Oral Rabies Vaccine Strain SPBN GASGAS: Genetic Stability after Serial In Vitro and In Vivo Passaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Virus
2.2. In Vitro Studies
2.3. In Vivo Studies
2.4. Assay
2.4.1. Virus Titration
2.4.2. Next Generation Sequencing (NGS)
2.4.3. Total Nucleic Acid (TNA) Extraction
2.4.4. cDNA Synthesis
2.4.5. Specific Amplification
2.4.6. Gel Electrophoresis and Gel Extraction
2.4.7. DNA Quantification and Sequencing
2.4.8. Sequencing Data Analysis
2.4.9. Ethics Statement
3. Results
3.1. In Vitro
3.2. In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Neglect. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Rosatte, R. Evolution of rabies wildlife control tactics. Adv. Virus Res. 2011, 79, 397–419. [Google Scholar] [PubMed]
- Freuling, C.M.; Hampson, K.; Selhorst, T.; Schröder, R.; Meslin, F.X.; Mettenleiter, T.C.; Müller, T. The elimination of fox rabies in Europe: Determinants of success and lessons for the future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120142. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.F.; Schröder, R.; Wysocki, P.; Mettenleiter, T.C.; Freuling, C.M. Spatio-temporal use of oral rabies vaccines in fox rabies elimination programs in Europe. PLoS Negl. Trop. Dis. 2015, 9, e0003953. [Google Scholar] [CrossRef] [PubMed]
- Fehlner-Gardiner, C.; Nadin-Davis, S.; Armstrong, J.; Muldoon, F.; Bachmann, P.; Wandeler, A. ERA vaccine-derived cases of rabies in wildlife and domestic animals in Ontario, Canada, 1989–2004. J. Wildl. Dis. 2008, 44, 71–85. [Google Scholar] [CrossRef]
- Pfaff, F.; Müller, T.; Freuling, C.M.; Fehlner-Gardiner, C.; Nadin-Davis, S.; Robardet, E.; Cliquet, F.; Vuta, V.; Hostnik, P.; Mettenleiter, T.C.; et al. In-depth genome analyses of viruses from vaccine-derived rabies cases and corresponding live-attenuated oral rabies vaccines. Vaccine 2019, 37, 4758–4765. [Google Scholar] [CrossRef]
- Müller, T.; Freuling, C.M.; Wysocki, P.; Roumiantzeff, M.; Freney, J.; Mettenleiter, T.C.; Vos, A. Terrestrial rabies control in the European Union: Historical achievements and challenges ahead. Vet. J. 2015, 203, 10–17. [Google Scholar] [CrossRef]
- Maki, J.; Guiot, A.L.; Aubert, M.; Brochier, B.; Cliquet, F.; Hanlon, C.A.; King, R.; Oertli, E.H.; Rupprecht, C.E.; Schumacher, C.; et al. Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): A global review. Vet. Res. 2017, 48, 57. [Google Scholar] [CrossRef]
- Vos, A.; Neubert, A.; Aylan, O.; Schuster, P.; Pommerening, E.; Müller, T.; Chivatsi, D.C. An update on safety studies of SAD B19 rabies virus vaccine in target and nontarget species. Epidemiol. Infect. 1999, 123, 165–175. [Google Scholar] [CrossRef]
- Artois, M.; Guittre, C.; Thomas, I.; Leblois, H.; Brochier, B.; Barrat, J. Potential pathogenicity for rodents of vaccines intended for oral vaccination against rabies: A comparison. Vaccine 1992, 10, 524–528. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Charlton, K.M.; Artois, M.; Casey, G.A.; Webster, W.A.; Campbell, J.B.; Lawson, K.F.; Schneider, L.G. Ineffectiveness and comparative pathogenicity of attenuated rabies virus vaccines for the striped skunk (Mephitis mephitis). J. Wildl. Dis. 1990, 26, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Bingham, J.; Foggin, C.M.; Gerber, H.; Hill, F.W.; Kappeler, A.; King, A.A.; Perry, B.D.; Wandeler, A.I. Pathogenicity of SAD rabies vaccine given orally in chacma baboons (Papio ursinus). Vet. Rec. 1992, 131, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Bätza, H.J.; Beckert, A.; Bunzenthal, C.; Cox, J.H.; Freuling, C.M.; Fooks, A.R.; Frost, J.; Geue, L.; Hoeflechner, A.; et al. Analysis of vaccine-virus-associated rabies cases in red foxes (Vulpes vulpes) after oral rabies vaccination campaigns in Germany and Austria. Arch. Virol. 2009, 154, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Hostnik, P.; Picard-Meyer, E.; Rihtaric, D.; Toplak, I.; Cliquet, F. Vaccine-induced rabies in a red fox (Vulpes vulpes): Isolation of vaccine virus in brain tissue and salivary glands. J. Wildl. Dis. 2014, 50, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Robardet, E.; Picard-Meyer, E.; Dobroštana, M.; Jaceviciene, I.; Mähar, K.; Muižniece, Z.; Pridotkas, G.; Masiulis, M.; Niin, E.; Olševskis, E.; et al. Rabies in the baltic states: Decoding a process of control and elimination. PLoS Negl. Trop. Dis. 2016, 10, e0004432. [Google Scholar] [CrossRef]
- Vuta, V.; Picard-Meyer, E.; Robardet, E.; Barboi, G.; Motiu, R.; Barbuceanu, F.; Vlagioiu, C.; Cliquet, F. Vaccine-induced rabies case in a cow (Bos taurus): Molecular characterization of vaccine strain in brain tissue. Vaccine 2016, 34, 5021–5025. [Google Scholar] [CrossRef]
- Hanley, K.A. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evolution 2011, 4, 635–643. [Google Scholar] [CrossRef]
- Cliquet, F.; Picard-Meyer, E.; Mojzis, M.; Dirbakova, Z.; Muizniece, Z.; Jaceviciene, I.; Mutinelli, F.; Matulova, M.; Frolichova, J.; Rychlik, I.; et al. In-Depth Characterization of Live Vaccines Used in Europe for Oral Rabies Vaccination of Wildlife. PLoS ONE 2015, 10, e0141537. [Google Scholar] [CrossRef]
- Höper, D.; Freuling, C.M.; Müller, T.; Hanke, D.; Von Messling, V.; Duchow, K.; Beer, M.; Mettenleiter, T.C. High definition viral vaccine strain identity and stability testing using full-genome population data–The next generation of vaccine quality control. Vaccine 2015, 33, 5829–5837. [Google Scholar] [CrossRef]
- Yarosh, O.K.; Wandeler, A.I.; Graham, F.L.; Campbell, J.B.; Prevec, L. Human adenovirus type 5 vectors expressing rabies glycoprotein. Vaccine 1996, 14, 1257–1264. [Google Scholar] [CrossRef]
- Mähl, P.; Cliquet, F.; Guiot, A.L.; Niin, E.; Fournials, E.; Saint-Jean, N.; Aubert, M.; Rupprecht, C.E.; Gueguen, S. Twenty year experience of the oral rabies vaccine SAG2 in wildlife: A global review. Vet. Res. 2014, 45, 77. [Google Scholar] [CrossRef] [PubMed]
- Dietzschold, B.; Faber, M.; Schnell, M.J. New approaches to the prevention and eradication of rabies. Expert. Rev. Vaccines 2003, 2, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Dietzschold, B.; Li, J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health 2009, 56, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ramezanpour, B.; De Foucauld, J.; Kortekaas, J. Emergency deployment of genetically engineered veterinary vaccines in Europe. Vaccine 2016, 34, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Rabitec—Rabies Vaccine (Live, Oral) for Foxes and Raccoon Dogs. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/rabitec#overview-section (accessed on 8 August 2021).
- USDA. Veterinary Memorandum No. 800.201; USDA/APHIS/VS: Washington, DC, USA, 2018. [Google Scholar]
- Council of Europe. §5.2.6–Evaluation of safety of veterinary vaccine and immunosera. In European Pharmacopoeia 7.7; EDQM: Strasbourg, France, 2013; pp. 5275–5277. [Google Scholar]
- Vos, A.; Freuling, C.M.; Hundt, B.; Kaiser, C.; Nemitz, S.; Neubert, A.; Nolden, T.; Teifke, J.P.; Te Kamp, V.; Ulrich, R.; et al. Oral vaccination of wildlife against rabies: Differences among host species in vaccine uptake efficiency. Vaccine 2017, 35, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.; Freuling, C.; Ortmann, S.; Kretzschmar, A.; Mayer, D.; Schliephake, A.; Müller, T. An assessment of shedding with the oral rabies virus vaccine strain SPBN GASGAS in target and non-target species. Vaccine 2018, 36, 811–817. [Google Scholar] [CrossRef]
- Ortmann, S.; Vos, A.; Kretzschmar, A.; Walther, N.; Kaiser, C.; Freuling, C.; Lojkic, I.; Müller, T. Safety studies with the oral rabies virus vaccine SPBN GASGAS in the small Indian mongoose (Herpestes auropunctatus). BMC Vet. Res. 2018, 14, 90. [Google Scholar] [CrossRef]
- Council of Europe. Rabies vaccine (live, oral) for foxes and raccoon dogs-0746. In European Pharmacopoeia 8.0; EDQM: Strasbourg, France, 2014; pp. 1011–1012. [Google Scholar]
- Posada-Cespedes, S.; Seifert, D.; Beerenwinkel, N. Recent Advances in Inferring Viral Diversity from High-throughput Sequencing Data. Virus Res. 2017, 239, 17–32. [Google Scholar] [CrossRef]
- Neverov, A.; Chumakov, K. Massively parallel sequencing for monitoring genetic consistency and quality control of live viral vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 20063–20068. [Google Scholar] [CrossRef]
- Tuffereau, C.; Leblois, H.; Bénéjean, J.; Coulon, P.; Lafay, F.; Flamand, A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology 1989, 172, 206–212. [Google Scholar] [CrossRef]
- Dietzschold, B.; Wunner, W.H.; Wiktor, T.J.; Lopes, A.D.; Lafon, M.; Smith, C.L.; Koprowski, H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc. Natl. Acad. Sci. USA 1983, 80, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Faber, M.; Faber, M.L.; Papaneri, A.; Bette, M.; Weihe, E.; Dietzschold, B.; Schnell, M.J. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J. Virol. 2005, 79, 14141–14148. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Faber, M.L.; Li, J.; Preuss, M.A.; Schnell, M.J.; Dietzschold, B. Dominance of a nonpathogenic glycoprotein gene over a pathogenic glycoprotein gene in rabies virus. J. Virol. 2007, 81, 7041–7047. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Pulmanausahakul, R.; Hodawadekar, S.S.; Spitsin, S.; McGettigan, J.P.; Schnell, M.J.; Dietzschold, B. Overexpression of the Rabies Virus Glycoprotein Results in Enhancement of Apoptosis and Antiviral Immune Response. JVI 2002, 76, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef]
- Knyazev, S.; Tsyvina, V.; Shankar, A.; Melnyk, A.; Artyomenko, A.; Malygina, T.; Porozov, Y.B.; Campbell, E.M.; Switzer, W.M.; Skums, P.; et al. Accurate assembly of minority viral haplotypes from next-generation sequencing through efficient noise reduction. Nucleic Acids Res. 2021, 49, e102. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol. Biol. 2014, 1079, 105–116. [Google Scholar]
- Zulkower, V.; Rosser, S. DNA Features Viewer: A sequence annotation formatting and plotting library for Python. Bioinformatics 2020, 36, 4350–4352. [Google Scholar] [CrossRef]
- Elena, S.F.; Sanjuan, R. Adaptive value of high mutation rates of RNA viruses: Separating causes from consequences. J. Virol. 2005, 79, 11555–11558. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.A.; de la Torre, J.C.; Meier, E.J.J. Extreme heterogeneity in populations of vesicular stomatitis virus. J. Virol. 1989, 63, 2072–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggerbauer, E.; Pfaff, F.; Finke, S.; Höper, D.; Beer, M.; Mettenleiter, T.C.; Nolden, T.; Teifke, J.P.; Müller, T.; Freuling, C.M. Comparative analysis of European bat lyssavirus 1 pathogenicity in the mouse model. PLoS Negl. Trop. Dis. 2017, 11, e0005668. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, C.J.R.; Roy, S.; Beale, M.A.; Tutill, H.; Williams, R.; Breuer, J. On the effective depth of viral sequence data. Virus Evol. 2017, 3, vex030. [Google Scholar] [CrossRef]
- Gallet, R.; Fabre, F.; Michalakis, Y.; Blanc, S. The Number of Target Molecules of the Amplification Step Limits Accuracy and Sensitivity in Ultradeep-Sequencing Viral Population Studies. J. Virol. 2017, 91, e00561-17. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Marth, G.T. Primer-site SNPs mask mutations. Nat. Methods 2007, 4, 192. [Google Scholar] [CrossRef]
- Marston, D.A.; Horton, D.L.; Nunez, J.; Ellis, R.J.; Orton, R.J.; Johnson, N.; Banyard, A.C.; McElhinney, L.M.; Freuling, C.M.; Fırat, M.; et al. Genetic analysis of a rabies virus host shift event reveals within-host viral dynamics in a new host. Virus Evol. 2017, 3, vex038. [Google Scholar] [CrossRef]
- Goller, K.V.; Dräger, C.; Höper, D.; Beer, M.; Blome, S. Classical swine fever virus marker vaccine strain CP7_E2alf: Genetic stability in vitro and in vivo. Arch. Virol. 2015, 160, 3121–3125. [Google Scholar] [CrossRef]
- Sobel Leonard, A.; Weissman, D.B.; Greenbaum, B.; Ghedin, E.; Koelle, K. Transmission Bottleneck Size Estimation from Pathogen Deep-Sequencing Data, with an Application to Human Influenza A Virus. J. Virol. 2017, 91, e00171-17. [Google Scholar] [CrossRef]
- Te Kamp, V.; Freuling, C.M.; Vos, A.; Schuster, P.; Kaiser, C.; Ortmann, S.; Kretzschmar, A.; Nemitz, S.; Eggerbauer, E.; Ulrich, R.; et al. Oral rabies vaccination of wildlife: What drives vaccine uptake efficiency in different reservoir species? Sci. Rep. 2020, 10, 2919. [Google Scholar] [CrossRef]
- Holmes, E.C.; Dudas, G.; Rambaut, A.; Andersen, K.G. The evolution of Ebola virus: Insights from the 2013–2016 epidemic. Nature 2016, 528, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
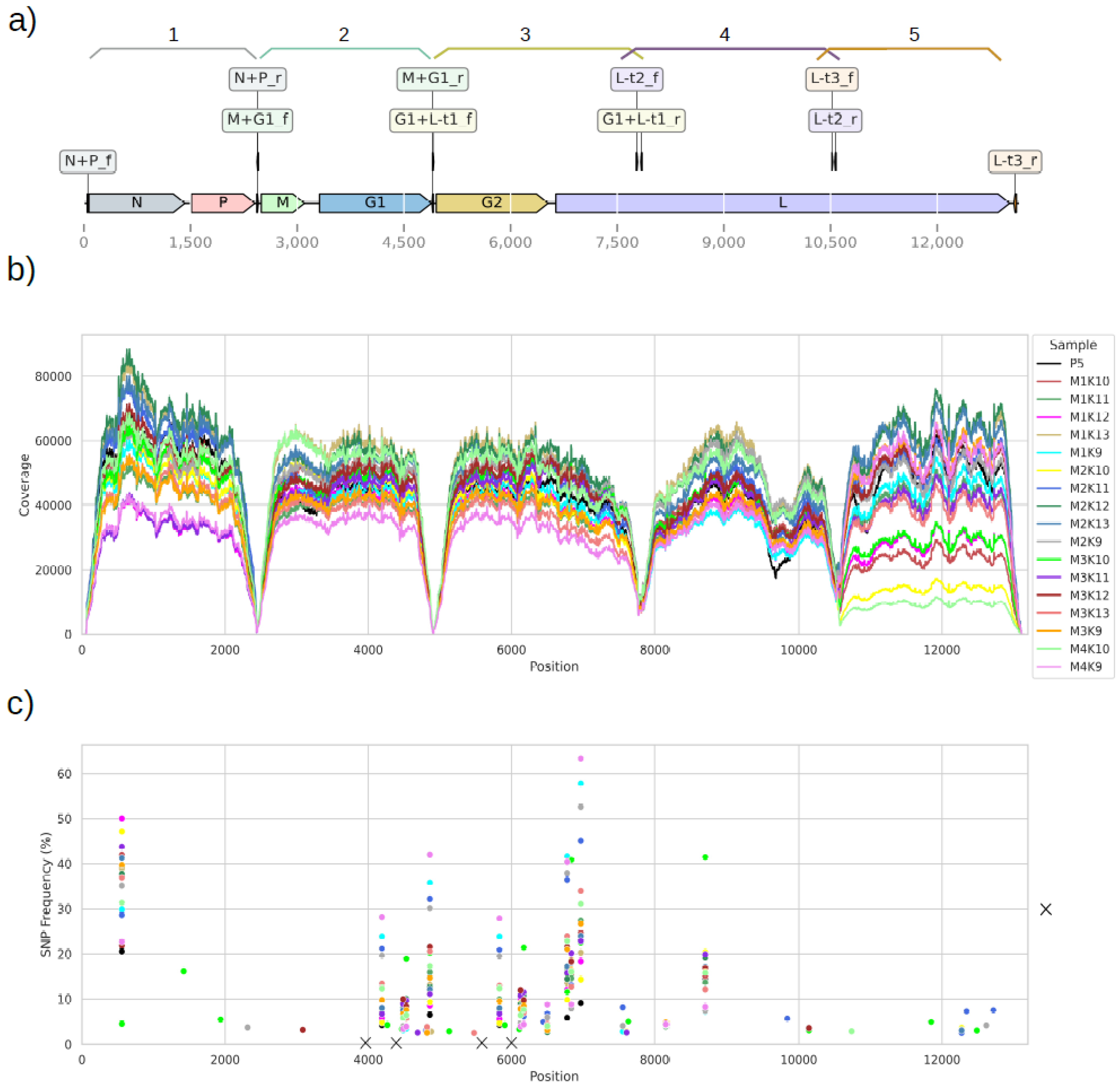


| Primer | Nucleotide Sequence | Resulting Fragment Size | |
|---|---|---|---|
| 1 | GASGAS_N+P_f | GCAAAAATGTAACACCCCTA | 2408 bp |
| GASGAS_N+P_r | GGGACATCTCGGATTTTATT | ||
| 2 | GASGAS_M+G1_f | ACAATAAAATCCGAGATGTCC | 2482 bp |
| GASGAS_M+G1_r | GATCGTTGAAAGGACGTTAAT | ||
| 3 | GASGAS_G1+L-t1_f | CCTTTCAACGATCCAAGTC | 2948 bp |
| GASGAS_G1+L-t1_r | CTTGCTAAGCACTCCTGGTA | ||
| 4 | GASGAS_L-t2_f | TATCGAAAGGGTCTGTCAAA | 2811 bp |
| GASGAS_L-t2_r | GTGCCAATGAAACGTAGAGT | ||
| 5 | GASGAS_L-t3_f | CACAGACATGGACATCAGAG | 2605 bp |
| GASGAS_L-t3_r | ATCAAACAACCAAAGGTTCA |
| Nucleotide Position | Reference Nucleotide | Alternative Nucleotide | Amino Acid Change | Gene | SNP Frequency (%) | |
|---|---|---|---|---|---|---|
| MSV | MSV + 5P | |||||
| 514 | T | C | - | N | 4.49 | 0.04 |
| 517 | G | A | - | N | 4.78 | 0.06 |
| 693 | G | A | G-->E | N | 4.5 | 0.04 |
| 1241 | G | A | D-->N | N | 5.14 | 0.06 |
| 1750 | G | T | E-->D | P | 4.12 | 0.05 |
| 9748 | G | T | L-->F | L | 0.44 | 2.75 |
| 12,454 | T | C | - | L | 3.7 | 1.20 |
| Passage | In Vivo | In Vitro |
|---|---|---|
| MSV | 7.6 | |
| 1st | 7.1 (15) | n.d. |
| 2nd | 8.0 (11) | n.d. |
| 3rd | 8.3 (10) | n.d. |
| 4th | 7.4 (9) | 7.6 |
| 5th | 7.3 (29) | 7.6 |
| Sample | Total Reads per Full Genome | % of Mapped Reads | Median of Reference Coverage (Reads) | Genomic Position of Changes in Consensus Sequence | Number of SNPs with >2.5% Frequency |
|---|---|---|---|---|---|
| P5 | 2,526,454 | 99.46 | 43,037 | none | 11 |
| M1K10 | 2,337,358 | 99.32 | 44,212 | none | 11 |
| M1K11 | 2,148,128 | 97.59 | 39,094 | none | 11 |
| M1K12 | 1,986,546 | 99.23 | 33,128 | 557 | 12 |
| M1K13 | 3,180,882 | 99.24 | 57,070 | none | 14 |
| M1K9 | 2,248,544 | 98.99 | 41,230 | 6969 | 11 |
| M2K10 | 2,021,776 | 99.33 | 37,857 | none | 13 |
| M2K11 | 2,824,706 | 99.01 | 47,369 | none | 17 |
| M2K12 | 3,190,578 | 99.27 | 55,116 | none | 13 |
| M2K13 | 3,025,394 | 99.11 | 52,629 | none | 11 |
| M2K9 | 2,771,734 | 99.08 | 49,271 | 6969 | 14 |
| M3K10 | 2,341,254 | 99.30 | 43,237 | none | 19 |
| M3K11 | 2,225,710 | 98.38 | 40,057 | none | 13 |
| M3K12 | 2,614,868 | 99.20 | 47,737 | none | 11 |
| M3K13 | 2,065,018 | 99.05 | 37,609 | none | 12 |
| M3K9 | 2,352,068 | 97.44 | 40,822 | none | 11 |
| M4K10 | 2,389,962 | 99.51 | 46,726 | none | 11 |
| M4K9 | 2,074,752 | 97.81 | 34,253 | 6969 | 10 |
| Position | Reference | Alternative | Amino Acid Change | Gene |
|---|---|---|---|---|
| 1420 | A | G | - | N |
| 1936 | A | C | - | P |
| 2314 | T | C | - | P |
| 3085 | C | T | S-->F | M |
| 3489/5130 | G | A | G-->R | G |
| 3837/5478 | A | C | S-->R | G |
| 4266/5907 | A | G | R-->G | G |
| 4468/6109 | G | A | G-->E | G |
| 4690/6331 | A | G | Y-->C | G |
| 4802/6443 | A | G | - | G |
| 4820/6461 | C | T | - | G |
| 4860/6501 | C | T | H-->Y | G |
| 4880/6521 | C | T | - | G |
| 7555 | G | A | - | L |
| 7633 | C | A | D-->E | L |
| 9838 | A | G | - | L |
| 10,141 | G | A | - | L |
| 10,736 | G | A | D-->N | L |
| 11,848 | C | A | - | L |
| 12,274 | C | T | - | L |
| 12,343 | C | T | - | L |
| 12,486 | A | C | K-->T | L |
| 12,619 | G | A | - | L |
| 12,718 | C | T | - | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borutzki, S.; Richter, B.; Proemmel, M.; Fabianska, I.; Tran, H.Q.; Hundt, B.; Mayer, D.; Kaiser, C.; Neubert, A.; Vos, A. Oral Rabies Vaccine Strain SPBN GASGAS: Genetic Stability after Serial In Vitro and In Vivo Passaging. Viruses 2022, 14, 2136. https://doi.org/10.3390/v14102136
Borutzki S, Richter B, Proemmel M, Fabianska I, Tran HQ, Hundt B, Mayer D, Kaiser C, Neubert A, Vos A. Oral Rabies Vaccine Strain SPBN GASGAS: Genetic Stability after Serial In Vitro and In Vivo Passaging. Viruses. 2022; 14(10):2136. https://doi.org/10.3390/v14102136
Chicago/Turabian StyleBorutzki, Stefan, Benjamin Richter, Matthias Proemmel, Izabela Fabianska, Hon Quang Tran, Boris Hundt, Dietmar Mayer, Christian Kaiser, Andreas Neubert, and Ad Vos. 2022. "Oral Rabies Vaccine Strain SPBN GASGAS: Genetic Stability after Serial In Vitro and In Vivo Passaging" Viruses 14, no. 10: 2136. https://doi.org/10.3390/v14102136




