Variable Normalization of Naïve CD4+ Lymphopenia and Markers of Monocyte and T Cell Activation over the Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C Virus Infection
Abstract
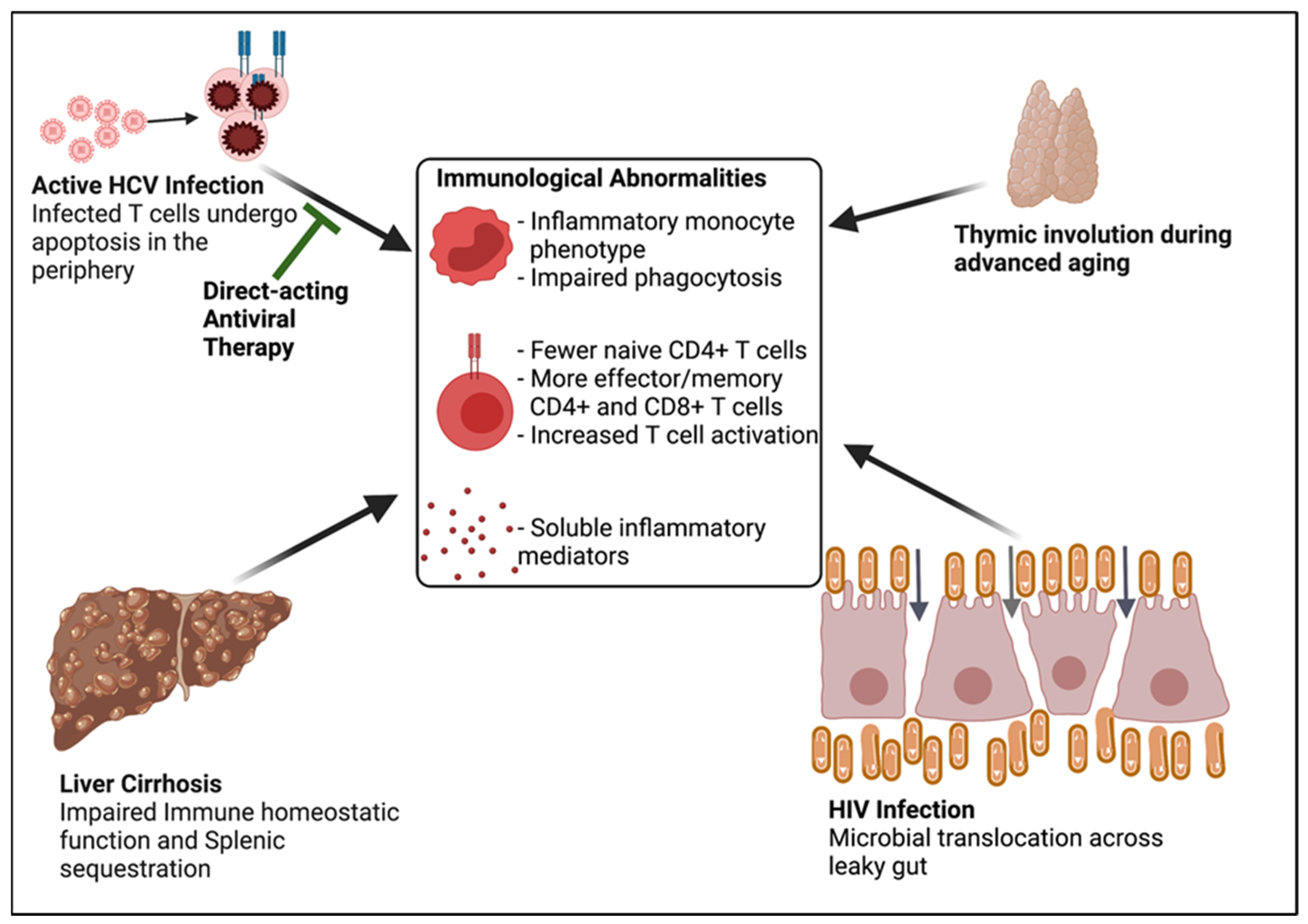
:1. Introduction
- Chronic HCV infection causes altered immune homeostasis, as defined by:
- Naïve CD4+ lymphopenia, and
- Systemic immune activation including markers of monocyte, macrophage, and T cell activation.
- Chronic HCV infected individuals frequently have concurrent HIV infection and age-associated disease.
- The combination of concurrent HIV infection and age-associated disease and the consequences of chronic HCV infection, such as liver cirrhosis, can lead to altered immune homeostasis independent of HCV infection.
- The residual altered immune homeostasis after DAA-associated HCV clearance is likely attributed in part to HIV infection, ageing, and liver cirrhosis.
2. Main Functions of Naïve CD4+ T Cells in T Cell Homeostasis
3. Factors That Contribute to Naïve CD4+ Lymphopenia in Chronic HCV Infection
3.1. HCV Viremia-Associated Peripheral T Cell Apoptosis
3.2. Liver Cirrhosis Is Associated with Altered Immune Homeostasis
3.3. Age-Associated Mechanisms of Altered T Cell Homeostasis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Auma, A.W.N.; Shive, C.; Damjanovska, S.; Kowal, C.; Cohen, D.E.; Bhattacharya, D.; Alston-Smith, B.; Osborne, M.; Kalayjian, R.; Balagopal, A.; et al. T-cell Activation Is Correlated with Monocyte Activation in HCV/HIV Coinfection and Declines During HCV Direct-Acting Antiviral Therapy. Open Forum Infect. Dis. 2021, 8, ofab079. [Google Scholar] [CrossRef]
- Auma, A.W.; Shive, C.L.; Lange, A.; Damjanovska, S.; Kowal, C.; Zebrowski, E.; Pandiyan, P.; Wilson, B.; Kalayjian, R.C.; Canaday, D.H.; et al. Naïve CD4+ T Cell Lymphopenia and Apoptosis in Chronic Hepatitis C Virus Infection Is Driven by the CD31+ Subset and Is Partially Normalized in Direct-Acting Antiviral Treated Persons. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Bougatsos, C.; Blazina, I.; Khangura, J.; Zakher, B. Screening for Hepatitis B Virus Infection in Adolescents and Adults: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2014, 161, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, M.G.; Rosenthal, E.M.; Barker, L.K.; Rosenberg, E.S.; Barranco, M.A.; Hall, E.W.; Edlin, B.R.; Mermin, J.; Ward, J.W.; Ryerson, A.B. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016. Hepatology 2019, 69, 1020–1031. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Schillie, S.; Barker, L.K.; Kupronis, B.A.; Wester, C. Vital Signs: Newly reported acute and chronic hepatitis C cases―United States, 2009–2018. Morb. Mortal. Wkly. Rep. 2020, 69, 399. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.L.; Morgan, T.R. The Natural History of Hepatitis C Virus (HCV) Infection. Int. J. Med. Sci. 2006, 3, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.D.; Morgan, R.L.; A Beckett, G.; Falck-Ytter, Y.; Holtzman, D.; Teo, C.-G.; Jewett, A.; Baack, B.; Rein, D.B.; Patel, N.; et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR. Recomm. Rep. 2012, 61, 1–32. [Google Scholar]
- Lawton, G. You’re only as young as your immune system. New Sci. 2020, 245, 44–48. [Google Scholar] [CrossRef]
- Garg, S.; Brooks, J.T.; Luo, Q.; Skarbinski, J. 1588Prevalence of and Factors Associated with Hepatitis C Virus Testing and Infection Among HIV-infected Adults Receiving Medical Care in the United States. Open Forum Infect. Dis. 2014, 1, S423. [Google Scholar] [CrossRef]
- Yehia, B.R.; Herati, R.S.; Fleishman, J.A.; Gallant, J.E.; Agwu, A.L.; Berry, S.A.; Korthuis, P.T.; Moore, R.D.; Metlay, J.P.; Gebo, K.A.; et al. Hepatitis C Virus Testing in Adults Living with HIV: A Need for Improved Screening Efforts. PLoS ONE 2014, 9, e102766. [Google Scholar]
- Spradling, P.R.; Richardson, J.T.; Buchacz, K.; Moorman, A.C.; Finelli, L.; Bell, B.P.; Brooks, J.T. Trends in Hepatitis C Virus Infection Among Patients in the HIV Outpatient Study, 1996–2007. JAIDS J. Acquir. Immune Defic. Syndr. 2010, 53, 388–396. [Google Scholar] [CrossRef]
- Parmigiani, A.; Alcaide, M.L.; Freguja, R.; Pallikkuth, S.; Frasca, D.; Fischl, M.A.; Pahwa, S. Impaired Antibody Response to Influenza Vaccine in HIV-Infected and Uninfected Aging Women Is Associated with Immune Activation and Inflammation. PLoS ONE 2013, 8, e79816. [Google Scholar]
- Avelino-Silva, V.I.; Ho, Y.-L.; Avelino-Silva, T.J.; Santos, S.D.S. Aging and HIV infection. Ageing Res. Rev. 2011, 10, 163–172. [Google Scholar] [CrossRef]
- Gustafson, C.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef]
- Liu, W.M.; Van der Zeijst, B.; Boog, C.J.; Soethout, E.C. Aging and impaired immunity to influenza viruses: Implications for vaccine development. Hum. Vaccines 2011, 7, 94–98. [Google Scholar] [CrossRef]
- Lee, J.; Linterman, M. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol. Lett. 2021, 241, 1–14. [Google Scholar] [CrossRef]
- Yonkers, N.L.; Sieg, S.; Rodriguez, B.; Anthony, D.D. Reduced Naive CD4 T Cell Numbers and Impaired Induction of CD27 in Response to T Cell Receptor Stimulation Reflect a State of Immune Activation in Chronic Hepatitis C Virus Infection. J. Infect. Dis. 2011, 203, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, R.D.; Rickabaugh, T.; Hultin, L.E.; Hultin, P.; Hausner, M.A.; Detels, R.; Phair, J.; Jamieson, B.D. Homeostasis of the Naive CD4+ T Cell Compartment during Aging. J. Immunol. 2008, 180, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Rickabaugh, T.M.; Kilpatrick, R.D.; Hultin, L.E.; Hultin, P.M.; Hausner, M.A.; Sugar, C.A.; Althoff, K.N.; Margolick, J.B.; Rinaldo, C.R.; Detels, R.; et al. The Dual Impact of HIV-1 Infection and Aging on Naïve CD4+ T-Cells: Additive and Distinct Patterns of Impairment. PLoS ONE 2011, 6, e16459. [Google Scholar] [CrossRef] [Green Version]
- Kostadinova, L.; Shive, C.L.; Judge, C.; Zebrowski, E.; Compan, A.; Rife, K.; Hirsch, A.; Falck-Ytter, Y.; Schlatzer, D.M.; Li, X.; et al. During Hepatitis C Virus (HCV) Infection and HCV-HIV Coinfection, an Elevated Plasma Level of Autotaxin Is Associated With Lysophosphatidic Acid and Markers of Immune Activation That Normalize During Interferon-Free HCV Therapy. J. Infect. Dis. 2016, 214, 1438–1448. [Google Scholar] [CrossRef] [Green Version]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2004, 53, 1–40. [Google Scholar]
- Solana, R.; Pawelec, G. The Neuroendocrine Immune Network in Ageing; Elsevier: Cham, Switzerland, 2004; Volume 9. [Google Scholar]
- Shive, C.L.; Judge, C.J.; Clagett, B.; Kalayjian, R.C.; Osborn, M.; Sherman, K.E.; Fichtenbaum, C.; Gandhi, R.T.; Kang, M.; Popkin, D.L.; et al. Pre-vaccine plasma levels of soluble inflammatory indices negatively predict responses to HAV, HBV, and tetanus vaccines in HCV and HIV infection. Vaccine 2018, 36, 453–460. [Google Scholar] [CrossRef]
- Taiwo, B.; Barcena, L.; Tressler, R. Understanding and controlling chronic immune activation in the HIV-infected patients suppressed on combination antiretroviral therapy. Curr. HIV/AIDS Rep. 2013, 10, 21–32. [Google Scholar] [CrossRef]
- McGovern, B.H.; Golan, Y.; Lopez, M.; Pratt, D.; Lawton, A.; Moore, G.; Epstein, M.; Knox, T.A. The Impact of Cirrhosis on CD4+ T Cell Counts in HIV-Seronegative Patients. Clin. Infect. Dis. 2007, 44, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Kimmig, S.; Przybylski, G.K.; Schmidt, C.A.; Laurisch, K.; Möwes, B.; Radbruch, A.; Thiel, A. Two Subsets of Naive T Helper Cells with Distinct T Cell Receptor Excision Circle Content in Human Adult Peripheral Blood. J. Exp. Med. 2002, 195, 789–794. [Google Scholar] [CrossRef]
- Bousso, P.; Wahn, V.; Douagi, I.; Horneff, G.; Pannetier, C.; Le Deist, F.; Zepp, F.; Niehues, T.; Kourilsky, P.; Fischer, A.; et al. Diversity, functionality, and stability of the T cell repertoire derived in vivo from a single human T cell precursor. Proc. Natl. Acad. Sci. USA 2000, 97, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Woodland, D.L.; Kotzin, B.L.; Palmer, E. Functional consequences of a T cell receptor D beta 2 and J beta 2 gene segment deletion. J. Immunol. 1990, 144, 379–385. [Google Scholar]
- Funauchi, M.; Farrant, J.; Moreno, C.; Webster, A. Defects in antigen-driven lymphocyte responses in common variable immunodeficiency (CVID) are due to a reduction in the number of antigen-specific CD4+ T cells. Clin. Exp. Immunol. 1995, 101, 82–88. [Google Scholar] [CrossRef]
- Kohler, S.; Wagner, U.; Pierer, M.; Kimmig, S.; Oppmann, B.; Möwes, B.; Jülke, K.; Romagnani, C.; Thiel, A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur. J. Immunol. 2005, 35, 1987–1994. [Google Scholar] [CrossRef]
- Gomez, I.; Hainz, U.; Jenewein, B.; Schwaiger, S.; Wolf, A.; Grubeck-Loebenstein, B. Changes in the expression of CD31 and CXCR3 in CD4+ naıve T cells in elderly persons. Mech. Ageing Dev. 2003, 124, 395–402. [Google Scholar] [CrossRef]
- Wertheimer, A.; Bennett, M.S.; Park, B.; Uhrlaub, J.; Martinez, C.; Pulko, V.; Currier, N.L.; Nikolich-Žugich, D.; Kaye, J.; Nikolich-Žugich, J. Aging and Cytomegalovirus Infection Differentially and Jointly Affect Distinct Circulating T Cell Subsets in Humans. J. Immunol. 2014, 192, 2143–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, B.; Lazuardi, L.; Weiskirchner, I.; Keller, M.; Neuner, C.; Fischer, K.-H.; Neuman, B.; Würzner, R.; Grubeck-Loebenstein, B. Healthy Aging and Latent Infection with CMV Lead to Distinct Changes in CD8+ and CD4+ T-Cell Subsets in the Elderly. Hum. Immunol. 2007, 68, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Douek, D.C.; McFarland, R.D.; Keiser, P.H.; Gage, E.A.; Massey, J.M.; Haynes, B.F.; Polis, M.; Haase, A.T.; Feinberg, M.B.; Sullivan, J.L.; et al. Changes in thymic function with age and during the treatment of HIV infection. Nat. Cell Biol. 1998, 396, 690–695. [Google Scholar] [CrossRef]
- Zhang, L.; Lewin, S.R.; Markowitz, M.; Lin, H.-H.; Skulsky, E.; Karanicolas, R.; He, Y.; Jin, X.; Tuttleton, S.; Vesanen, M.; et al. Measuring Recent Thymic Emigrants in Blood of Normal and HIV-1–Infected Individuals before and after Effective Therapy. J. Exp. Med. 1999, 190, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Shmagel, K.V.; Saidakova, E.V.; Korolevskaya, L.B.; Shmagel, N.G.; Chereshnev, V.A.; Anthony, D.D.; Lederman, M.M. Influence of hepatitis C virus coinfection on CD4+ T cells of HIV-infected patients receiving HAART. AIDS 2014, 28, 2381–2388. [Google Scholar] [CrossRef]
- Toubi, E.; Kessel, A.; Goldstein, L.; Slobodin, G.; Sabo, E.; Shmuel, Z.; Zuckerman, E. Enhanced peripheral T-cell apoptosis in chronic hepatitis C virus infection: Association with liver disease severity. J. Hepatol. 2001, 35, 774–780. [Google Scholar] [CrossRef]
- Norbury, C.J.; Hickson, I.D. Cellular Responses to DNA Damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, G.N. Host defense, viruses and apoptosis. Cell Death Differ. 2001, 8, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Iannello, A.; Debbeche, O.; Martin, E.; Attalah, L.H.; Samarani, S.; Ahmad, A. Viral strategies for evading antiviral cellular immune responses of the host. J. Leukoc. Biol. 2005, 79, 16–35. [Google Scholar] [CrossRef]
- Wack, A.; Soldaini, E.; Tseng, C.-T.K.; Nuti, S.; Klimpel, G.R.; Abrignani, S. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur. J. Immunol. 2001, 31, 166–175. [Google Scholar] [CrossRef]
- Levy, S. Function of the tetraspanin molecule CD81 in B and T cells. Immunol. Res. 2014, 58, 179–185. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, S.; Shin, E.-C. Significance of bystander T cell activation in microbial infection. Nat. Immunol. 2022, 23, 13–22. [Google Scholar] [CrossRef] [PubMed]
- El-Bendary, M.; Hawas, S.; Elhammady, D.; Al-Hadidy, A.-H.M.; Rizk, H. Expression of Fas (CD95) and Bcl-2 in peripheral blood mononuclear cells in patients with chronic HCV and schistosomiasis. Egypt. J. Basic Appl. Sci. 2014, 1, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Taya, N.; Torimoto, Y.; Shindo, M.; Hirai, K.; Hasebe, C.; Kohgo, Y. Fas-mediated apoptosis of peripheral blood mononuclear cells in patients with hepatitis C. Br. J. Haematol. 2000, 110, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mita, E.; Hayashi, N.; Iio, S.; Takehara, T.; Hijioka, T.; Kasahara, A.; Fusamoto, H.; Kamada, T. Role of Fas Ligand in Apoptosis Induced by Hepatitis C Virus Infection. Biochem. Biophys. Res. Commun. 1994, 204, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Ruiz, P.; Schreiber, A.D. Impaired Function of Macrophage Fcγ Receptors and Bacterial Infection in Alcoholic Cirrhosis. N. Engl. J. Med. 1994, 331, 1122–1128. [Google Scholar] [CrossRef]
- Cook, R.T.; Waldschmidt, T.J.; Cook, B.L.; Labrecque, D.R.; McLatchie, K. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clin. Exp. Immunol. 1996, 103, 304–310. [Google Scholar] [CrossRef]
- Seidler, S.; Zimmermann, H.W.; Weiskirchen, R.; Trautwein, C.; Tacke, F. Elevated circulating soluble interleukin-2 receptor in patients with chronic liver diseases is associated with non-classical monocytes. BMC Gastroenterol. 2012, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Lario, M.; Muñoz, L.; Ubeda, M.; Borrero, M.-J.; Martínez, J.; Monserrat, J.; Díaz, D.; Álvarez-Mon, M.; Albillos, A. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J. Hepatol. 2013, 59, 723–730. [Google Scholar] [CrossRef]
- Morita, K.; Fukuda, Y.; Nakano, I.; Katano, Y.; Hayakawa, T. Peripheral lymphocyte subsets vary with stage of hepatitis C virus-associated liver disease. Hepato-Gastroenterol. 2005, 52, 1803–1808. [Google Scholar]
- Perrin, D.; Bignon, J.D.; Beaujard, E.; Cheneau, M.L. Populations of circulating T lymphocytes in patients with alcoholic cirrhosis. Gastroentérologie Clin. Biol. 1984, 8, 907–910. [Google Scholar]
- Laguno, M.; Martínez-Rebollar, M.; Casanova, M.; de Lazzari, E.; González-Cordón, A.; Torres, B.; Inciarte, A.; de la Mora, L.; Ugarte, A.; Ambrosioni, J.; et al. Long-term evolution in liver fibrosis and immune profile after direct-acting antivirals therapy in hepatitis C virus-human immunodeficiency virus co-infected patients. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Everson, G.T.; Flamm, S.L.; Kumar, P.; Landis, C.; Brown, R.S.; Fried, M.W.; Terrault, N.A.; O’Leary, J.G.; Vargas, H.E.; et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology 2015, 149, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Manns, M.; Samuel, D.; Gane, E.J.; Mutimer, D.; Mccaughan, G.; Buti, M.; Prieto, M.; Calleja-Panero, J.L.; Peck-Radosavljevic, M.; Mullhaupt, B.; et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: A multicentre, open-label, randomised, phase 2 trial. Lancet Infect. Dis. 2016, 16, 685–697. [Google Scholar] [CrossRef]
- Fabbri, G.; Mastrorosa, I.; Vergori, A.; Timelli, L.; Lorenzini, P.; Zaccarelli, M.; Cicalini, S.; Bellagamba, R.; Plazzi, M.M.; Mazzotta, V.; et al. Liver stiffness reduction and serum fibrosis score improvement in HIV/hepatitis C virus-coinfected patients treated with direct-acting antivirals. HIV Med. 2018, 19, 578–584. [Google Scholar] [CrossRef]
- Giannini, E.G.; Crespi, M.; DeMarzo, M.; Bodini, G.; Furnari, M.; Marabotto, E.; Torre, F.; Zentilin, P.; Savarino, V. Improvement in hepatitis C virus patients with advanced, compensated liver disease after sustained virological response to direct acting antivirals. Eur. J. Clin. Investig. 2018, 49, e13056. [Google Scholar] [CrossRef]
- Medrano, L.M.; Berenguer, J.; Salgüero, S.; González-García, J.; Díez, C.; Hontañón, V.; Garcia-Broncano, P.; Ibañez-Samaniego, L.; Bellón, J.M.; Jiménez-Sousa, M.A.; et al. Successful HCV Therapy Reduces Liver Disease Severity and Inflammation Biomarkers in HIV/HCV-Coinfected Patients with Advanced Cirrhosis: A Cohort Study. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Moussalli, J.; Munteanu, M.; Thabut, D.; Lebray, P.; Rudler, M.; Ngo, Y.; Thibault, V.; Mkada, H.; Charlotte, F.; et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J. Hepatol. 2013, 59, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Girón-Ortega, J.-A.; Márquez-Coello, M.; Gutiérrez-Saborido, D.; Arizcorreta, A.; Cuesta-Sancho, S.; Girón-González, J.-A. Modifications of CD4 T cells, CD4/CD8 ratio and serum levels of soluble CD14 in HIV-HCV-coinfected patients after sustained HCV response induced by direct-acting antiviral agents: Influence of liver cirrhosis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1863–1871. [Google Scholar] [CrossRef]
- Bartoletti, M.; Giannella, M.; Lewis, R.E.; Viale, P. Bloodstream infections in patients with liver cirrhosis. Virulence 2016, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.N.A.; Yau, R.; Russo, H.P.; Putney, K.; Restrepo, A.; Garey, K.W.; Sofjan, A.K. Bacteremia in Patients with Liver Cirrhosis. J. Clin. Gastroenterol. 2018, 52, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.D.; Sulkowski, M.S.; Smeaton, L.M.; Damjanovska, S.; Shive, C.L.; Kowal, C.M.; E Cohen, D.; Bhattacharya, D.; Alston-Smith, B.L.; Balagopal, A.; et al. Hepatitis C Virus (HCV) Direct-Acting Antiviral Therapy in Persons with Human Immunodeficiency Virus–HCV Genotype 1 Coinfection Resulting in High Rate of Sustained Virologic Response and Variable in Normalization of Soluble Markers of Immune Activation. J. Infect. Dis. 2020, 222, 1334–1344. [Google Scholar] [CrossRef]
- Kostadinova, L.; Shive, C.L.; Zebrowski, E.; Fuller, B.; Rife, K.; Hirsch, A.; Compan, A.; Moreland, A.; Falck-Ytter, Y.; Popkin, D.L.; et al. Soluble Markers of Immune Activation Differentially Normalize and Selectively Associate with Improvement in AST, ALT, Albumin, and Transient Elastography During IFN-Free HCV Therapy. Pathog. Immun. 2018, 3, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Mascia, C.; Vita, S.; Zuccalà, P.; Marocco, R.; Tieghi, T.; Savinelli, S.; Rossi, R.; Iannetta, M.; Pozzetto, I.; Furlan, C.; et al. Changes in inflammatory biomarkers in HCV-infected patients undergoing direct acting antiviral-containing regimens with or without interferon. PLoS ONE 2017, 12, e0179400. [Google Scholar] [CrossRef] [Green Version]
- Arjona, M.M.D.O.; Marquez, M.; Soto, M.J.; Rodriguez-Ramos, C.; Terron, A.; Vergara, A.; Arizcorreta, A.; Fernandez-Gutierrez, C.; Giron-González, J.A. Bacterial Translocation in HIV-Infected Patients with HCV Cirrhosis: Implication in Hemodynamic Alterations and Mortality. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 56, 420–427. [Google Scholar] [CrossRef]
- Kashani, A.; Salehi, B.; Anghesom, D.; Kawayeh, A.M.; Rouse, G.A.; Runyon, B.A.; Kashani, A.; Salehi, B.; Anghesom, D.; Kawayeh, A.M.; et al. Spleen Size in Cirrhosis of Different Etiologies. J. Ultrasound Med. 2015, 34, 233–238. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia–Tsao, G.; Bosch, J.; Burroughs, A.K.; Ripoll, C.; Maurer, R.; Planas, R.; Escorsell, A.; et al. Incidence, Prevalence, and Clinical Significance of Abnormal Hematologic Indices in Compensated Cirrhosis. Clin. Gastroenterol. Hepatol. 2009, 7, 689–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [Green Version]
- Nikolich-Žugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Martin, G.E.; Angelovich, T.; Cheng, W.-J.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012, 11, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Candore, G.; Caruso, C.; Colomba, C.; Di Bona, D.; Duro, G.; Gambino, C.M.; Ligotti, M.E.; Pandey, J.P. Role of Immunogenetics in the Outcome of HCMV Infection: Implications for Ageing. Int. J. Mol. Sci. 2019, 20, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Jamieson, B.D.; E Hultin, L.; Hultin, P.M.; Effros, R.B.; Detels, R. Premature Aging of T cells Is Associated with Faster HIV-1 Disease Progression. JAIDS J. Acquir. Immune Defic. Syndr. 2009, 50, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianesin, K.; Noguera-Julian, A.; Zanchetta, M.; Del Bianco, P.; Petrara, M.R.; Freguja, R.; Rampon, O.; Fortuny, C.; Camós, M.; Mozzo, E.; et al. Premature aging and immune senescence in HIV-infected children. AIDS 2016, 30, 1363–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auma, A.W.N.; Shive, C.L.; Kostadinova, L.; Anthony, D.D. Variable Normalization of Naïve CD4+ Lymphopenia and Markers of Monocyte and T Cell Activation over the Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C Virus Infection. Viruses 2022, 14, 50. https://doi.org/10.3390/v14010050
Auma AWN, Shive CL, Kostadinova L, Anthony DD. Variable Normalization of Naïve CD4+ Lymphopenia and Markers of Monocyte and T Cell Activation over the Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C Virus Infection. Viruses. 2022; 14(1):50. https://doi.org/10.3390/v14010050
Chicago/Turabian StyleAuma, Ann W. N., Carey L. Shive, Lenche Kostadinova, and Donald D. Anthony. 2022. "Variable Normalization of Naïve CD4+ Lymphopenia and Markers of Monocyte and T Cell Activation over the Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C Virus Infection" Viruses 14, no. 1: 50. https://doi.org/10.3390/v14010050
APA StyleAuma, A. W. N., Shive, C. L., Kostadinova, L., & Anthony, D. D. (2022). Variable Normalization of Naïve CD4+ Lymphopenia and Markers of Monocyte and T Cell Activation over the Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C Virus Infection. Viruses, 14(1), 50. https://doi.org/10.3390/v14010050





