Contribution of Serum Cytomegalovirus PCR to Diagnosis of Early CMV Primary Infection in Pregnant Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. CMV PCR in Serum and Whole Blood Collected from Pregnant Women with Recent PI
- positive CMV IgM with negative CMV IgG: approximately 15 days
- positive CMV IgM, positive CMV IgG and CMV IgG avidity index < 20%: PI between 2 and 6 weeks
- positive CMV IgM, positive CMV IgG and CMV IgG avidity index between 20 and 40%: PI between 6 weeks and 3 months
2.1.2. Comparison of CMV PCR in Whole Blood versus Serum
2.2. CMV DNA Detection and Quantification
2.3. Statistical Analysis
3. Results
3.1. Description of Results
3.1.1. Sensitivity and Quantitative Comparison of CMV PCR in Serum versus Whole Blood in Pregnant Women with Recent PI
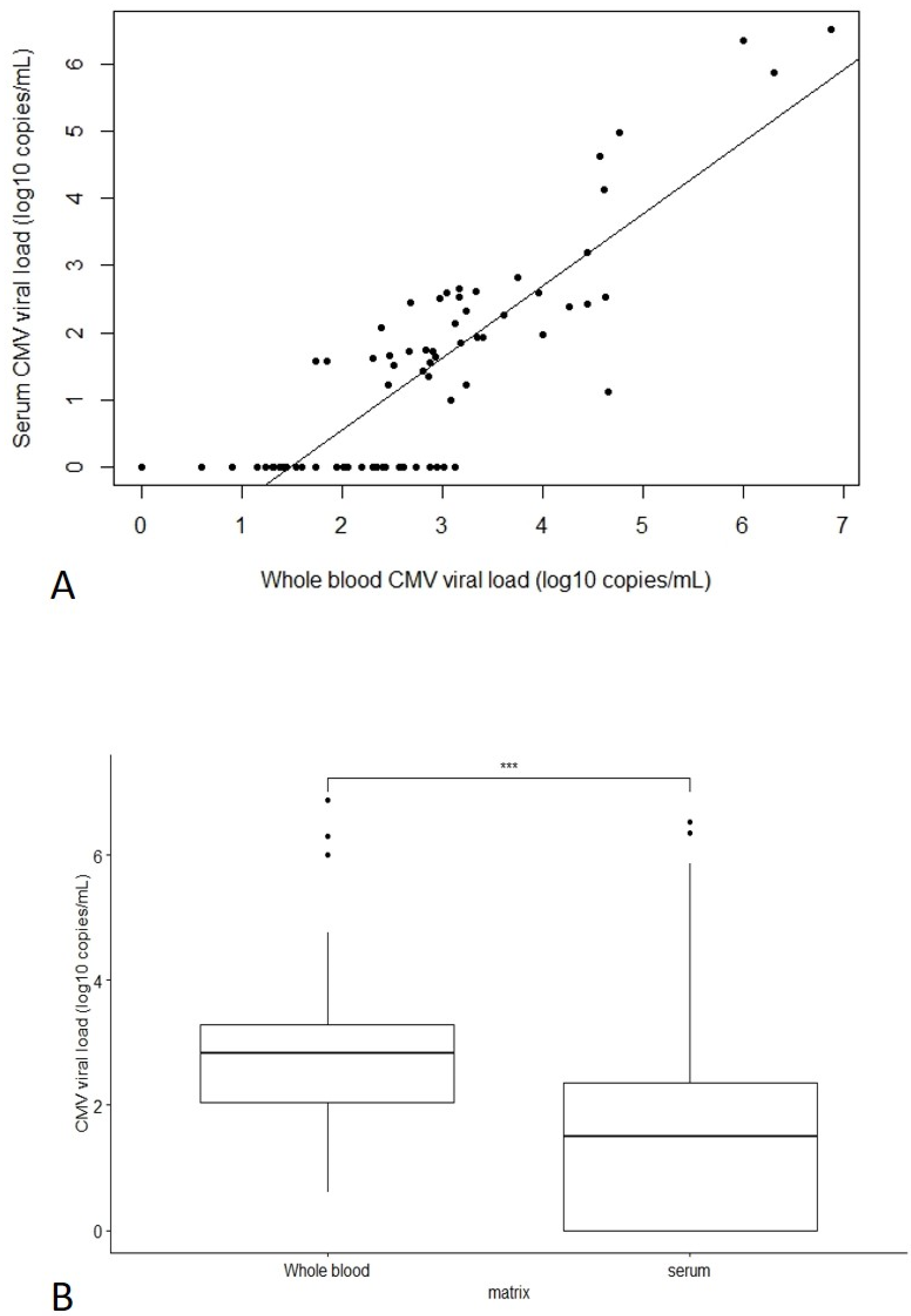
3.1.2. Comparison of CMV PCR in Whole Blood versus Serum
4. Discussion
- after CMV infection, CMV IgM is positive approximately 18 days later, which is concomitant with a positive CMV PCR both in whole blood and serum.
- IgG seroconversion appears at Day 20 and IgG avidity is reliable from Day 25/30.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenneson, A.; Cannon, M.J. Review and Meta-Analysis of the Epidemiology of Congenital Cytomegalovirus (CMV) Infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New Estimates of the Prevalence of Neurological and Sensory Sequelae and Mortality Associated with Congenital Cytomegalovirus Infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef]
- Stagno, S.; Pass, R.F.; Cloud, G.; Britt, W.J.; Henderson, R.E.; Walton, P.D.; Veren, D.A.; Page, F.; Alford, C.A. Primary Cytomegalovirus Infection in Pregnancy. Incidence, Transmission to Fetus, and Clinical Outcome. JAMA 1986, 256, 1904–1908. [Google Scholar] [CrossRef]
- Ross, S.A.; Fowler, K.B.; Ashrith, G.; Stagno, S.; Britt, W.J.; Pass, R.F.; Boppana, S.B. Hearing Loss in Children with Congenital Cytomegalovirus Infection Born to Mothers with Preexisting Immunity. J. Pediatr. 2006, 148, 332–336. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Magny, J.-F.; Couderc, S.; Pichon, C.; Parodi, M.; Bussières, L.; Guilleminot, T.; Ghout, I.; Ville, Y. Risk Factors for Congenital Cytomegalovirus Infection Following Primary and Nonprimary Maternal Infection: A Prospective Neonatal Screening Study Using Polymerase Chain Reaction in Saliva. Clin. Infect. Dis. 2017, 65, 398–404. [Google Scholar] [CrossRef]
- de Vries, J.J.C.; van Zwet, E.W.; Dekker, F.W.; Kroes, A.C.M.; Verkerk, P.H.; Vossen, A.C.T.M. The Apparent Paradox of Maternal Seropositivity as a Risk Factor for Congenital Cytomegalovirus Infection: A Population-Based Prediction Model: Maternal Seropositivity as a Risk Factor for CCMV. Rev. Med. Virol. 2013, 23, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to Prevent Vertical Transmission of Cytomegalovirus after Maternal Primary Infection during Pregnancy: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2020, 396, 779–785. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Fourgeaud, J.; Stirnemann, J.; Leruez-Ville, M.; Ville, Y. Secondary Prevention of Congenital CMV Infection with Valaciclovir Following Maternal Primary Infection in Early Pregnancy. Ultrasound Obs. Gynecol. 2021, 58, 576–581. [Google Scholar] [CrossRef]
- Egloff, C.; Sibiude, J.; Vauloup-Fellous, C.; Benachi, A.; Bouthry, E.; Biquard, F.; Hawkins-Villarreal, A.; Houhou-Fidouh, N.; Mandelbrot, L.; Vivanti, A.J.; et al. New Data on Efficacy of Valaciclovir in Secondary Prevention of Maternal-Fetal Transmission of CMV. Ultrasound Obs. Gynecol. 2022. accepted and unedited article. [Google Scholar] [CrossRef]
- Vauloup-Fellous, C.; Lazzarotto, T.; Revello, M.G.; Grangeot-Keros, L. Clinical Evaluation of the Roche Elecsys CMV IgG Avidity Assay. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1365–1369. [Google Scholar] [CrossRef] [Green Version]
- Périllaud-Dubois, C.; Bouthry, E.; Jadoui, A.; Leng, A.-L.; Roque-Afonso, A.-M.; Vauloup-Fellous, C. Positive Predictive Values of CMV-IgM and Importance of CMV-IgG Avidity Testing in Detecting Primary Infection in Three Different Clinical Settings. A French Retrospective Cohort Study. J. Clin. Virol. 2020, 132, 104641. [Google Scholar] [CrossRef]
- Vauloup-Fellous, C.; Berth, M.; Heskia, F.; Dugua, J.-M.; Grangeot-Keros, L. Re-Evaluation of the VIDAS® Cytomegalovirus (CMV) IgG Avidity Assay: Determination of New Cut-off Values Based on the Study of Kinetics of CMV–IgG Maturation. J. Clin. Virol. 2013, 56, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Sellier, Y.; Salomon, L.J.; Stirnemann, J.J.; Jacquemard, F.; Ville, Y. Prediction of Fetal Infection in Cases With Cytomegalovirus Immunoglobulin M in the First Trimester of Pregnancy: A Retrospective Cohort. Clin. Infect. Dis. 2013, 56, 1428–1435. [Google Scholar] [CrossRef]
- Garrigue, I.; Doussau, A.; Asselineau, J.; Bricout, H.; Couzi, L.; Rio, C.; Merville, P.; Fleury, H.; Lafon, M.-E.; Thiebaut, R. Prediction of Cytomegalovirus (CMV) Plasma Load from Evaluation of CMV Whole-Blood Load in Samples from Renal Transplant Recipients. J. Clin. Microbiol. 2008, 46, 493–498. [Google Scholar] [CrossRef]
- Razonable, R.R.; Brown, R.A.; Wilson, J.; Groettum, C.; Kremers, W.; Espy, M.; Smith, T.F.; Paya, C.V. The Clinical Use of Various Blood Compartments for Cytomegalovirus (CMV) DNA Quantitation in Transplant Recipients with CMV Disease. Transplantation 2002, 73, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de santé–Argumentaire–Evaluation de la Mesure de la Charge Virale du CytoméGalovirus par Amplification Génique chez les Receveurs d’Allogreffes. 2015, p. 74. Available online: https://www.has-sante.fr/jcms/c_2027970/fr/evaluation-de-la-mesure-de-la-charge-virale-du-cytomegalovirus-par-amplification-genique-chez-les-receveurs-d-allogreffes (accessed on 12 December 2020).
- Andrade, P.; Fioravanti, M.; Anjos, E.; De Oliveira, C.; Albuquerque, D.; Costa, S. Peripheral Blood Leukocytes and Serum Nested Polymerase Chain Reaction Are Complementary Methods for Monitoring Active Cytomegalovirus Infection in Transplant Patients. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, e69–e74. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Hou, P.F.; Bi, J.; Ying, C.M. Detection of Human Cytomegalovirus DNA in Various Blood Components after Liver Transplantation. Braz. J. Med. Biol. Res. 2014, 47, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Sidoti, F.; Mantovani, S.; Gregori, G.; Proietti, A.; Ghisetti, V.; Cavallo, R. Comparison of Two Molecular Assays for Detection of Cytomegalovirus DNA in Whole Blood and Plasma Samples from Transplant Recipients. New Microbiol. 2016, 39, 186–191. [Google Scholar] [PubMed]
- Berth, M.; Benoy, I.; Christensen, N. Evaluation of a Standardised Real-Time PCR Based DNA-Detection Method (Realstar®) in Whole Blood for the Diagnosis of Primary Human Cytomegalovirus (CMV) Infections in Immunocompetent Patients. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Zavattoni, M.; Sarasini, A.; Percivalle, E.; Simoncini, L.; Gerna, G. Human Cytomegalovirus in Blood of Immunocompetent Persons during Primary Infection: Prognostic Implications for Pregnancy. J. Infect. Dis. 1998, 177, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Micieli, M.; Votino, C.; Visconti, F.; Quaresima, P.; Strazzulla, A.; Torti, C.; Zullo, F. Knowledge of Human Cytomegalovirus Infection and Prevention in Pregnant Women: A Baseline, Operational Survey. Infectious Diseases in Obstetrics and Gynecology 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Kagan, K.O.; Enders, M.; Schampera, M.S.; Baeumel, E.; Hoopmann, M.; Geipel, A.; Berg, C.; Goelz, R.; De Catte, L.; Wallwiener, D.; et al. Prevention of Maternal-Fetal Transmission of Cytomegalovirus after Primary Maternal Infection in the First Trimester by Biweekly Hyperimmunoglobulin Administration. Ultrasound Obstet Gynecol 2019, 53, 383–389. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Périllaud-Dubois, C.; Bouthry, E.; Mouna, L.; Pirin, C.; Vieux-Combe, C.; Picone, O.; Roque-Afonso, A.-M.; Vivanti, A.J.; Vauloup-Fellous, C. Contribution of Serum Cytomegalovirus PCR to Diagnosis of Early CMV Primary Infection in Pregnant Women. Viruses 2022, 14, 2137. https://doi.org/10.3390/v14102137
Périllaud-Dubois C, Bouthry E, Mouna L, Pirin C, Vieux-Combe C, Picone O, Roque-Afonso A-M, Vivanti AJ, Vauloup-Fellous C. Contribution of Serum Cytomegalovirus PCR to Diagnosis of Early CMV Primary Infection in Pregnant Women. Viruses. 2022; 14(10):2137. https://doi.org/10.3390/v14102137
Chicago/Turabian StylePérillaud-Dubois, Claire, Elise Bouthry, Lina Mouna, Christine Pirin, Corinne Vieux-Combe, Olivier Picone, Anne-Marie Roque-Afonso, Alexandre J. Vivanti, and Christelle Vauloup-Fellous. 2022. "Contribution of Serum Cytomegalovirus PCR to Diagnosis of Early CMV Primary Infection in Pregnant Women" Viruses 14, no. 10: 2137. https://doi.org/10.3390/v14102137
APA StylePérillaud-Dubois, C., Bouthry, E., Mouna, L., Pirin, C., Vieux-Combe, C., Picone, O., Roque-Afonso, A.-M., Vivanti, A. J., & Vauloup-Fellous, C. (2022). Contribution of Serum Cytomegalovirus PCR to Diagnosis of Early CMV Primary Infection in Pregnant Women. Viruses, 14(10), 2137. https://doi.org/10.3390/v14102137





