, A.; Linhardt, R.J.; Shukla, D.; Liu, J. Using a 3-O-sulfated heparin octasaccharide to inhibit the entry of herpes simplex virus type 1. Biochemistry 2008, 47, 5774–5783. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.; Coombe, D.R. Heparin Mimetics: Their Therapeutic Potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef]
- El Masri, R.; Seffouh, A.; Lortat-Jacob, H.; Vives, R.R. The “in and out” of glucosamine 6-O-sulfation: The 6th sense of heparan sulfate. Glycoconj. J. 2017, 34, 285–298. [Google Scholar] [CrossRef]
- Singh, A.; Kett, W.C.; Severin, I.C.; Agyekum, I.; Duan, J.; Amster, I.J.; Proudfoot, A.E.I.; Coombe, D.R.; Woods, R.J. The interaction of heparin tetrasaccharides with chemokine CCL5 is modulated by sulfation pattern and pH. J. Biol. Chem. 2015, 290, 15421–15436. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Fung Ka, W.; Rodriguez, E.; Patel, R.; Gor, J.; Mulloy, B.; Perkins Stephen, J. The solution structure of heparan sulfate differs from that of heparin: Implications for function. J. Biol. Chem. 2013, 288, 27737–27751. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef]
- Du, B.; Zeng, H.; Yang, Y.; Bian, Z.; Xu, B. Anti-inflammatory activity of polysaccharide from Schizophyllum commune as affected by ultrasonication. Int. J. Biol. Macromol. 2016, 91, 100–105. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, F.; Xu, X.; Zhang, L. Extended chain conformation of β-glucan and its effect on antitumor activity. J. Mater. Chem. B 2017, 5, 5623–5631. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Szymanski, C.M.; Schnaar, R.L.; Aebi, M. Bacterial and viral infections. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hard, G.W., Aebi, M., Darvill, A.G., Kinoshitaet, T., et al., Eds.; Cold Spring Harbor: Long Island, NY, USA, 2015; pp. 527–538. [Google Scholar]
- Mitsuya, H.; Looney, D.J.; Kuno, S.; Ueno, R.; Wongstaal, F.; Broder, S. Dextran sulfate suppresion of viruses in the HIV family: Inhibition of virion binding to CD4+ cells. Science 1988, 240, 646–649. [Google Scholar] [CrossRef]
- Batinic, D.; Robey, F.A. The V3 Region of the Envelope Glycoprotein of Human Immunodeficiency Virus Type 1 Binds Sulfated Polysacchardies and CD4-Derived Synthetic Peptides. J. Biol. Chem. 1992, 267, 6664–6671. [Google Scholar] [CrossRef]
- Callahan, L.N.; Phelan, M.; Mallinson, M.; Norcross, M.A. Dextran Sulfate Blocks Antibody Binding to the Principal Neutralizing Domain of Human Immunodeficiency Virus Type 1 without Interfering with GP120-CD4 Interactions. J. Virol. 1991, 65, 1543–1550. [Google Scholar] [CrossRef] [Green Version]
- Schols, D.; Pauwels, R.; Desmyter, J.; Declercq, E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral GP120 glycoprotein express by T-cells persistently infected with HIV-1. Virology 1990, 175, 556–561. [Google Scholar] [CrossRef]
- Watson, K.; Gooderham, N.J.; Davies, D.S.; Edwards, R.J. Interaction of the transactivating protein HIV-1 tat with sulfated polysaccharides. Biochem. Pharmacol. 1999, 57, 775–783. [Google Scholar] [CrossRef]
- Moelling, K.; Schulze, T.; Diringer, H. Inhibition of human immunodeficiency virus type-1 RNase-h by sulfated polyanions. J. Virol. 1989, 63, 5489–5491. [Google Scholar] [CrossRef] [Green Version]
- Pirrone, V.; Wigdahl, B.; Krebs, F.C. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antivir. Res. 2011, 90, 168–182. [Google Scholar] [CrossRef]
- Shieh, M.T.; WuDunn, D.; Montgomery, R.I.; Esko, J.D.; Spear, P.G. Cell Surface Receptors for Herpes Simplex Virus are Heparan Sulfate Proteoglycans. J. Cell Biol. 1992, 116, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- WuDunn, D.; Spear, P.G. Initial Interaction of Herpes Simplex Virus with Cells is Binding to Heparan Sulfate. J. Virol. 1989, 63, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, T.; Hayashi, T.; Hayashi, K.; Hamada, J.; Lee, J.-B.; Sankawa, U. An Antivirally Active Sulfated Polysaccharide from Sargassum horneri (TURNER) C. AGARDH. Biol. Pharm. Bull. 1998, 21, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Darmani, N.A.; Yue, B.Y.J.T.; Shukla, D. In vitro antiviral activity of neem (Azardirachta indica L.) bark extract against herpes simplex virus type-1 infection. Phytother. Res. 2009, 24, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Hu, B.; Huang, X.; Chai, Y.; Zhou, D.; Wang, Y.; Shuai, H.; Yang, D.; Hou, Y.; Zhang, X.; et al. Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nat. Commun. 2021, 12, 134. [Google Scholar] [CrossRef]
- Hao, W.; Ma, B.; Li, Z.; Wang, X.; Gao, X.; Li, Y.; Qin, B.; Shang, S.; Cui, S.; Tan, Z. Binding of the SARS-CoV-2 Spike Protein to Glycans. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yan, L.; Song, Y.; Xia, K.; He, P.; Zhang, F.; Chen, S.; Pouliot, R.; Weiss, D.J.; Tandon, R.; Bates, J.T.; et al. Heparan sulfates from bat and human lung and their binding to the spike protein of SARS-CoV-2 virus. Carbohydr. Polym. 2021, 260, 117797. [Google Scholar] [CrossRef]
- Yue, J.; Jin, W.; Yang, H.; Faulkner, J.; Song, X.; Qiu, H.; Teng, M.; Azadi, P.; Zhang, F.; Linhardt, R.J.; et al. Heparan Sulfate Facilitates Spike Protein-Mediated SARS-CoV-2 Host Cell Invasion and Contributes to Increased Infection of SARS-CoV-2 G614 Mutant and in Lung Cancer. Front. Mol. Biosci. 2021, 8, 649575. [Google Scholar] [CrossRef]
- Papa, G.; Mallery, D.L.; Albecka, A.; Welch, L.G.; Cattin-Ortolá, J.; Luptak, J.; Paul, D.; McMahon, H.T.; Goodfellow, I.G.; Carter, A.; et al. Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell-cell fusion. PLoS Pathog. 2021, 17, e1009246. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Liu, S.-L. Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 2021, 297, 100847. [Google Scholar] [CrossRef]
- Shajahan, A.; Pepi, L.E.; Rouhani, D.S.; Heiss, C.; Azadi, P. Glycosylation of SARS-CoV-2: Structural and functional insights. Anal. Bioanal. Chem. 2021, 413, 7179–7193. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CL (pro) inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg. Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef]
- Rechter, S.; König, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dörnenburg, H.; Walter, C.; Marschall, M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antivir. Res. 2006, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Magnan, S.; Tota, J.E.; El-Zein, M.; Burchell, A.N.; Schiller, J.T.; Ferenczy, A.; Tellier, P.P.; Coutlée, F.; Slavetchva, N. Efficacy of a Carrageenan gel Against Transmission of Cervical HPV (CATCH): Interim analysis of a randomized, double-blind, placebo-controlled, phase 2B trial. Clin. Microbiol. Infect. 2019, 25, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Perino, A.; Consiglio, P.; Maranto, M.; De Franciscis, P.; Marci, R.; Restivo, V.; Manzone, M.; Capra, G.; Cucinella, G.; Calagna, G. Impact of a new carrageenan-based vaginal microbicide in a female population with genital HPV-Infection: First experimental results. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6744–6752. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (US). Lubricant Investigation in Men to Inhibit Transmission of HPV Infection (Limit-HPV); National Library of Medicine (US): Bethesda, MD, USA, 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT02354144 (accessed on 29 January 2021).
- Eccles, R.; Meier, C.; Jawad, M.; Weinmüllner, R.; Grassauer, A.; Prieschl-Grassauer, E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: A randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir. Res. 2010, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, M.; Enzenhofer, E.; Schneider, S.; Rauch, M.; Bodenteich, A.; Neumann, K.; Prieschl-Grassauer, E.; Grassauer, A.; Lion, T.; Mueller, C.A. Efficacy of a Carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir. Res. 2013, 14, 124. [Google Scholar] [CrossRef] [Green Version]
- Koenighofer, M.; Lion, T.; Bodenteich, A.; Prieschl-Grassauer, E.; Grassauer, A.; Unger, H.; Mueller, C.A.; Fazekas, T. Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials. Multidiscip. Respir. Med. 2014, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Graf, C.; Bernkop-Schnürch, A.; Egyed, A.; Koller, C.; Prieschl-Grassauer, E.; Morokutti-Kurz, M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int. J. Gen. Med. 2018, 11, 275–283. [Google Scholar] [CrossRef] [Green Version]
- BETADINE Cold Defense Nasal Spray. Available online: http://sg.betadine.com/en/sg/cold-and-flu/betadine-cold-defence-nasal-spray (accessed on 21 November 2021).
- Instructions for Use: Coldamaris Prophylactic Nasal Spray. Available online: http://www.faam-zarin.com/images/Coldamarisprophylactic-GA_Englisch (accessed on 21 November 2021).
- Dogliotti, A. Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Prophylaxis of COVID-19 Disease in Health Personnel Dedicated to Patients Care with COVID-19 Disease (CARR-COV-02). 2020. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04521322 (accessed on 30 August 2020).
- Moakes, R.J.A.; Davies, S.P.; Stamataki, Z.; Grover, L.M. Formulation of a composite nasal spray enabling enhanced surface coverage and prophylaxis of SARS-CoV-2. Adv. Mater. 2021, 33, e2008304. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A viruses. Carbohydr. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.-B.; Nakano, T.; Hayashi, T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013, 15, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, P.J. Crossing barriers: Infections of the lung and the gut. Mucosal Immunol. 2009, 2, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef]
- Barton, C.; Kouokam, J.C.; Hurst, H.; Palmer, K.E. Pharmacokinetics of the Antiviral Lectin Griffithsin Administered by Different Routes Indicates Multiple Potential Uses. Viruses 2016, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.; Birse, K.; Holm, J.B.; Gajer, P.; Humphrys, M.S.; Garber, D.; Guenthner, P.; Noël-Romas, L.; Abou, M.; McCorrister, S.; et al. Impact of the griffithsin anti-HIV microbicide and placebo gels on the rectal mucosal proteome and microbiome in non-human primates. Sci. Rep. 2018, 8, 8059. [Google Scholar] [CrossRef]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS ONE 2011, 6, e22635. [Google Scholar] [CrossRef] [Green Version]
- Kouokam, J.C.; Lasnik, A.B.; Palmer, K.E. Studies in a Murine Model Confirm the Safety of Griffithsin and Advocate Its Further Development as a Microbicide Targeting HIV-1 and Other Enveloped Viruses. Viruses 2016, 8, 311. [Google Scholar] [CrossRef] [Green Version]
- Mazalovska, M.; Kouokam, J.C. Lectins as Promising Therapeutics for the Prevention and Treatment of HIV and Other Potential Coinfections. BioMed Res. Int. 2018, 2018, 3750646. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C., 2nd; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W., Jr.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; Liwang, P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: Evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef]
- Lal, M.; Lai, M.; Ugaonkar, S.; Wesenberg, A.; Kizima, L.; Rodriguez, A.; Levendosky, K.; Mizenina, O.; Fernández-Romero, J.; Zydowsky, T. Development of a Vaginal Fast-Dissolving Insert Combining Griffithsin and Carrageenan for Potential Use Against Sexually Transmitted Infections. J. Pharm. Sci. 2018, 107, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Griffithsin-Based Rectal Microbicide for Prevention of Viral Entry (Prevent); National Library of Medicine (U.S.): Bethesda, MD, USA, 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT04032717 (accessed on 25 July 2019).
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Mader, J.; Gallo, A.; Schommartz, T.; Handke, W.; Nagel, C.-H.; Günther, P.; Brune, W.; Reich, K. Calcium spirulan derived from Spirulina platensis inhibits herpes simplex virus 1 attachment to human keratinocytes and protects against herpes labialis. J. Allergy Clin. Immunol. 2016, 137, 197–203.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.L.; Ding, H.; Geng, M.Y.; Liang, P.F.; Li, Y.X.; Guan, H.S. Studies of the anti- AIDS effects of marine polysaccharide drug 911 and its related mechanisms of action. Chin. J. Mar. Drugs 2000, 6, 4–8. [Google Scholar]
- Liu, H.; Geng, M.; Xin, X.; Li, F.; Zhang, Z.; Li, J.; Ding, J. Multiple and multivalent interactions of novel anti-AIDS drug candidates, sulfated polymannuronate (SPMG)-derived oligosaccharides, with gp120 and their anti-HIV activities. Glycobiology 2005, 15, 501–510. [Google Scholar] [CrossRef]
- Umemura, M.; Itoh, M.; Makimura, Y.; Yamazaki, K.; Umekawa, M.; Masui, A.; Matahira, Y.; Shibata, M.; Ashida, H.; Yamamoto, K. Design of a Sialylglycopolymer with a Chitosan Backbone Having Efficient Inhibitory Activity against Influenza Virus Infection. J. Med. Chem. 2008, 51, 4496–4503. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Makimura, Y.; Itoh, M.; Yamamoto, T.; Mine, T.; Mitani, S.; Simizu, I.; Ashida, H.; Yamamoto, K. One-step synthesis of efficient binding-inhibitor for influenza virus through multiple addition of sialyloligosaccharides on chitosan. Carbohydr. Polym. 2010, 81, 330–334. [Google Scholar] [CrossRef]
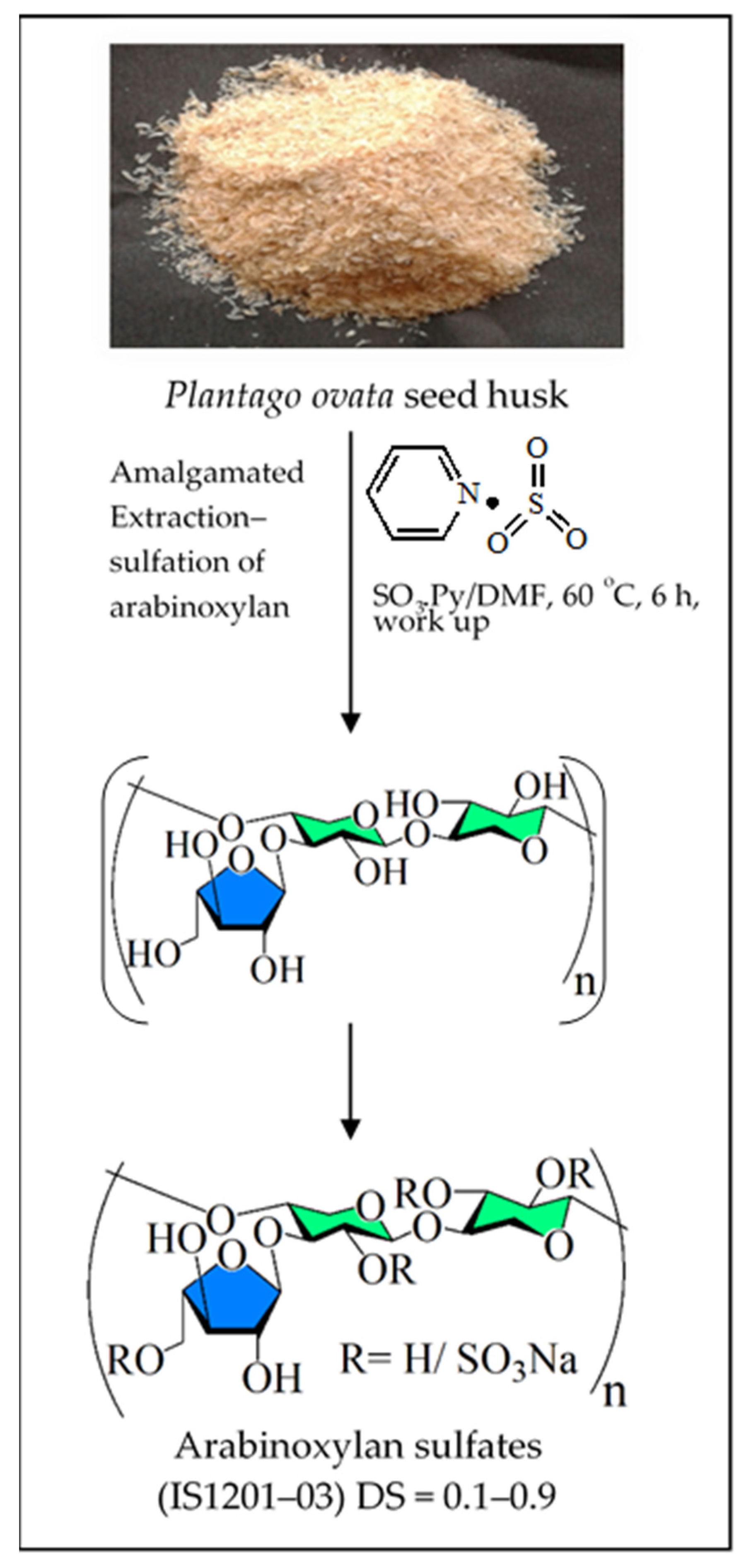
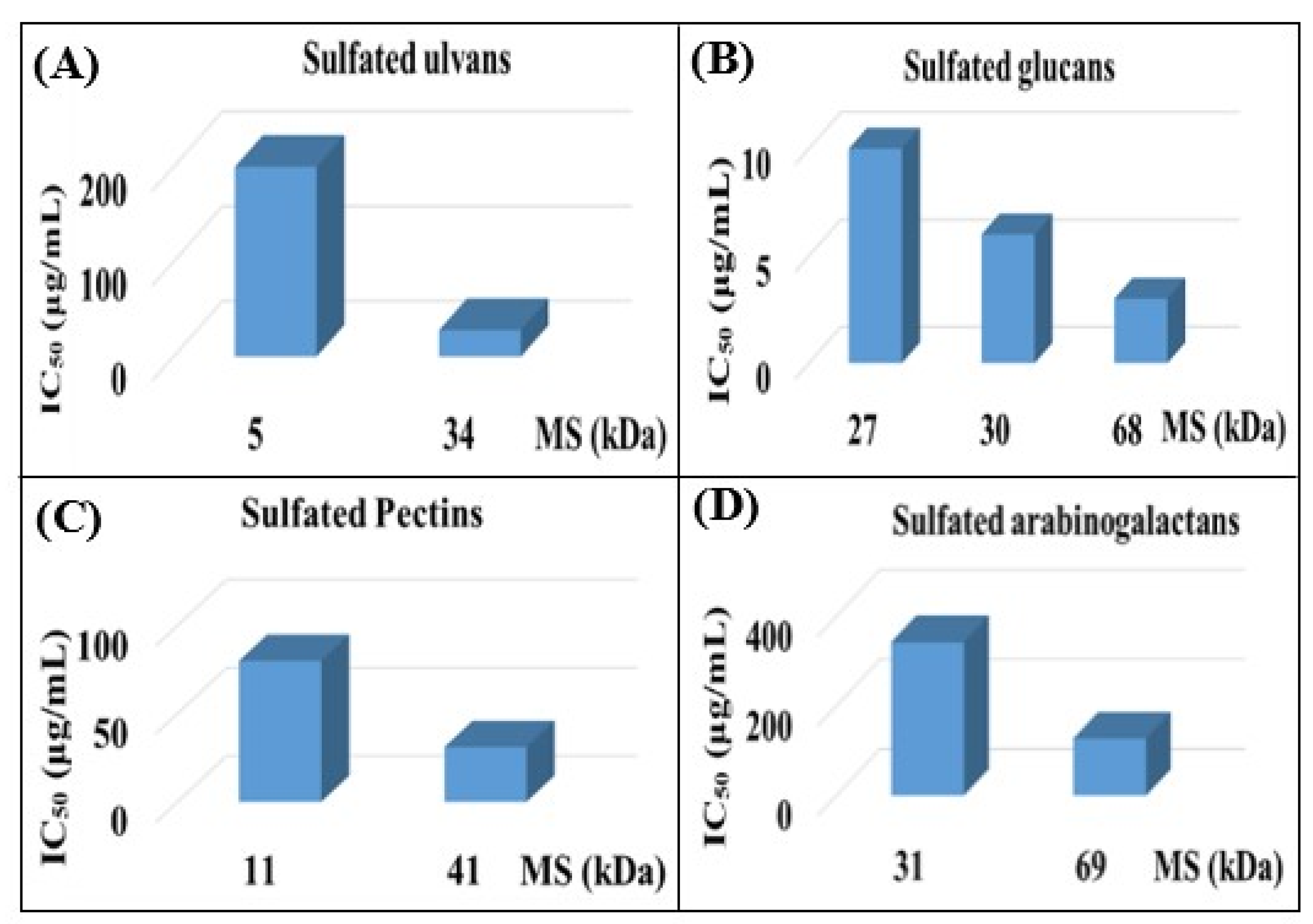
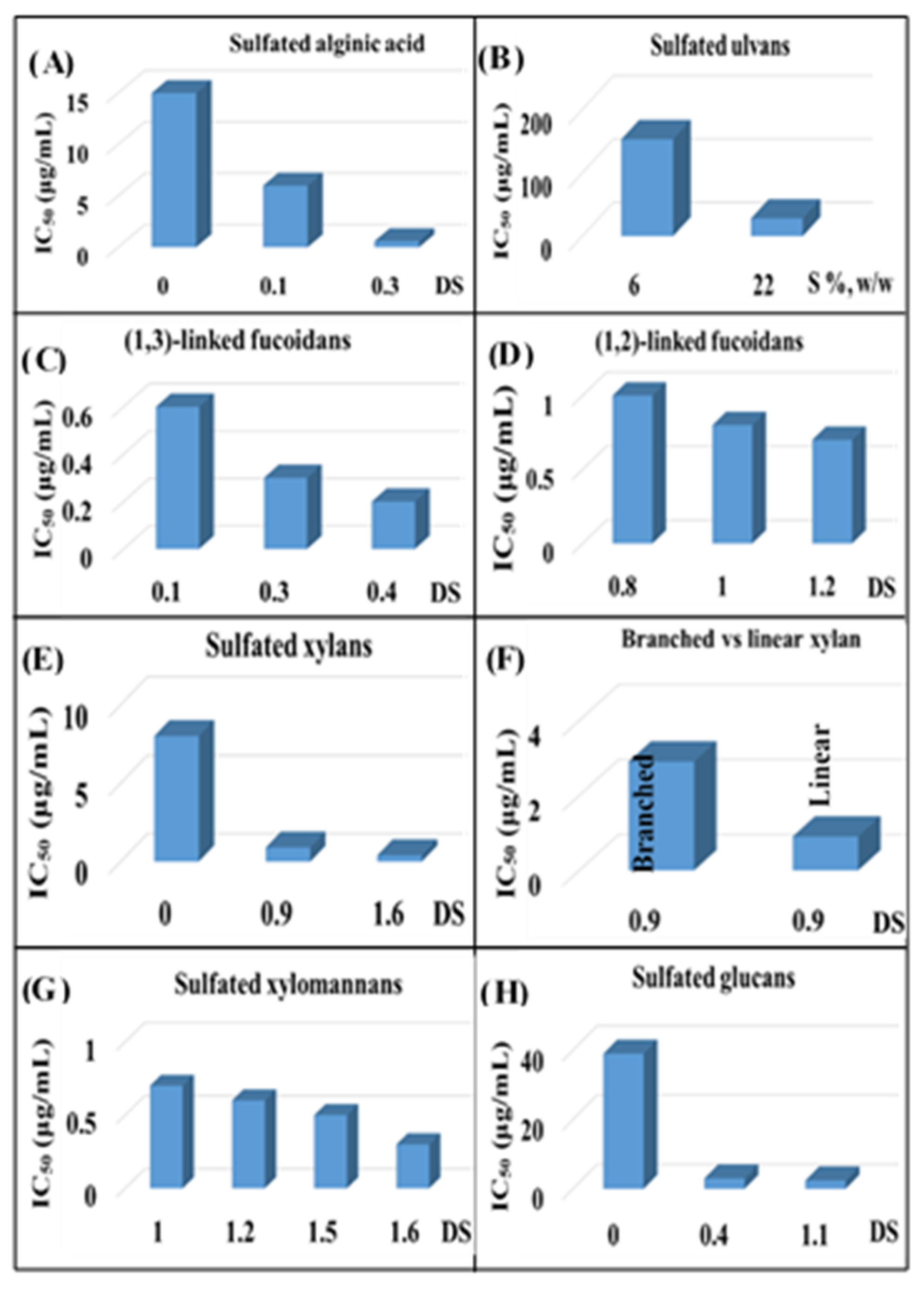
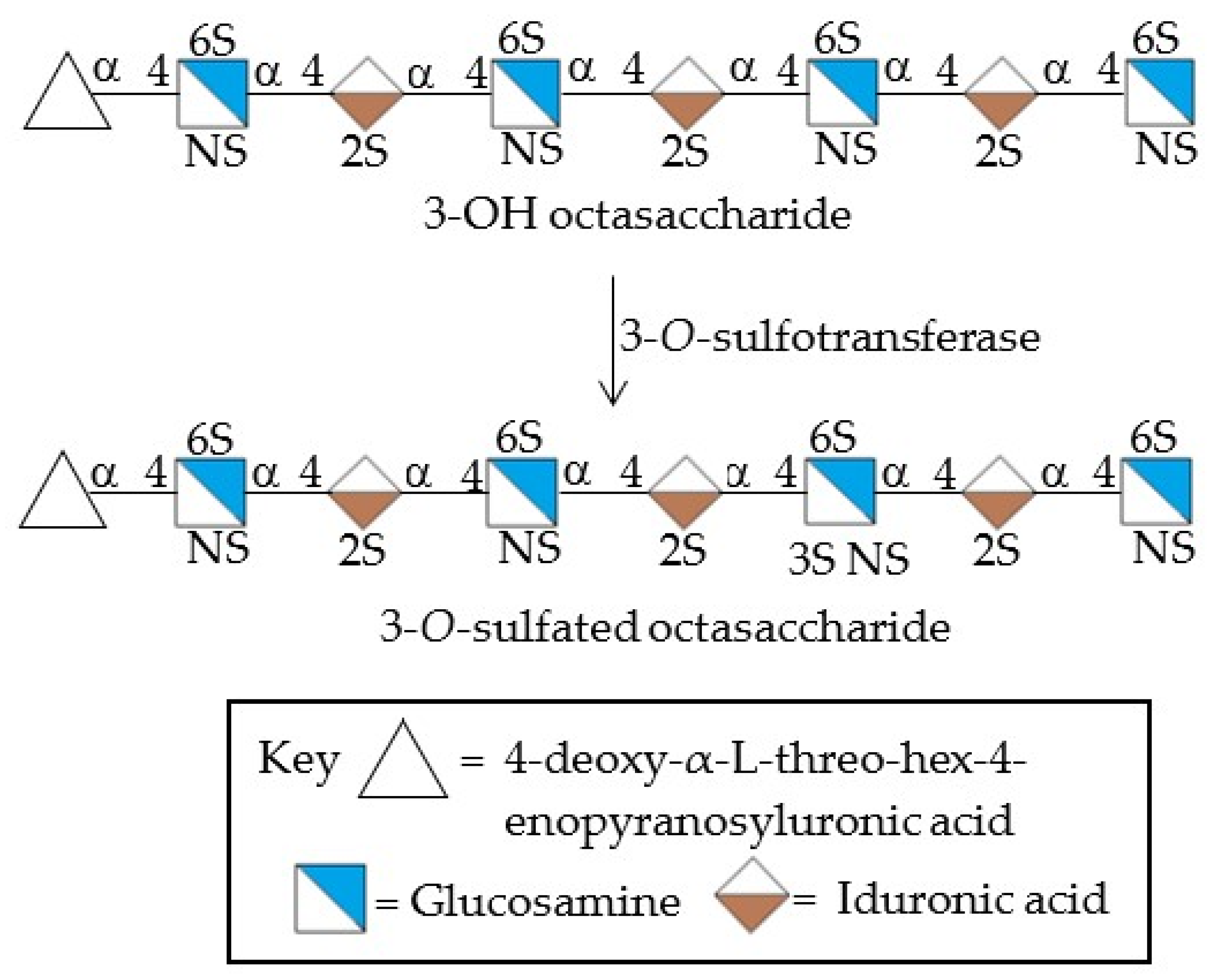
| Entry and Compound | Origin | Molecular Mass a | Sulfate (mole %)/DS | Analyzed Viruses | IC50/EC50 Value (μg/mL) | Comments on Antiviral Activity | References |
|---|---|---|---|---|---|---|---|
| 1. Fucoidan |  Adapted from [71] and [72] | ||||||
| Padina tetrastromatica | - | 0 | HSV-1 (HSV-2) | >100 (50) | Inhibition of viral adsorption to the cell, DS represents the key parameter | [73] | |
| 0.8 | 1 (0.4) | ||||||
| 1.0 | 0.8 (0.3) | ||||||
| 1.2 | 0.7 (0.3) | ||||||
| Sargassum tenerrimum | 30 | 2 | HSV-1 | 1.4 | Block of viral entry | [74] | |
| - | 6 | 0.5 | |||||
| Undaria pinnatifida | 9 | 10.4 | HSV-1 | 2.5 | Interference with virus-host cell binding, broad-spectrum activity | [75] | |
| HSV-2 | 2.6 | ||||||
| HCMV | 1.5 | ||||||
| IV-A | 15 | ||||||
| Sphacelaria indica | - | 0 | HSV-1 | N. D | Interfere with viral attachment and entry | [76] | |
| 26 | 4 | 1.3 | |||||
| - | 7 | 1.5 | |||||
| Laminaria angustata | 56 | 4.2 | HSV-1 | 0.6 | Direct interaction with viral particles | [77] | |
| - | 6.7 | 0.32 | |||||
| - | 7.3 | 0.21 | |||||
| Cystoseira indica | - | HSV-1 (HSV-2) | 35 (20) | Inhibition of viral attachment, desulfation removes antiviral effect | [78] | ||
| - | 8 | 2.1 (0.5) | |||||
| 35 | 0 | >100 | |||||
| - | 9 | 3 (1.3) | |||||
| Stoechospermm marginatum | 40 | 1–13 | HSV-1 (HSV-2) | 1.15–50 (0.78–50) | Interference with the HSV-1 replication cycle | [71] | |
| Sargassum henslowianum | 66, 59 | 32, 32 | HSV-1 (HSV-2) | 0.9,0.5 (0.8,0.5) | Block of HSV-2 adsorption to the cell | [79] | |
| Fucus evanescens | - | - | HSV-1 (HSV-2){ECHO-1} [HIV-1] | 80 (65) {110} [25] | Inhibition of the early stage of virus replication, broad-spectrum activity | [80] | |
| - | - | 100 (85) {93} [25] | |||||
| Sargassum swartzii | 45 | 19.2 | HIV-1 | 3.1 | DS may be the key parameter | [81] | |
| 30 | 24.5 | 1.6 | |||||
| >100 | 24 | 0.6 | |||||
| Saccharina japonica | 100 | - | SARS-CoV-2 | 8.3 | Binding to the S protein of SARS-CoV-2 | [82] | |
| 12 | - | 16 | |||||
| 2. Galactan sulfates | 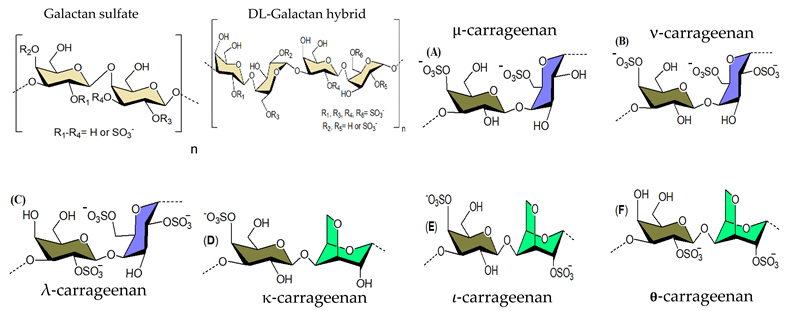 | ||||||
| Gracilaria corticata | - | 0 | HSV-1 (HSV-2) | 56 (24) | Inhibition of virus adsorption, interfering with the interaction of viral glycoproteins | [83] | |
| - | 1 | 16 (8) | |||||
| - | 1.2 | 15 (7) | |||||
| - | 1.5 | 4(1.7) | |||||
| - | 0 | >100 (37) | |||||
| 30 | 0.9 | 27 (14.6) | |||||
| - | 2 | 1.6 (1.1) | |||||
| - | 2.1 | 1.6 (1.5) | |||||
| Bostrychia montagnei | 6–46 | 11.2–24 | HSV-1 (HSV-2) | 13–50 (11–50) | Shielding off the positively charged sites | [84] | |
| Gracilaria corticata | 165 | 11.6 | HSV-1 (HSV-2) | 0.19 (0.24) | Inhibition of virus entry by interaction with viral glycoprotein | [85] | |
| 62 | 2.6 | 27.5 (38.5) | |||||
| 54 | 2.5 | 50 (45.9) | |||||
| Gymnogongrus torulosus | 18–77 | - | HSV-1 (DEN-2) | 0.6–16 (0.19–1.7) | Binding of the surface envelope glycoprotein | [86] | |
| Schizymenia binderi | 380 | 22.2 | HSV-1 | 0.76 | Interference with the HSV–HS interaction | [87] | |
| HSV-2 | 0.63 | ||||||
| Solieria chordalis (𝜄-carrageenan) | - | 0.3–5.1 | HSV-1 | 0.3–19 | - | [88] | |
| Meristiella gelidium- 𝜄/Ϗ/ν carrageenan | - | 29 | HSV-2 (DENV-2) | 0.06 (0.79) | - | [89] | |
| - | 33 | 0.05 (0.14) | |||||
| - | 29 | 0.04 (0.21) | |||||
| Ϗ, λ-carrageenan | - | - | HSV-1 | 3.7, 1.6 | Inhibition of virus adsorption to the host cell, broad-spectrum activity | [90] | |
| HSV-2 | 2, 1.5 | ||||||
| HIV-1 | 12, 1.9 | ||||||
| CMV | 2.8, 0.3 | ||||||
| VSV | 0.3, 0.2 | ||||||
| 𝜄-carrageenan | - | - | HSV-1 | 2 | Inhibition of an undefined step in virus replication, broad-spectrum antiviral activity | [91] | |
| HSV-2 | 10 | ||||||
| SFV | 10 | ||||||
| Vaccinia | 10 | ||||||
| ASF | 10 | ||||||
| EMC | 10 | ||||||
| κ-carrageenan | 1–4 | 4–30 | IAV | 14.9–142 | Inhibition of IAV multiplication | [92] | |
| ι-, λ-, κ-carrageenan | - | - | DENV-1 (DENV-2) {DENV-3} [DENV-4] | 40.7 (0.4) {4.1} [8.2] | Inhibitors of DENV-2 and 3 multiplications in Vero and HepG2 cells, broad-spectrum activities | [93] | |
| >50 (0.1) {2} [4.2] | |||||||
| >50 (1.8) {6.3} [50] | |||||||
| ι- carrageenan | - | - | H3N2 | 0.04 | Surface block of epithelia in IAV-infected animals | [94] | |
| H1N1 | 0.20 | ||||||
| ι- carrageenan | - | 32–39 | IV-A, B | 0.3–1.4 | Inhibition of viral entry | [95] | |
| SARS- CoV-2 | 0.9 | ||||||
| 3. Xylomannan sulfate | 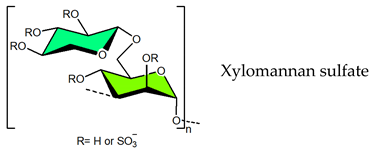 | ||||||
| Nemalion helminthoides | 14 | 19.4 | HSV-1 (HSV-2) {DENV-2} | 9.68 (3.72) {8.22} | DS may play an important role | [96] | |
| 12 | 22.9 | 5.43 (2.79) {16.1} | |||||
| Scinaia hatei | - | N. D | HSV-1 (HSV-2) | 8 (12) | Mode of action directed to viral entry | [97] | |
| - | 0.93 | 0.9 (0.4) | |||||
| - | 1.42 | 1.2 (0.22) | |||||
| - | 1.64 | 0.4(0.3) | |||||
| - | 1.95 | 1.4(0.4) | |||||
| Sebdenia polydactyla | - | 0 | HSV-1 | >10 | DS may play an important role, DS of 1 is sufficient for antiviral activity | [98] | |
| 150 | 0.6 | 2.8 | |||||
| - | 1 | 0.7 | |||||
| - | 1.2 | 0.6 | |||||
| - | 1.5 | 0.47 | |||||
| - | 1.6 | 0.35 | |||||
| Scinaia hatei | - | 0 | HSV-1 (HSV-2) | >100 (100) | Inhibition of virus-cell attachment | [99] | |
| 160 | 8 | 0.5 (0.5) | |||||
| Scinaia hatei | - | 9 | DENV-2 | 1.1 | Interference with viral multiplication cycle | [100] | |
| 160 | 8 | 0.6 | |||||
| 4. Rhamnan sulfate | 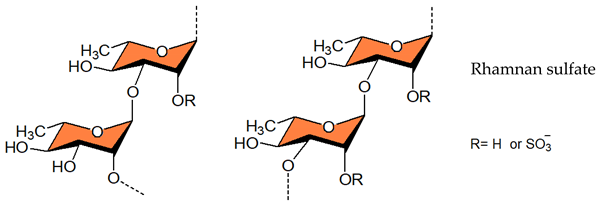 | ||||||
| Monostroma nitidum | - | 31.7 | HSV-2 | 0.87 | Inhibitor of HSV-2 entry | [101] | |
| Monostroma Iatissimum | - | - | HSV-1 | 0.78 | Inhibition of virus adsorption, board-spectrum activity | [102] | |
| HCMV | 1.7 | ||||||
| HIV-1 | 1.5 | ||||||
| Monostroma latissimum | 513 | 26.1 | EV71 | - | Inhibition of viral replication | [103] | |
| 5. Ulvan | 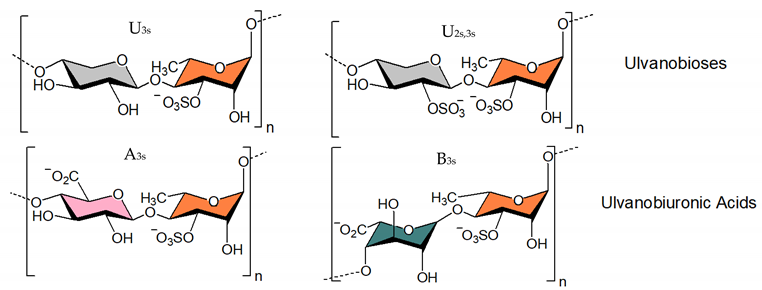 | ||||||
| Adapted from [104] | |||||||
| Ulva intestinalis | - | - | MV | 3.6 | - | [105] | |
| Ulva pertusa | 1068 | 17.7 | VSV | - | Interaction with viral envelope glycoprotein | [106] | |
| 39 | 17.9 | 0.6 | |||||
| 18 | 18.1 | 15 | |||||
| 5 | 17.1 | 6 | |||||
| Ulva clathrata | 360 | 9.5 | NDV | 0.1 | Inhibition of viral entry | [107] | |
| Ulva armoricana | - | - | HSV-1 | 373 321 | Antiviral activity correlated to high levels of Rhap | [108] | |
| 6. Alginic acid | 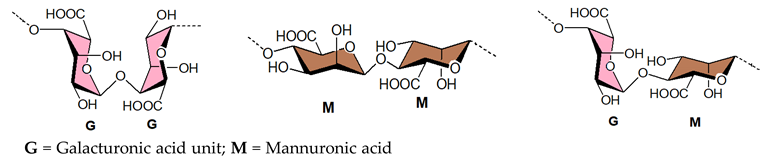 | ||||||
| Sphacelaria indica | 21 | 0 | HSV-1 | 10 | Interfere with viral attachment and entry | [76] | |
| - | 8 | 0.65 | |||||
| - | 9 | 0.6 | |||||
| Sargassum tenerrimum | 26 | 0 | HSV-1 | 15 | Block of viral entry | [74] | |
| - | 2 | 6 | |||||
| 7. Heparin | 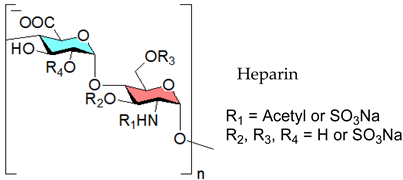 | ||||||
| Heparin | - | 15 | SARS-CoV-2 | 5.99 | Affinity to SGP | [109] | |
| Heparin | - | - | SARS-CoV | - | Protein binding responsible for SARS-CoV inhibition | [110] | |
| Heparin | - | - | SARS-CoV-2 | - | Heparin may bind to viral protein | [111] | |
| Heparin | - | 6.4 | HIV-1 (HIV-2) | 0.52 (1.7) | Block of virus adsorption | [112] | |
| 8. Chondroitin sulfates types A, C, D and E (CS-A, CS-C, CS-D, CS-E) |  | ||||||
| Thelenota anana | - | - | HIV-1 | 0.24–31.8 | Potently binds viral gp120 protein | [113] | |
| Chondroitin sulfate | - | 2.41 | HSV-2 | 74.8 | - | [114] | |
| - | - | 26.6 | |||||
| Chondroitin sulfate | - | - | DENV-1 | 0.53 | Entry inhibitor targeting viral E protein, broad spectrum activity | [115] | |
| DENV-2 | 3.80 | ||||||
| DENV-3 | 1.38 | ||||||
| DENV-4 | 0.30 | ||||||
| JEV | 0.93 | ||||||
| Entry and Compound | Origin/Preparation Notes | Molecular Mass | Sulfate (mol %)/DS | Analyzed Viruses | IC50, EC50 Value (μg mL−1) | Comments on Antiviral Activity | References |
|---|---|---|---|---|---|---|---|
| Chemically sulfated plant/fungal/bacterial polysaccharides | |||||||
 | |||||||
| 1. Lentinan sulfate | Lentinus edodes in HSO₃Cl-Py | - | 0 | TMV | - | Affinity of the polyanion towards positive ions on viral particles | [252] |
| - | 0.69 | - | |||||
| - | 0.98 | - | |||||
| - | 1.37 | - | |||||
| Lentinus edodes In HSO₃Cl-Py | - | 0 | TMV | - | Affinity towards TMV coat protein | [253] | |
| - | 0.98 | - | |||||
| Lentinula edodes In HSO₃Cl-Py | - | 0 | NDV | - | [254] | ||
| - | 0.69 | - | |||||
| - | 0.98 | - | |||||
| - | 1.37 | - | |||||
| Lentinus edodes In HSO₃Cl-Py | - | 0 | IBV | - | Activity refers to DS (up to DS value 0.98) | [255] | |
| - | 0.69 | - | |||||
| - | 0.98 | - | |||||
| - | 1.37 | - | |||||
| 2. Dextran sulfate | Leuconostocmesenteroid In HSO₃Cl-Py | 1–70 | 81 | HIV-1 HIV-2 | 0.2–7.1 0.1–3.9 | Activity by shielding off the positively charged sites in the V3 loop of the viral envelope glycoprotein gp120 | [31] |
| 3. Curdlan sulfate | Curdlan in DMSO In SO3-py | 6.2–10.8 | 0.66–1.55 | HIV | 0.04–0.4 | DS is an antiviral determinant, but not the position of sulfate groups | [256] |
| Curdlan In SO3-py | 14 | 1.4 | DENV-2 | 0.26 | Inhibition of viral infection at the step of virus-host cell binding | [257] | |
| 6 | 1.5 | 0.37 | |||||
| Curdlan In SO3-py | 172 | 9.23 | HBV | - | Interference with virus binding to host cells surfaces | [258] | |
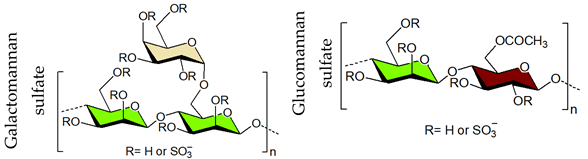 | 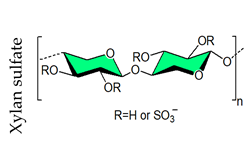 | ||||||
| 4. Pectin sulfate | Azadirachta indica In SO3-pyridine | 41 | 4 | HSV-1 | 31.1 | Interference at an early stage of the viral replication cycle | [259] |
| 11 | 4 | 80.5 | |||||
| 80 | - | BoHV-1 | 32.1 | Inhibition of virus-cell adsorption | [260] | ||
| 41 | 4 | 105.2 | |||||
| 41 | 4 | PV-1 | 37.5 | Inhibition of the initial stage of viral replication | [261] | ||
| 11 | 4 | 12.1 | |||||
| 5. Galactomannan sulfates | Fenugreek gum In PSA and SO3-Py | 7–24 | 0.7–1.4 | HIV | 0.4–1.6 | Electrostatic interaction between negatively charged sulfate groups and positively charged amino groups of viral surface proteins | [262] |
| Guar gum In PSA and SO3-Py | 8–23 | 1.1–1.3 | 0.3–0.6 | ||||
| Locust bean gum In PSA and SO3-P | 9–23 | 1–1.4 | 0.3–0.7 | ||||
| Tara gum In PSA, SO3-Py | 6–24 | 0.7–1.3 | 0.2–8 | ||||
| Adenanthera pavonina In HSO₃Cl-Py | 700 | 1.21 | PV-1 | 1.18 | Inhibition mainly the initial stages of viral infection | [263] | |
| A. pavonina C. ferrea D. gardneriana In HSO₃Cl-Py | - | 0.72–0.82 | DENV -2 | - | Entry inhibitor of DENV-2 | [264] | |
| Mimosa scabrella In HSO₃Cl-Py | - | 0 | HSV-1 | n.a. | Inhibition of the virus attachment step | [265] | |
| 620 | 0.62 | <2.5 | |||||
| Leucaena leucocephala In HSO₃Cl-Py | - | 0 | YFV | n.a. | Block of early stages of viral replication | [266] | |
| 574 | 0.50 | 200 | |||||
| Commercial Galactomannans In PSA | 4 | 1.11 | HIV | 2.14 | Electrostatic interaction between sulfate and amino groups | [267] | |
| 4.6 | 1.12 | 1.93 | |||||
| 5.2 | 1.15 | 0.44 | |||||
| 6.5 | 1.16 | 0.23 | |||||
| 7.5 | 1.52 | 0.18 | |||||
| 6. Glucomannan sulfate | Konjac glucomanna In PSA and SO3-Py | 8 | 1.3 | HIV | 1.4 | Electrostatic interaction between sulfate and amino groups | [268] |
| 8 | 1.4 | 1.3 | |||||
| 8 | 1.9 | 1.6 | |||||
| 56 | 1.6 | 0.7 | |||||
| Konjac glucomannan In HSO₃Cl-Py | - | 33.11 | CVB | 148 | Block of virus invading function | [269] | |
| Agaricus brasiliensis In HSO₃Cl-Py | 86 | 14.77 | HSV-1 HSV-2(vivo) | 17.27 4.73 | Inhibition of viral attachment and entry | [270] | |
| Agaricus brasiliensis In HSO₃Cl-Py | 86 | 14.77 | HSV-1 HSV-2 | 1.24 0.39 | Inhibition of viral attachment | [271] | |
| 7. Xylan sulfate | Scinaia hatei In SO3-py | 0–1.95 | HSV-1 HSV-2 | 0.4–7.6 0.22–11.7 | Inhibition of viral entry | [97] | |
| 8. Ophiopogon polysaccharide | Ophiopogon japonicus In HSO₃Cl-Py | 0.83–1.52 | NDV | - | Inhibition of viral adsorption | [272] | |
| 9. Glycosa minoglycan | Pseudomonas In H2SO4-DMF | - | - | IVA | >100 | Inhibition of viral attachment to the cell prior to viral penetration | [273] |
| 130 | 4.3 | 16 | |||||
| 150 | 8 | 5.2 | |||||
| Sulfated polysaccharide generated by combined extraction-sulfation from natural sources | |||||||
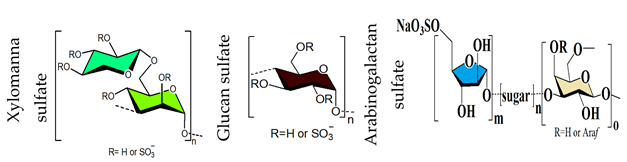 | 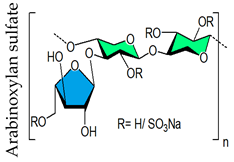 | ||||||
| 10. Xylomannan sulfate | Scinaia hatei In SO3⋅Py | 12–74 | 11.3–50.1 | HSV-1 HSV-2 | 0.67–88 (0.22–38.55) | Sulfate groups represent hallmark of activity | [43] |
| 11. Glucan sulfate | Rice bran In Oleum-DMF | 68 | 1.6 | HSV-1 GPCMV MCMV HCMV | 3–>10 8.1–>10 3.4–8.1 2.4–6.5 | Inhibition of viral entry | [47] |
| 30.5 | 1.7 | ||||||
| 27.3 | 1.2 | ||||||
| Rice bran In SO3-Py | - | 0.3–0.4 | HCMV | n.a. | Sulfate groups represent hallmark of activity | [274] | |
| - | 2 | 3.46 | |||||
| Eleocharis dulcis fruit In oleum–DMF | - | 0 | HCMV | >30 | Mode of antiviral action mostly based on the inhibition of viral entry | [275] | |
| - | 1.2 | - | |||||
| 94 | 1.7 | 2.3 | |||||
| - | 0.7 | - | |||||
| Oat Bran In HSO₃Cl-Py | 500 | 0 | HIV-1 | n.a. | Negative compound charges bind to positively charged amino acids | [276] | |
| 686 | 36.5 | 5.98 | |||||
| Gastrodia elata Bl In HSO₃Cl-Py | 280 | 0 | DENV-2 | n.a. | DS is the determining factor of antiviral activity | [277] | |
| 65 | 0.206 | 20.6 | |||||
| 190 | 1.68 | 10.7 | |||||
| Gastrodia elata Bl In HSO₃Cl-Py | 190 | 1.68 | DENV-2 | 0.68 | Interference with viral adsorption | [278] | |
| Agaricus brasiliensis In HSO₃Cl-Py | 609 | 0 | HSV-1 (HSV-2) | n.a. (n.a.) | Inhibition of viral adsorption and penetration | [279] | |
| 127 | 1.88 | 6.7 (4.6) | |||||
| Botryosphaeran In HSO₃Cl-Py | - | 0 | HSV DENV | 39.3 or n.a. | Electrostatic interaction between sulfate and amino groups | [280] | |
| - | 0.4 | 3.0 (66) | |||||
| - | 1.1 | 2.4 (78) | |||||
| 12.Sulfated Ulvan | Enteromorpha compressa In Oleum-DMF | 5 | - | HSV-1 | 200 | Electrostatic interference with the positive charge of viral glycoprotein | [45] |
| 34 | 22 | 28.2 | |||||
| 13.Arabinogalactan sulfate | Anogeissus latifolia gum In SO3⋅Py | - | 0 | HSV-1 | n.a. | Inhibition of viral attachment and entry | [44] |
| 69 | 0.1 | 127 | |||||
| 35 | 0.3 | 630 | |||||
| 31 | 0.5 | 342 | |||||
| 14.Arabinoxylan sulfate | Plantago ovata seed husk In SO3⋅Py | 31.3 | 0.1 | HSV-1 | n.a. | DS determines antiviral activity | [46] |
| 26.7 | 0.4 | 11.5 | |||||
| 18.4 | 0.9 | 2.9 | |||||
| Study Title | Identifier | Status | Results | Primary Outcome | |
|---|---|---|---|---|---|
| 1 | Study to Investigate if Sucking a Coldamaris Lozenge Elutes Sufficient Iota-carrageenan to Inactivate Usual Common Cold Viruses | NCT04533906 | Completed | Pending | Iota-carrageenan concentration in saliva |
| 2 | USEFULNESS of Topic Ivermectin and Carrageenan to Prevent Contagion of COVID 19 (IVERCAR) | NCT04425850 | Completed | Published | Number of participants testing positive for COVID-19 |
| 3 | Prophylaxis COVID-19 in Healthcare Agents by Intensive Treatment With Ivermectin and Iota-carrageenan (Ivercar-Tuc) | NCT04701710 | Completed | Pending | Number of subjects who were diagnosed with COVID-19 in EG and CG |
| 4 | Carrageenan Nasal Spray for COVID-19 Prophylaxis | NCT04590365 | Recruiting | Pending | Rate of COVID-19 infection |
| 5 | Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Prophylaxis of COVID-19 Disease in Health Personnel Dedicated to Patients with COVID-19 Disease | NCT04521322 | Recruiting | Pending | Diagnosis of COVID19 disease |
| 6 | Effect of Local Treatment(Carrageenan Nasal Spray and PVP-I Mouthwash) in Reducing Viral Load in Patients With COVID-19 (LT-COVID19) | NCT05049213 | Recruiting | Pending | Change from baseline naso-pharyngeal viral load quantified by RT-PCR at Day 8 |
| 7 | Prophylactic Treatment With Carragelose Nasal Spray to Prevent SARS-CoV-2, COVID-19, Infections in Health Care Workers | NCT04681001 | Recruiting | Pending | Presence of COVID-19 symptoms including symptoms of respiratory viral infection |
| 8 | Efficacy and Safety Evaluation of Inhaleen Inhalation in Hospitalized COVID-19 Patients | NCT04793984 | Recruiting | Pending | Clinical status of subjects as expressed on the WHO-8-Category ordinal scale |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, B.; Ali, I.; Jana, S.; Mukherjee, S.; Pal, S.; Ray, S.; Schütz, M.; Marschall, M. Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses. Viruses 2022, 14, 35. https://doi.org/10.3390/v14010035
Ray B, Ali I, Jana S, Mukherjee S, Pal S, Ray S, Schütz M, Marschall M. Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses. Viruses. 2022; 14(1):35. https://doi.org/10.3390/v14010035
Chicago/Turabian StyleRay, Bimalendu, Imran Ali, Subrata Jana, Shuvam Mukherjee, Saikat Pal, Sayani Ray, Martin Schütz, and Manfred Marschall. 2022. "Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses" Viruses 14, no. 1: 35. https://doi.org/10.3390/v14010035






