Comparative HIV-1 Phylogenies Characterized by PR/RT, Pol and Near-Full-Length Genome Sequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Sample Requirements
2.2. Plasma Isolation and HIV-1 RNA Extraction
2.3. PCR Amplification and Sanger Sequencing of the HIV-1 Pol Region
2.4. Subtyping of HIV-1 PR/RT and Pol Region Nucleotide Sequences
2.5. Phylogenetic Analyses of HIV-1 PR/RT and Pol Region Nucleotide Sequences
2.6. PCR Amplification and Sanger Sequencing of the Near-Full-Length HIV-1 Genome
2.7. Subtyping and Phylogenetic Analyses of Near-Full-Length HIV-1 Genome Nucleotide Sequences
3. Results
3.1. Clinical, Epidemiological and Behavioral Information of the Study Participants
3.2. Discrepancies among the HIV-1 Subtyping Tools
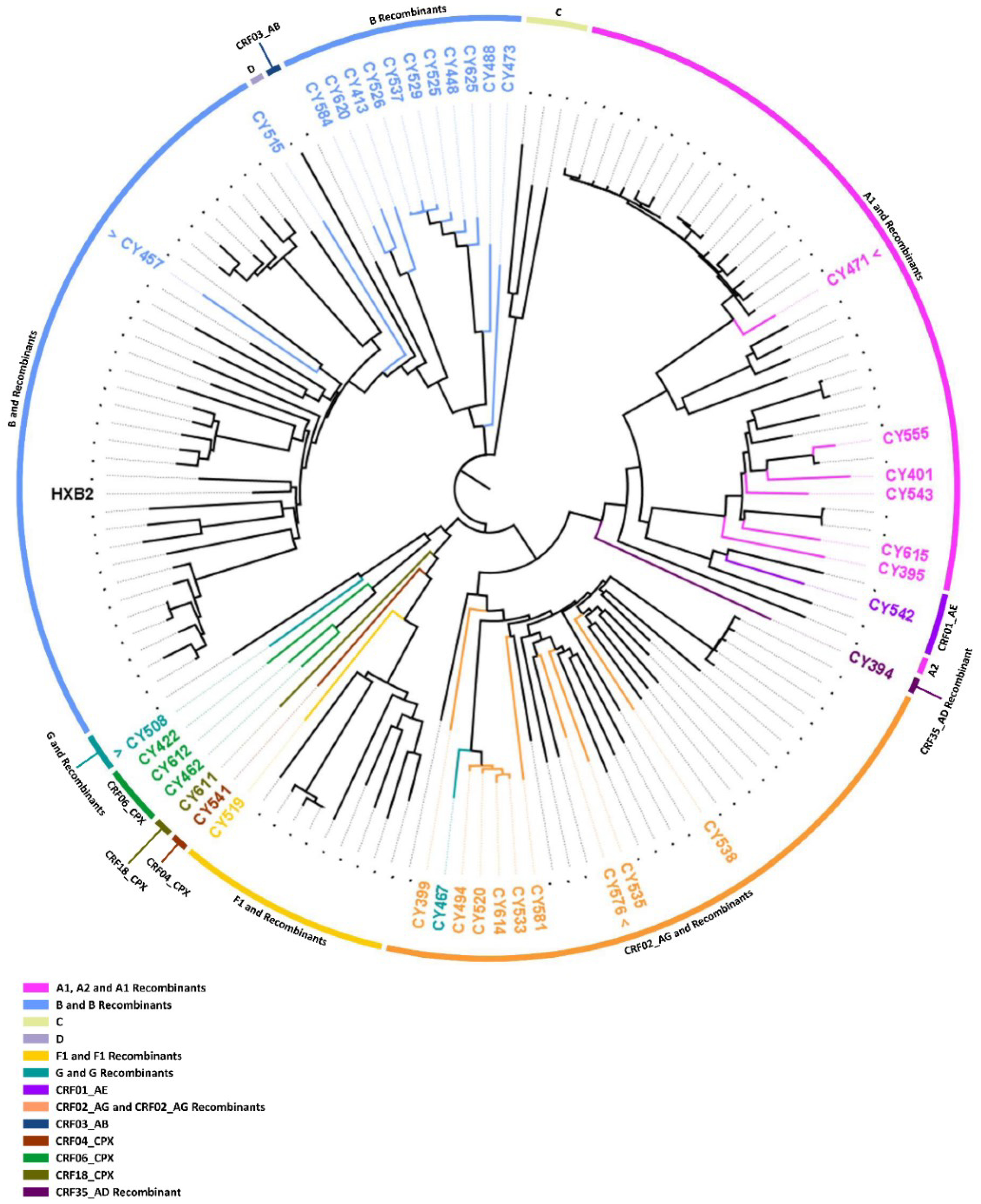
3.3. Comparative Phylogenetic Analyses
3.4. Characterization by Near-Full-Length Genome Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 4 April 2022).
- Lee, C.-Y.; Lin, Y.-P.; Wang, S.-F.; Lu, P.-L. Late cART Initiation Consistently Driven by Late HIV Presentation: A Multicenter Retrospective Cohort Study in Taiwan from 2009 to 2019. Infect. Dis. Ther. 2022, 11, 1033–1056. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Kirtley, S.; Gouws-Williams, E.; Ghys, P.D. Global and regional epidemiology of HIV-1 recombinants in 1990–2015: A systematic review and global survey. Lancet. HIV 2020, 7, e772–e781. [Google Scholar] [CrossRef]
- Robertson, D.L.; Anderson, J.P.; Bradac, J.A.; Carr, J.K.; Foley, B.; Funkhouser, R.K.; Gao, F.; Hahn, B.H.; Kalish, M.L.; Kuiken, C.; et al. HIV-1 nomenclature proposal. Science 2000, 288, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; McArthur, C.; Vallari, A.; Sthreshley, L.; Cloherty, G.A.; Berg, M.G.; Rodgers, M.A. Complete genome sequence of CG-0018a-01 establishes HIV-1 subtype L. J. Acquir. Immune Defic. Syndr. 2019, 83, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Peña, A.-C.; Theys, K.; Stylianou, D.C.; Demetriades, I.; Abecasis, A.B.; Kostrikis, L.G. HIV-1 Infection in Cyprus, the Eastern Mediterranean European Frontier: A Densely Sampled Transmission Dynamics Analysis from 1986 to 2012. Sci. Rep. 2018, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Kostrikis, L.G.; Hezka, J.; Stylianou, D.C.; Kostaki, E.; Andreou, M.; Kousiappa, I.; Paraskevis, D.; Demetriades, I. HIV-1 transmission networks across Cyprus (2010–2012). PLoS ONE 2018, 13, e0195660. [Google Scholar] [CrossRef]
- Robertson, D.L.; Hahn, B.H.; Sharp, P.M. Recombination in AIDS viruses. J. Mol. Evol. 1995, 40, 249–259. [Google Scholar] [CrossRef]
- Los Alamos HIV Sequence Database HIV Circulating Recombinant Forms (CRFs). Available online: https://www.hiv.lanl.gov/content/sequence/HIV/CRFs/crfs.comp (accessed on 12 September 2022).
- The EuroGUidelines Group for HIV Resistance. EuroGuidelines Group for HIV Resistance Clinical and laboratory guidelines for the use of HIV-1 drug resistance testing as part of treatment management: Recommendations for the European setting. AIDS 2001, 15, 309–320. [Google Scholar] [CrossRef]
- World Health Organization Update of Recommendations on First- and Second-Line Antiretroviral Regimens. Available online: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1 (accessed on 1 November 2019).
- Chrysostomou, A.C.; Topcu, C.; Stylianou, D.C.; Hezka, J.; Kostrikis, L.G. Development of a new comprehensive HIV-1 genotypic drug resistance assay for all commercially available reverse transcriptase, protease and integrase inhibitors in patients infected with group M HIV-1 strains. Infect. Genet. Evol. 2020, 81, 104243. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Pineda-Peña, A.-C.; Faria, N.R.; Imbrechts, S.; Libin, P.; Abecasis, A.B.; Deforche, K.; Gómez-López, A.; Camacho, R.J.; de Oliveira, T.; Vandamme, A.-M. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: Performance evaluation of the new REGA version 3 and seven other tools. Infect. Genet. Evol. 2013, 19, 337–348. [Google Scholar] [CrossRef]
- Struck, D.; Lawyer, G.; Ternes, A.-M.; Schmit, J.-C.; Bercoff, D.P. COMET: Adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014, 42, e144. [Google Scholar] [CrossRef]
- Schultz, A.-K.; Zhang, M.; Bulla, I.; Leitner, T.; Korber, B.; Morgenstern, B.; Stanke, M. jpHMM: Improving the reliability of recombination prediction in HIV-1. Nucleic Acids Res. 2009, 37, W647–W651. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Posada, D.; Stawiski, E.; Chappey, C.; Poon, A.F.Y.; Hughes, G.; Fearnhill, E.; Gravenor, M.B.; Leigh Brown, A.J.; Frost, S.D.W. An evolutionary model-based algorithm for accurate phylogenetic breakpoint mapping and subtype prediction in HIV-1. PLoS Comput. Biol. 2009, 5, e1000581. [Google Scholar] [CrossRef]
- Tang, M.W.; Liu, T.F.; Shafer, R.W. The HIVdb System for HIV-1 Genotypic Resistance Interpretation. Intervirology 2012, 55, 98–101. [Google Scholar] [CrossRef]
- Liu, T.F.; Shafer, R.W. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42, 1608–1618. [Google Scholar] [CrossRef]
- Beerenwinkel, N.; Däumer, M.; Oette, M.; Korn, K.; Hoffmann, D.; Kaiser, R.; Lengauer, T.; Selbig, J.; Walter, H. Geno2pheno: Estimating phenotypic drug resistance from HIV-1 genotypes. Nucleic Acids Res. 2003, 31, 3850–3855. [Google Scholar] [CrossRef]
- Kousiappa, I.; van de Vijver, D.A.M.C.; Demetriades, I.; Kostrikis, L.G. Genetic Analysis of HIV Type 1 Strains from Newly Infected Untreated Patients in Cyprus: High Genetic Diversity and Low Prevalence of Drug Resistance. AIDS Res. Hum. Retrovir. 2009, 25, 23–35. [Google Scholar] [CrossRef]
- Thomson, M.M.; Casado, G.; Posada, D.; Sierra, M.; Nájera, R. Identification of a novel HIV-1 complex circulating recombinant form (CRF18_cpx) of Central African origin in Cuba. AIDS 2005, 19, 1155–1163. [Google Scholar] [CrossRef]
- Topcu, C.; Georgiou, V.; Hezka Rodosthenous, J.; Demetriades, I.; Foley, B.T.; Kostrikis, L.G. Characterization of a novel HIV-1 circulating recombinant form, CRF91_cpx, comprising CRF02_AG, G, J, and U, mostly among men who have sex with men. Virulence 2022, 13, 1331–1348. [Google Scholar] [CrossRef]
- Kousiappa, I.; Van De Vijver, D.A.M.C.; Kostrikis, L.G. Near Full-Length Genetic Analysis of HIV Sequences Derived from Cyprus: Evidence of a Highly Polyphyletic and Evolving Infection. AIDS Res. Hum. Retrovir. 2009, 25, 727–740. [Google Scholar] [CrossRef]
- Kousiappa, I.; Achilleos, C.; Hezka, J.; Lazarou, Y.; Othonos, K.; Demetriades, I.; Kostrikis, L.G. Molecular characterization of HIV type 1 strains from newly diagnosed patients in Cyprus (2007–2009) recovers multiple clades including unique recombinant strains and lack of transmitted drug resistance. AIDS Res. Hum. Retrovir. 2011, 27, 1183–1199. [Google Scholar] [CrossRef]
- Fabeni, L.; Berno, G.; Fokam, J.; Bertoli, A.; Alteri, C.; Gori, C.; Forbici, F.; Takou, D.; Vergori, A.; Zaccarelli, M.; et al. Comparative Evaluation of Subtyping Tools for Surveillance of Newly Emerging HIV-1 Strains. J. Clin. Microbiol. 2017, 55, 2827–2837. [Google Scholar] [CrossRef]
- Giovanetti, M.; Ciccozzi, M.; Parolin, C.; Borsetti, A. Molecular Epidemiology of HIV-1 in African Countries: A Comprehensive Overview. Pathogens 2020, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lunar, M.M.; Mlakar, J.; Zorec, T.M.; Poljak, M. HIV-1 Unique Recombinant Forms Identified in Slovenia and Their Characterization by Near Full-Length Genome Sequencing. Viruses 2020, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.; Deforche, K.; Cassol, S.; Salminen, M.; Paraskevis, D.; Seebregts, C.; Snoeck, J.; van Rensburg, E.J.; Wensing, A.M.J.; van de Vijver, D.A.; et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics 2005, 21, 3797–3800. [Google Scholar] [CrossRef] [PubMed]
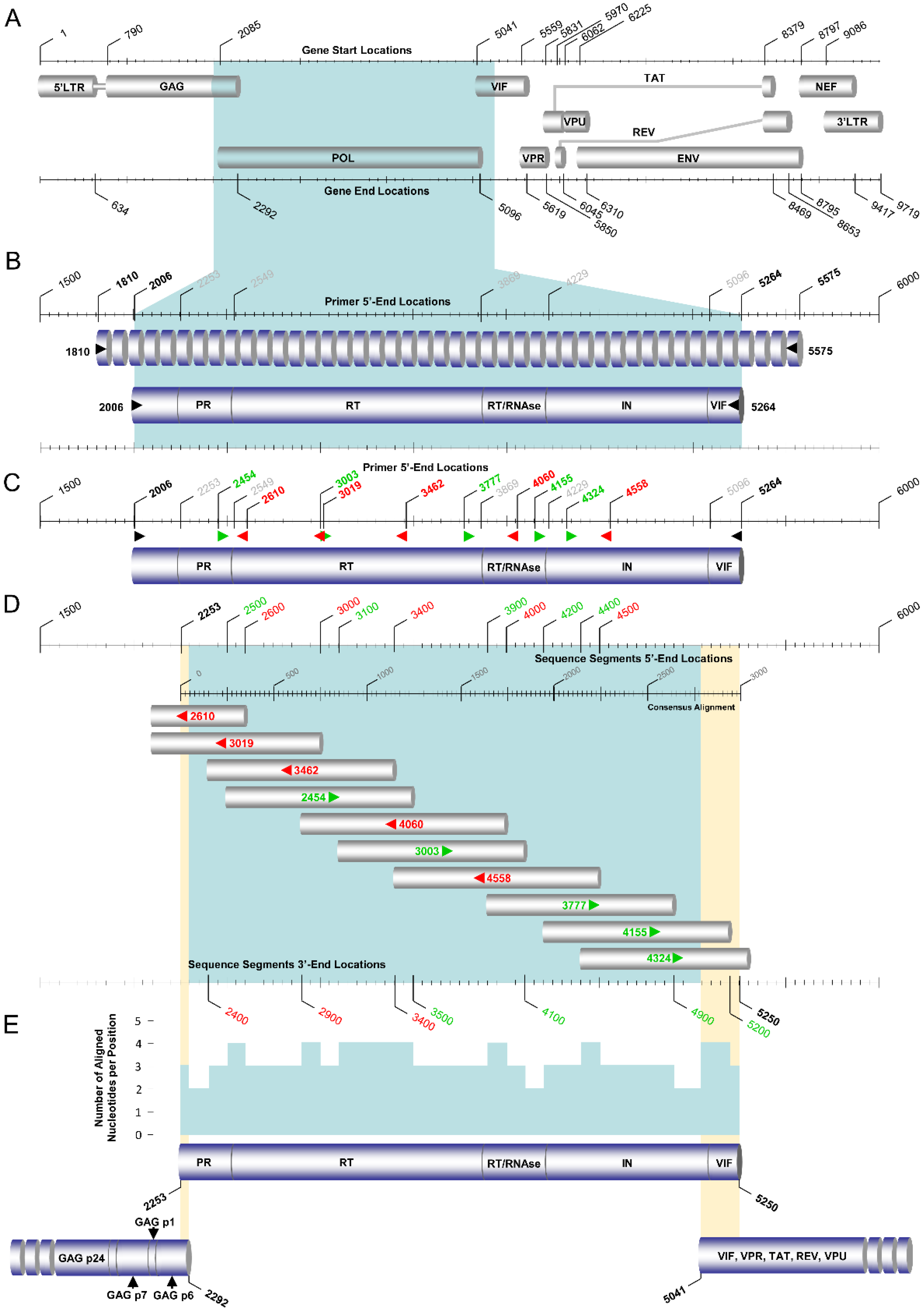



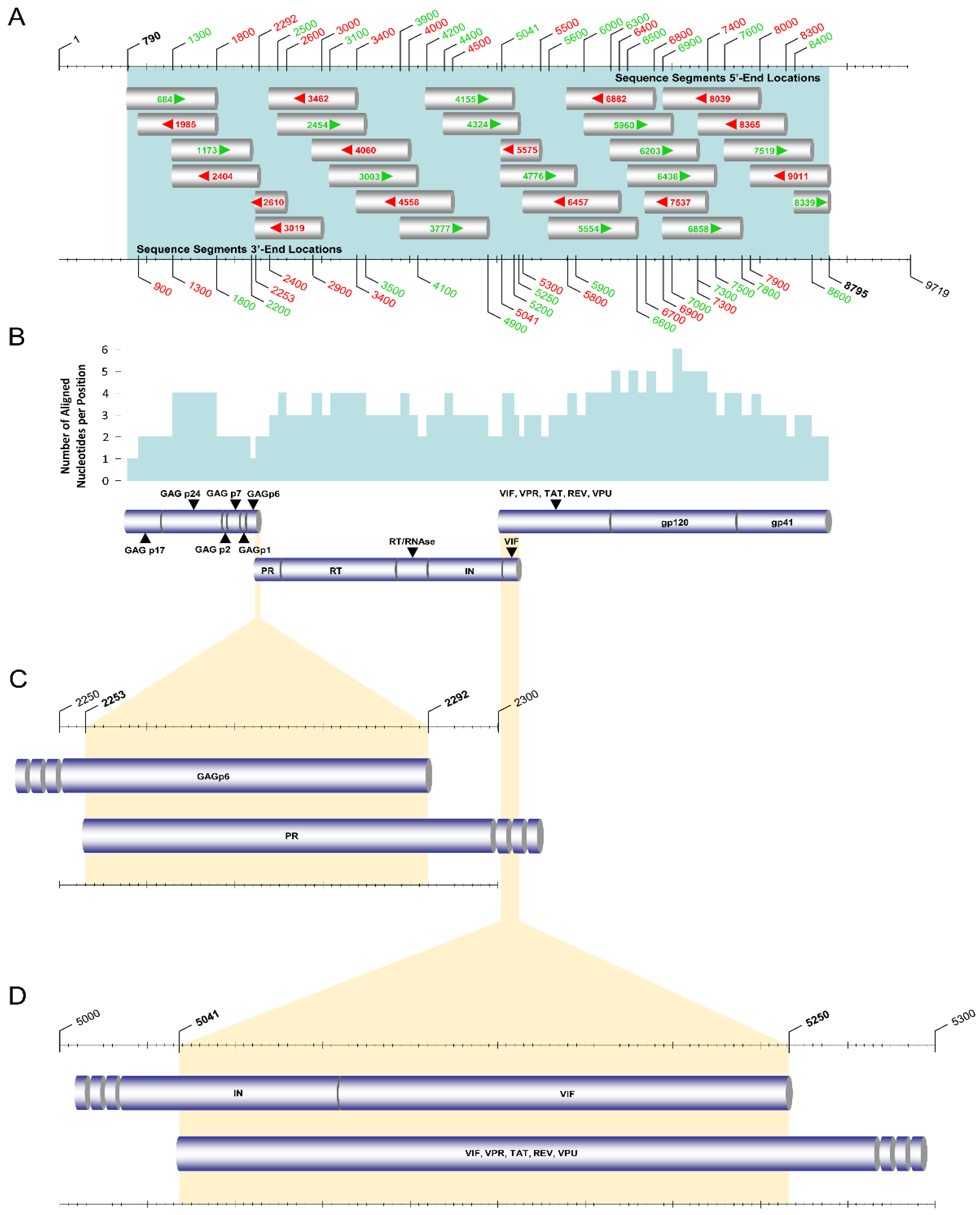


| Primary Touchdown RT–PCR a | |||
| Step c | Temperature (°C) | Time (min) | Number of Cycles |
| Reverse Transcription | 50 | 10:00 | 1× |
| RT Inactivation/Initial Denaturation | 98 | 2:00 | 1× |
| Initial Amplification | 98 | 0:10 | 10× |
| 61–52 (ΔΤ = −1 °C) d | 0:10 | ||
| 72 | 2:00 | ||
| Final Amplification | 98 | 0:10 | 30× |
| 52 | 0:10 | ||
| 72 | 2:00 | ||
| Final Extension | 72 | 5:00 | 1× |
| Reaction Stop | 4 | Indefinitely | Hold |
| Nested Touchdown PCR b | |||
| Step | Temperature (°C) | Time (min) | Number of Cycles |
| Initial Denaturation/Tag Polymerase Activation | 94 | 2:00 | 1× |
| Initial Amplification | 94 | 0:30 | 10× |
| 62–53 (ΔΤ = −1 °C) | 0:30 | ||
| 72 | 3:30 | ||
| Final Amplification | 94 | 0:30 | 30× |
| 53 | 0:30 | ||
| 72 | 3:30 | ||
| Final Extension | 72 | 5:00 | 1× |
| Reaction Stop | 4 | Indefinitely | Hold |
| Designation a | Target Gene | Sequence b | Position c | Amplicon Length (nts) | Reference |
|---|---|---|---|---|---|
| PCR Primers | |||||
| 630 (F) | gag | TAGCAGTGGCGCCC | 630–643 | 1872 | This study |
| 2501 (R) | gag | GTTGACAGGTGTAGGTCCTAC | 2481–2501 | [23] | |
| 684 * (F) | gag | TCTCGACGCAGGACTCG | 684–700 | 1721 | [23] |
| 2404 * (R) | gag | CCAATTCCYCCTATCATTTTTGGTTTCC | 2377–2404 | This study | |
| 1810 (F) | pol | GCTACAYTAGAAGAAATGATGACAGCATG | 1810–1838 | 3766 | [13] |
| 5575 * (R) | pol | TCTGGGGCTTGTTCCATCTATC | 5554–5575 | [13] | |
| 2006 (F) | pol | GGGCCCCTAGGAAAAAGGG | 2006–2024 | 3259 | [13] |
| 5264 (R) | pol | CCTGTATGCAGACCCCAATATGTT | 5241–5264 | [13] | |
| 3777 * (F) | env | TGGATTCCTGARTGGGARTTTG | 3777–3798 | 5405 | [13] |
| 9181 (R) | env | GTGTGTARTTYTGCCAATCAGG | 9160–9181 | This study | |
| 4155 * (F) | env | GTACCAGCACACAAAGGRATTG | 4155–4176 | 4883 | [13] |
| 9038 (R) | env | TAAGTCATTGGTCTTARAGGYACYTG | 9013–9038 | This study | |
| Sequencing Primers | |||||
| 684 * (F) | gag | TCTCGACGCAGGACTCG | 684–700 | [23] | |
| 1173 (F) | gag | CAGYCAAAATTAYCCTATAGTGCA | 1173–1196 | [23] | |
| 1985 (R) | gag | CCTTCYTTGCCACARTTGAAACAY | 1962–1985 | [23] | |
| 2404 * (R) | gag | CCAATTCCYCCTATCATTTTTGGTTTCC | 2377–2404 | This study | |
| 2454 (F) | pol | GGAMAWAARGCTATAGGTACAG | 2454–2475 | [13] | |
| 2610 (R) | pol | CYTTTGGGCCATCCATTC | 2593–2610 | [13] | |
| 3003 (F) | pol | GGATGGAAAGGATCACC | 3003–3019 | [13] | |
| 3019 (R) | pol | GGTGATCCTTTCCATCC | 3003–3019 | [13] | |
| 3462 (R) | pol | CTGCCARTTCTARYTCTGCTTC | 3441–3462 | [13] | |
| 3777 * (F) | pol | TGGATTCCTGARTGGGARTTTG | 3777–3798 | [13] | |
| 4060 (R) | pol | CCTAATGCATAYTGTGAGTCTGTTAC | 4035–4060 | [13] | |
| 4155 * (F) | pol | GTACCAGCACACAAAGGRATTG | 4155–4176 | [13] | |
| 4324 (F) | pol | TAGCAAAAGAAATAGTAGCCAGCTG | 4324–4348 | [13] | |
| 4558 (R) | pol | ACTGGCCATCTTCCTGCTAATTTTA | 4534–4558 | [13] | |
| 4776 (F) | env | CACAATTTTAAAAGAAAAGGGGGGATTG | 4776–4803 | This study | |
| 5554 (F) | env | GATAGATGGAACAAGCCCCAGA | 5554–5575 | This study | |
| 5575 * (R) | env | TCTGGGGCTTGTTCCATCTATC | 5554–5575 | [13] | |
| 5960 (F) | env | GGCATHTCCTATGGCAGGAAG | 5960–5980 | This study | |
| 6203 (F) | env | GAAAGAGCAGAAGAYAGTGGMA | 6203–6224 | This study | |
| 6438 (F) | env | CATGCCTGTGTACCCACAGA | 6438–6457 | [23] | |
| 6457 (R) | env | TCTGTGGGTACACAGGCATG | 6438–6457 | This study | |
| 6858 (F) | env | CCAATTCCYATACATTATTGTGCYC | 6858–6882 | [23] | |
| 6882 (R) | env | GRGCACAATAATGTATRGGAATTGG | 6858–6882 | This study | |
| 7519 (F) | env | AAGCAATGTATGCCCCTCC | 7519–7537 | This study | |
| 7537 (R) | env | GGAGGGGCATACATTGCTT | 7519–7537 | This study | |
| 8039 (R) | env | GGTGCARATGWGTTTTCCAGAGC | 8017–8039 | [23] | |
| 8339 (F) | env | AATAGAGTTAGGCAGGGATACTCACC | 8339–8364 | This study | |
| 8365 (R) | env | GGTGAGTATCCCTGCCTAACTCTATT | 8340–8365 | This study | |
| 9011 (R) | env | GGYCTGACTGGAAARCCYAC | 8992–9011 | This study |
| Primary Touchdown RT–PCR a | |||
| Step c | Temperature (°C) | Time (min) | Number of Cycles |
| Reverse Transcription | 50 | 10:00 | 1× |
| RT Inactivation/Initial Denaturation | 98 | 2:00 | 1× |
| Initial Amplification | 98 | 0:10 | 10× |
| 64–55 (ΔΤ = −1 °C) d | 0:10 | ||
| 72 | 1:00 | ||
| Final Amplification | 98 | 0:10 | 15× |
| 55 | 0:10 | ||
| 72 | 1:00 | ||
| Final Extension | 72 | 5:00 | 1× |
| Reaction Stop | 4 | Indefinitely | Hold |
| Nested Touchdown PCR b | |||
| Step | Temperature (°C) | Time (min) | Number of Cycles |
| Initial Denaturation/Tag Polymerase Activation | 94 | 2:00 | 1× |
| Initial Amplification | 94 | 0:30 | 10× |
| 64–55 (ΔΤ = −1 °C) | 0:30 | ||
| 72 | 1:00 | ||
| Final Amplification | 94 | 0:30 | 30× |
| 55 | 0:30 | ||
| 72 | 1:00 | ||
| Final Extension | 72 | 5:00 | 1× |
| Reaction Stop | 4 | Indefinitely | Hold |
| Primary Touchdown RT–PCR a | |||
| Step c | Temperature (°C) | Time (min) | Number of Cycles |
| Reverse Transcription | 58 | 10:00 | 1× |
| RT Inactivation/Initial Denaturation | 98 | 2:00 | 1× |
| Initial Amplification | 98 | 0:10 | 10× |
| 69–60 (ΔΤ = −1 °C) d | 0:10 | ||
| 72 | 3:00 | ||
| Final Amplification | 98 | 0:10 | 30× |
| 60 | 0:10 | ||
| 72 | 3:00 | ||
| Final Extension | 72 | 5:00 | 1× |
| Reaction Stop | 4 | Indefinitely | Hold |
| Nested PCR b | |||
| Step | Temperature (°C) | Time (min) | Number of Cycles |
| Initial Denaturation/Tag Polymerase Activation | 98 | 0:30 | 1× |
| Amplification | 98 | 0:10 | 40× |
| 60 | 0:10 | ||
| 72 | 3:00 | ||
| Final Extension | 72 | 5:00 | 1× |
| Reaction Stop | 4 | Indefinitely | Hold |
| No. | Samples 1 | GenBank Acession Number | REGA 3.0 4 | COMET 2.3 5 | jpHMM 6 | SCUEAL 7 | Stanford 8 | Geno2pheno 3.4 9 | Discrepancy 10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR/RT 2 | POL 3 | PR/RT | POL | PR/RT | POL | PR/RT | POL | PR/RT | POL | PR/RT | POL | PR/RT | POL | Regions | |||
| 3 | CY394 | ON989215 | CRF 35_AD | Rec. of 35_AD, G | 35_AD | Unassigned; 35_AD, 14_BG, G, 20_BG, G, 24_BG, G, 14_BG, G | A1 D | A1 D G | A1, D recombinant | Complex | CRF35_AD | A + D | A1 | A1 | YES | YES | YES |
| 4 | CY395 | ON989216 | Rec. of 14_BG, A1 | Rec. of A1, G | Unassigned; G, A1 | Unassigned; A1, G | A1 G | A1 G | A1, G recombinant | A1, G recombinant | G | A | 14_BG | A1 | YES | YES | YES |
| 7 | CY399 | ON989219 | G-like | Rec. of 02_AG, G, A1 | 02_AG | 02_AG | G | A1 G | G | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 8 | CY401 | ON989220 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A1, G recombinant | A | A | 01_AE | A1 | YES | YES | YES |
| 14 | CY413 | ON989226 | B | Rec. of B, A1 | Unassigned; B, A1 | Unassigned; B, A1 | B | A1 B | B, G recombinant | A1, B recombinant | B | B | B | B | YES | YES | YES |
| 18 | CY422 | ON989230 | CRF06_CPX | CRF06_CPX | 06_cpx | 06_cpx | D | A1 C D G J | CRF32-like | Complex | CRF06_cpx | CRF06_cpx | 06_CPX | 06_CPX | YES | YES | YES |
| 27 | CY448 | ON989239 | Rec. of B, G | Rec. of B, A1, G | Unassigned; 02_AG, 56_cpx | Unassigned; 56_cpx, 02_AG, 63_02A1, 02_AG, 71_BF1, 25_cpx, 02_AG, C, 43_02G, 02_AG, 71_BF1, 05_DF, 71_BF1, 05_DF, 02_AG, A1, B | B G | A1 B G | B, G recombinant | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | B | YES | YES | YES |
| 33 | CY457 | ON989245 | B | Rec. of B, A1 | B | Unassigned; B, A1 | B | A1 B | B | A3, B recombinant | B | B | B | B | - | YES | YES |
| 36 | CY462 | ON989248 | CRF 06_CPX | CRF 06_CPX | 06_cpx | 06_cpx | A1 C G | A1 C G J | G, K recombinant | CRF06-like | CRF06_cpx | CRF06_cpx | 06_CPX | 06_CPX | YES | YES | YES |
| 40 | CY467 | OK584018 | Rec. of G, A1 | Rec. of G, A1, B | 02_AG | Unassigned; 02_AG, 11_cpx, 01_AE, 90_BF1, B, D, B, D, B, D, B | G | A1 B G | G | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 42 | CY471 | ON989253 | A1 | Rec. of A1, B | A1 | Unassigned; A1, B | A1 | A1 B | A1 | A1, B recombinant | A | A | A1 | A1 | - | YES | YES |
| 44 | CY473 | ON989255 | B | Rec. of B, G, A1 | B | Unassigned; 02_AG, 38_BF1, B, 94_cpx, 08_BC, B, 08_BC, B, 07_BC, 90_BF1, 07_BC, 52_01B, 07_BC, 90_BF1, B, 68_01B, B, 90_BF1, B | B G | A1 B G | Complex | Complex | B | B | B | B | YES | YES | YES |
| 54 | CY488 | ON989265 | Rec. of B, A1 | Rec. of B, A1 | Unassigned; 56_cpx, 02_AG, 90_BFI, 72_BFI, 90_BFI, 83_cpx | Unassigned; 56_cpx, 02_AG, 90_BF1, 72_BF1, 90_BF1, 83_cpx, 90_BF1, A1, 02_AG, 19_cpx, B, 90_BF1 | A1 B | A1 B | Complex | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | 03_AB | YES | YES | YES |
| 58 | CY494 | OK283056 | Rec. of G, A1 | Rec. of 02_AG, G | 02_AG | Unassigned; 02_AG, 11_cpx, 01_AE | G | A1 G | G | A-ancestral, G recombinant | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 65 | CY508 | ON989275 | G | Rec. of G, K | G | Unassigned; G, A1, D | G | B G | G | G, J recombinant | G | G | G | G | - | YES | YES |
| 68 | CY515 | ON989278 | Rec. of F1, B | Rec. of B, F1 | F1 | Unassigned; B, F1, A1, F1, F2 | B F1 | B F1 | Complex | B, F1 recombinant | B | B | 12_BF | B | YES | YES | YES |
| 70 | CY519 | ON989280 | F1 | Rec. of F1, A1 | Unassigned; 71_BF1, A1 | Unassigned; F1, A1, 01_AE, A1, G | F1 | A1 F1 | F1 | Complex | F | F | F1 | F2 | YES | YES | YES |
| 71 | CY520 | OK283057 | Rec. of G, A1 | Rec. of 02_AG, G | 02_AG | Unassigned; 02_AG, 11_cpx, 01_AE | G | A1 G | G | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 73 | CY525 | ON989282 | Rec. of G, B | Rec. of B, A1, G | Unassigned; 02_AG, 56_cpx | Unassigned; 02_AG, 56_cpx, 90_BF1, B, 90_BF1, B, 56_cpx, B, 56_cpx, B, 39_BF, 51_01B, B, 51_01B, B, 90_BF1, 56_cpx, 90_BF1 | B C G | A1 B G | Complex | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | B | YES | YES | YES |
| 74 | CY526 | ON989283 | Rec. of G, B | Rec. of B, A1, G | Unassigned; 02_AG, 56_cpx | Unassigned; 02_AG, 56_cpx, 90_BF1, B, 90_BF1, B, 56_cpx, B, 39_BF, 90_BF1, 39_BF, B, 69_01B, 90_BF1, 56_cpx | B G | A1 B G | Complex | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | B | YES | YES | YES |
| 76 | CY529 | ON989285 | Rec. of G, B | Rec. of B, A1, G | Unassigned; 02_AG, 56_cpx | Unassigned; 02_AG, 56_cpx, 90_BF1, B, 90_BF1, B, 56_cpx, B, 56_cpx, B, 39_BF, 90_BF1, B, 90_BF1, 56_cpx | B C G | A1 B G | Complex | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | B | YES | YES | YES |
| 78 | CY533 | OK283058 | Rec. of G, A1 | Rec. of 02_AG, G, A1 | 02_AG | Unassigned; 02_AG, 11_cpx, 01_AE | G | A1 G | G | A, G recombinant | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 80 | CY535 | ON989288 | CRF 02_AG | CRF 02_AG | 02_AG | 02_AG | A1 D G | A1 C G | Complex | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 81 | CY537 | ON989289 | Rec. of G, B | Rec. of B, A1, G | Unassigned; 02_AG, 90_BF1, 31_BC, 56_cpx | Unassigned; 02_AG, 90_BF1, 31_BC, 56_cpx, 90_BF1, B, 90_BF1, B, 56_cpx, B, 39_BF, 90_BF1, 39_BF, B, 69_01B, 90_BF1, 56_cpx | B C G | A1 B G | Complex | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | B | YES | YES | YES |
| 82 | CY538 | ON989290 | 02_AG | Rec. of 02_AG, A1 | 02_AG | 02_AG | G | A1 G | G | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 84 | CY541 | ON989292 | CRF 04_CPX | CRF 04_CPX | F1 (check for 04_cpx) | 04_cpx | A1 C | A1 C | Complex | Complex | CRF04_cpx | CRF04_cpx | 04_CPX | 04_CPX | YES | YES | YES |
| 85 | CY542 | ON989293 | 01_AE | 01_AE | 01_AE | 01_AE | 01_AE | 01_AE | AE, B recombinant | AE, D recombinant | CRF01_AE | CRF01_AE | 01_AE | 01_AE | YES | YES | YES |
| 86 | CY543 | ON989294 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A | A | 01_AE | 01_AE | YES | YES | YES |
| 95 | CY555 | ON989303 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A | A | 01_AE | 01_AE | YES | YES | YES |
| 107 | CY576 | ON989315 | CRF 02_AG | CRF 02_AG | 02_AG | 02_AG | A1 G | A1 B G | Complex | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | - | YES | YES |
| 110 | CY581 | ON989318 | CRF 02_AG | Rec. of 02_AG, G, A1 | 02_AG | 02_AG | A1 G | A1 G | G | A, G recombinant | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 112 | CY584 | ON989320 | B | Rec. of B, A1 | B | Unassigned; B, A1 | B | A1 B | B, D recombinant | A1, B recombinant | B | B | B | B | YES | YES | YES |
| 125 | CY611 | ON989333 | Rec. of 18_cpx, G | CRF 18_cpx | F1 (check for 18_cpx) | 18_cpx | B G | A1 C F1 G | AE, G recombinant | G, K recombinant | CRF18_cpx | CRF18_cpx | K | 02_AG | YES | YES | YES |
| 126 | CY612 | ON989334 | CRF 06_CPX | CRF 06_CPX | 06_cpx | 06_cpx | C G | A1 C G J | CRF06-like | Complex | CRF06_cpx | CRF06_cpx | 06_CPX | 06_CPX | YES | YES | YES |
| 127 | CY614 | OK283059 | Rec. of G, A1 | Rec. of 02_AG, G, A1 | 02_AG | Unassigned; 02_AG, 11_cpx, 01_AE | G | A1 G | G | Complex | CRF02_AG | CRF02_AG | 02_AG | 02_AG | YES | YES | YES |
| 128 | CY615 | ON989335 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | A | A | 01_AE | 01_AE | YES | YES | YES |
| 132 | CY620 | ON989339 | B | Rec. of B, A1 | B | Unassigned; B, A1 | B | A1 B | B, D recombinant | Complex | B | B | B | B | YES | YES | YES |
| 134 | CY625 | ON989341 | Rec. of B, G | Rec. of B, A1, G | Unassigned; 02_AG, B, 56_cpx | Unassigned; 02_AG, B, 56_cpx, 90_BF1, B, 56_cpx, B, 51_01B, B, 69_01B, 90_BF1 | A1 B G | A1 B G | Complex | Complex | B + CRF02_AG | B + CRF02_AG | 02_AG | B | YES | YES | YES |
| No. | Samples 1 | GenBank Acession Number | NFLG 2 |
|---|---|---|---|
| 1 | CY394 | ON989215 | Rec. of 14_BG, A1, D |
| 2 | CY395 | ON989216 | Rec. of A1, G |
| 3 | CY399 | ON989219 | Rec. of G, 43_02G, A1 |
| 4 | CY401 | ON989220 | A1 |
| 5 | CY413 | ON989226 | Rec. of A1, B |
| 6 | CY422 | ON989230 | CRF06_CPX |
| 7 | CY448 | ON989239 | Rec. of 02_AG, B, G, A1 |
| 8 | CY457 | ON989245 | B |
| 9 | CY462 | ON989248 | CRF06_CPX |
| 10 | CY467 | OK584018 | Rec. of 02_AG, G, B, J, D |
| 11 | CY471 | ON989253 | A1 |
| 12 | CY473 | ON989255 | Rec. of 02_AG, B |
| 13 | CY488 | ON989265 | Rec. of 02_AG, B, G |
| 14 | CY494 | OK283056 | Rec. of 02_AG, G, J |
| 15 | CY508 | ON989275 | Rec. of G, 06_CPX |
| 16 | CY515 | ON989278 | Rec. of B, F1 |
| 17 | CY519 | ON989280 | Rec. of F1, A1 |
| 18 | CY520 | OK283057 | Rec. of 02_AG, G, J |
| 19 | CY525 | ON989282 | Rec. of 02_AG, B, G |
| 20 | CY526 | ON989283 | Rec. of 02_AG, B, G |
| 21 | CY529 | ON989285 | Rec. of 02_AG, B, G |
| 22 | CY533 | OK283058 | Rec. of 02_AG, G, J, A1 |
| 23 | CY535 | ON989288 | CRF02_AG |
| 24 | CY537 | ON989289 | Rec. of 02_AG, B, G |
| 25 | CY538 | ON989290 | CRF02_AG |
| 26 | CY541 | ON989292 | CRF04_CPX |
| 27 | CY542 | ON989293 | Rec. of 01_AE, B |
| 28 | CY543 | ON989294 | A1 |
| 29 | CY555 | ON989303 | A1 |
| 30 | CY576 | ON989315 | CRF02_AG |
| 31 | CY581 | ON989318 | CRF02_AG |
| 32 | CY584 | ON989320 | Rec. of A1, B |
| 33 | CY611 | ON989333 | Rec. of 02_AG, F1, A1, G |
| 34 | CY612 | ON989334 | CRF06_CPX |
| 35 | CY614 | OK283059 | Rec. of 02_AG, G, J, A1 |
| 36 | CY615 | ON989335 | A1 |
| 37 | CY620 | ON989339 | Rec. of A1, B |
| 38 | CY625 | ON989341 | Rec. of 02_AG, B, G, A1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topcu, C.; Georgiou, V.; Rodosthenous, J.H.; Kostrikis, L.G. Comparative HIV-1 Phylogenies Characterized by PR/RT, Pol and Near-Full-Length Genome Sequences. Viruses 2022, 14, 2286. https://doi.org/10.3390/v14102286
Topcu C, Georgiou V, Rodosthenous JH, Kostrikis LG. Comparative HIV-1 Phylogenies Characterized by PR/RT, Pol and Near-Full-Length Genome Sequences. Viruses. 2022; 14(10):2286. https://doi.org/10.3390/v14102286
Chicago/Turabian StyleTopcu, Cicek, Vasilis Georgiou, Johana Hezka Rodosthenous, and Leondios G. Kostrikis. 2022. "Comparative HIV-1 Phylogenies Characterized by PR/RT, Pol and Near-Full-Length Genome Sequences" Viruses 14, no. 10: 2286. https://doi.org/10.3390/v14102286
APA StyleTopcu, C., Georgiou, V., Rodosthenous, J. H., & Kostrikis, L. G. (2022). Comparative HIV-1 Phylogenies Characterized by PR/RT, Pol and Near-Full-Length Genome Sequences. Viruses, 14(10), 2286. https://doi.org/10.3390/v14102286





