The CD8+ and CD4+ T Cell Immunogen Atlas of Zika Virus Reveals E, NS1 and NS4 Proteins as the Vaccine Targets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viral Strains and Mice
2.2. Splenocyte Isolation
2.3. Peptide Prediction Approaches and Peptide Synthesis
2.4. ELISPOT Assays
2.5. Flow Cytometry Analyses
2.6. Tetramer Preparation and Staining
2.7. Statistical Analysis
3. Results
3.1. The Distinct Immunogenic Hierarchy of Structural and Nonstructural Proteins of ZIKV in H-2b and H-2d Mice
3.2. The Profile Mapping of Antigenic Peptides across the Whole ZIKV Polyprotein in Mice
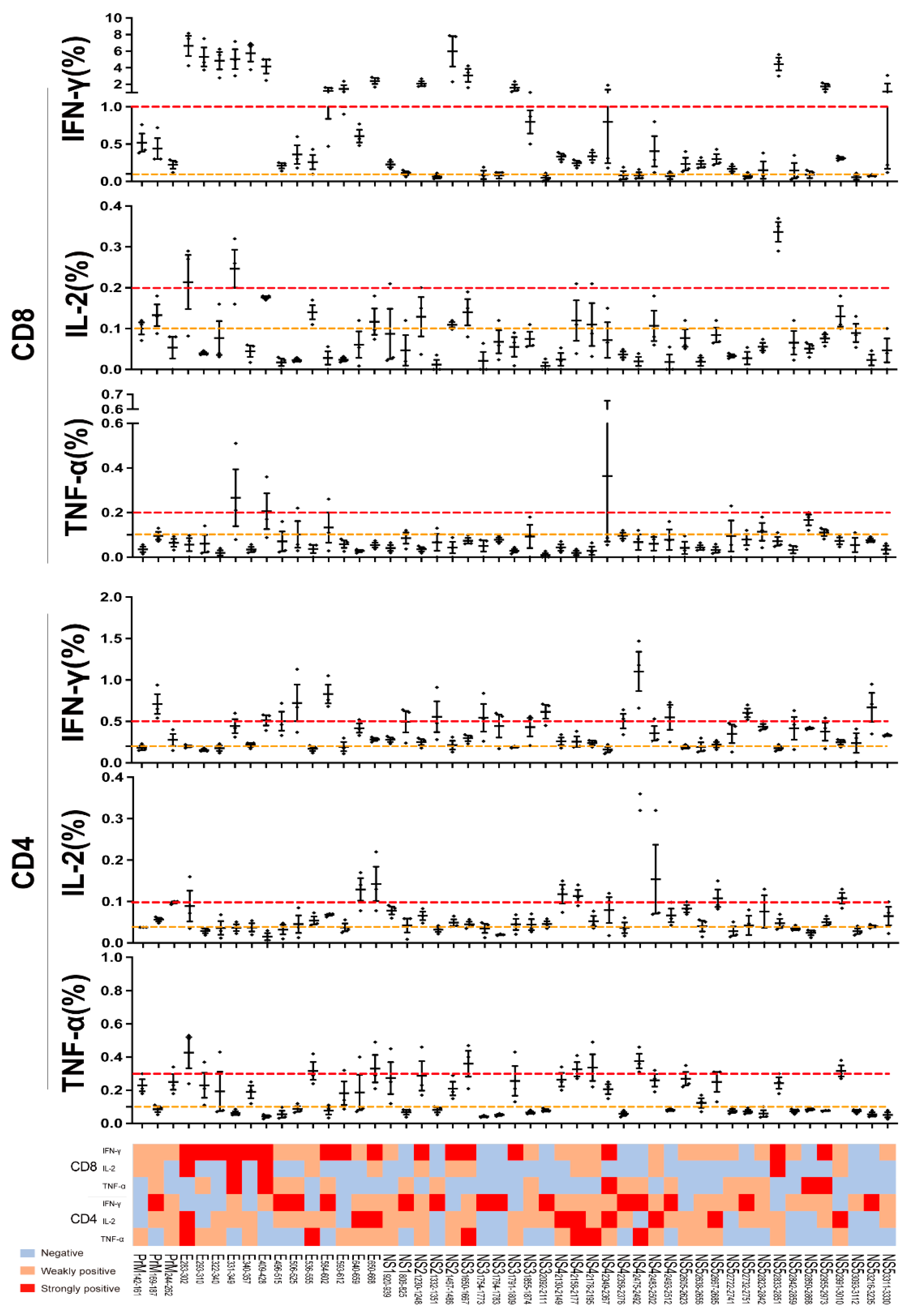
3.3. The CD8+ and CD4+ T Cell Recognition Features of the ZIKV Antigens
3.4. The Immunodominant Hotspots of ZIKV Recognized by CD8+ T Cells in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans R Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Hofer, U. Viral Pathogenesis: Tracing the steps of Zika virus. Nat. Rev. Microbiol. 2016, 14, 401. [Google Scholar] [CrossRef]
- Dos Santos, T.; Rodriguez, A.; Almiron, M.; Sanhueza, A.; Ramon, P.; de Oliveira, W.K.; Coelho, G.E.; Badaró, R.; Cortez, J.; Ospina, M.; et al. Zika Virus and the Guillain-Barré Syndrome—Case Series from Seven Countries. N. Engl. J. Med. 2016, 375, 1598–1601. [Google Scholar] [CrossRef]
- Brasil, P.; Sequeira, P.C.; Freitas, A.D.A.; Zogbi, H.E.; Calvet, G.A.; de Souza, R.V.; Siqueira, A.M.; de Mendonca, M.C.L.; Nogueira, R.M.R.; de Filippis, A.M.B.; et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet 2016, 387, 1482. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, W.K.; de França, G.V.A.; Carmo, E.H.; Duncan, B.B.; de Souza Kuchenbecker, R.; Schmidt, M.I. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: A surveillance-based analysis. Lancet 2017, 390, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabié, A.; Callier, C.; Carles, G.; Cassadou, S.; Césaire, R.; et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N. Engl. J. Med. 2018, 378, 985–994. [Google Scholar] [CrossRef]
- Hancock, W.T.; Marfel, M.; Bel, M. Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infect Dis. 2014, 20, 1960. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Barjas-Castro, M.L.; Angerami, R.N.; Cunha, M.S.; Suzuki, A.; Nogueira, J.S.; Rocco, I.M.; Maeda, A.Y.; Vasami, F.G.S.; Katz, G.; Boin, I.F.S.F.; et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016, 56, 1684–1688. [Google Scholar] [CrossRef]
- Joguet, G.; Mansuy, J.-M.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-Rainsford, N.; et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: A prospective observational study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2016, 167, 1511–1524.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Lai, L.; Rouphael, N.; Xu, Y.; Natrajan, M.S.; Beck, A.; Hart, M.; Feldhammer, M.; Feldpausch, A.; Hill, C.; Wu, H.; et al. Innate, T-, and B-Cell Responses in Acute Human Zika Patients. Clin. Infect Dis. 2018, 66, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J. Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, J.; Yang, M.; Gao, F.; Zhou, J.; Kitamura, Y.; Gao, B.; Tien, P.; Shu, Y.; Iwamoto, A.; et al. Identification and structural definition of H5-specific CTL epitopes restricted by HLA-A*0201 derived from the H5N1 subtype of influenza A viruses. J. Gen. Virol. 2010, 91 Pt 4, 919–930. [Google Scholar] [CrossRef]
- Liu, W.J.; Bi, Y.; Wang, D.; Gao, G.F. On the Centenary of the Spanish Flu: Being Prepared for the Next Pandemic. Virol. Sin. 2018, 33, 463–466. [Google Scholar] [CrossRef]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018, 14, e1007237. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.M.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, C.G.O.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.A.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical role of CD4 T cells and IFNγ signaling in antibody-mediated resistance to Zika virus infection. Nat. Commun. 2018, 9, 3136. [Google Scholar] [CrossRef] [Green Version]
- Hari, A.; Ganguly, A.; Mu, L.; Davis, S.P.; Stenner, M.D.; Lam, R.; Munro, F.; Namet, I.; Alghamdi, E.; Fürstenhaupt, T.; et al. Redirecting soluble antigen for MHC class I cross-presentation during phagocytosis. Eur. J. Immunol. 2015, 45, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, B.; Zhang, S.; Tan, S.; Sun, Y.; Chen, Z.; Qin, Y.; Sun, M.; Shi, G.; Wu, Y.; et al. Conserved epitopes dominate cross-CD8+ T-cell responses against influenza A H1N1 virus among Asian populations. Eur. J. Immunol. 2013, 43, 2055–2069. [Google Scholar] [CrossRef] [PubMed]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog. 2017, 13, e1006184. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Li, S.; Zhang, Y.; Han, X.; Jia, B.; Liu, H.; Liu, D.; Tan, S.; Wang, Q.; Bi, Y.; et al. CD8(+) T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J. Virol. 2017, 91, e00900-17. [Google Scholar] [CrossRef] [Green Version]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [Green Version]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef]
- Osuna, C.E.; Lim, S.-Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.W.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef] [Green Version]
- Manangeeswaran, M.; Ireland, D.D.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 2016, 12, e1006004. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liao, X.; Fan, D.; Wang, L.; Song, J.; Feng, K.; Li, M.; Wang, P.; Chen, H.; An, J. Maternal immunization with a DNA vaccine candidate elicits specific passive protection against post-natal Zika virus infection in immunocompetent BALB/c mice. Vaccine 2018, 36, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Medina-Magües, L.G.; Gergen, J.; Jasny, E.; Petsch, B.; Lopera-Madrid, J.; Medina-Magües, E.S.; Salas-Quinchucua, C.; Osorio, J.E. mRNA Vaccine Protects against Zika Virus. Vaccines 2021, 9, 1464. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Ke, C.; Wu, Q.; Lu, W.; Qin, Z.; He, X.; Liu, Y.; Deng, J.; Xu, S.; et al. Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection. Viruses 2017, 9, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, S. T cells in coxsackievirus-induced myocarditis. Viral Immunol. 2004, 17, 152–164. [Google Scholar] [CrossRef]
- Schirmbeck, R.; Reimann, J. Enhancing the immunogenicity of exogenous hepatitis B surface antigen-based vaccines for MHC-I-restricted T cells. Biol. Chem. 1999, 380, 285–291. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [Green Version]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef]
- Ikram, A.; Zaheer, T.; Awan, F.M.; Obaid, A.; Naz, A.; Hanif, R.; Paracha, R.Z.; Ali, A.; Naveed, A.K.; Janjua, H.A. Exploring NS3/4A, NS5A and NS5B proteins to design conserved subunit multi-epitope vaccine against HCV utilizing immunoinformatics approaches. Sci. Rep. 2018, 8, 16107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, S.; Oezkan, F.; Bedrejowski, M.; Kudla, M.; Reiser, M.; Viazov, S.; Scherbaum, N.; Roggendorf, M.; Timm, J. Degree of cross-genotype reactivity of hepatitis C virus-specific CD8+ T cells directed against NS3. Hepatology 2009, 50, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Eckels, D.D.; Zhou, H.; Bian, T.H.; Wang, H. Identification of antigenic escape variants in an immunodominant epitope of hepatitis C virus. Int. Immunol. 1999, 11, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elong Ngono, A.; Chen, H.W.; Tang, W.W.; Joo, Y.; King, K.; Weiskopf, D.; Sidney, J.; Sette, A.; Shresta, S. Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection. EBioMedicine 2016, 13, 284–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kümmerer, B.M. Establishment and Application of Flavivirus Replicons. Adv. Exp. Med. Biol. 2018, 1062, 165–173. [Google Scholar]
- Gomez-Perosanz, M.; Ras-Carmona, A.; Lafuente, E.M.; Reche, P.A. Identification of CD8(+) T cell epitopes through proteasome cleavage site predictions. BMC Bioinform. 2020, 21 (Suppl. 17), 484. [Google Scholar] [CrossRef]
- Cascio, P.; Hilton, C.; Kisselev, A.F.; Rock, K.L.; Goldberg, A.L. 26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. Embo J. 2001, 20, 2357–2366. [Google Scholar] [CrossRef]




| Name | Peptides Sequence | SCFs/106 | CD4/CD8 | Epitopes Sequence | MHC | SCFs/106 |
|---|---|---|---|---|---|---|
| C18-37 | KRGVARVSPFGGLKRLPAGL | 98 | NA | |||
| PrM142-161 | GEAISFPTTLGMNKCYIQIM | 76 | CD8 | ISFPTTLGM | Db | 43 |
| TLGMNKCYI | Db | 94 | ||||
| PrM169-187 | ATMSYECPMLDEGVEPDDV | 265 | CD8/CD4 | MSYECPML | Db | 42 |
| PrM244-262 | LIRVENWIFRNPGFALAAAA | 281 | CD8/CD4 | IFRNPGFAL | Kb | 90 |
| E283-302 | LLIAPAYSIRCIGVSNRDFV | 792 | CD8 | IGVSNRDFV | Db | 836 |
| E293-310 | CIGVSNRDFVEGMSGGTWV | 804 | CD8 | SNRDFVEGM | Db | 220 |
| E312-331 | DVVLEHGGCVTVMAQDKPTV | 118 | NA | |||
| E322-340 | TVMAQDKPTVDIELVTTTV | 284 | CD8 | |||
| E331-349 | VDIELVTTTVSNMAEVRSY | 238 | CD8 | TTVSNMAEV | Db | 104 |
| E340-357 | VSNMAEVRSYCYEASISDMA | 402 | CD8 | EVRSYCYEASI | Kb | 758 |
| RSYCYEASI | Db | 218 | ||||
| E350-369 | CYEASISDMASDSRCPTQGE | 284 | NA | |||
| E389-408 | RGWGNGCGLFGKGSLVTCAK | 156 | NA | |||
| E409-428 | FACSKKMTGKSIQPENLEYR | 78 | CD8 | |||
| E496-515 | MNNKHWLVHKEWFHDIPLPW | 60 | CD4 | |||
| E506-525 | EWFHDIPLPWHAGADTGTPH | 296 | CD8/CD4 | |||
| E536-555 | KDAHAKRQTVVVLGSQEGAV | 132 | CD8 | VLGSQEGAV | Kb | 110 |
| E575-593 | SSGHLKCRLKMDKLRLKGV | 184 | NA | |||
| E584-602 | KMDKLRLKGVSYSLCTAAF | 260 | CD8/CD4 | |||
| E593-612 | VSYSLCTAAFTFTKIPAETL | 42 | CD8 | VSYSLCTAA | Kb | 153 |
| AAFTFTKI | Kb | 214 | ||||
| E603-622 | TFTKIPAETLHGTVTVEVQY | 88 | NA | |||
| E630-649 | KVPAQMAVDMQTLTPVGRLI | 40 | NA | MAVDMQTLTPV | Db | 114 |
| E640-659 | QTLTPVGRLITANPVITEST | 357 | CD8/CD4 | |||
| E650-668 | TANPVITESTENSKMMLEL | 338 | CD8 | |||
| E679-697 | IGVGEKKITHHWHRSGSTI | 124 | NA | |||
| E688-706 | HHWHRSGSTIGKAFEATVR | 658 | NA | |||
| E705-724 | VRGAKRMAVLGDTAWDFGSV | 120 | NA | |||
| E744-763 | KSLFGGMSWFSQILIGTLLM | 704 | NA | |||
| E754-770 | SQILIGTLLMWLGLNTK | 144 | NA | |||
| E771-788 | NGSISLMCLALGGVLIFL | 216 | NA | |||
| NS1796-815 | VGCSVDFSKKETRCGTGVFV | 350 | NA | |||
| NS1806-825 | ETRCGTGVFVYNDVEAWRDR | 232 | CD8/CD4 | TGVFVYNDV | Kb | 144 |
| NS1816-835 | YNDVEAWRDRYKYHPDSPRR | 124 | NA | |||
| NS1835-854 | RLAAAVKQAWEDGICGISSV | 140 | NA | |||
| NS1864-883 | SVEGELNAILEENGVQLTVV | 418 | NA | |||
| NS1835-855 | EENGVQLTVVVGSVKNPMWR | 266 | NA | |||
| NS1874-893 | RGPQRLPVPVNELPHGWKAW | 174 | NA | |||
| NS1903-922 | NELPHGWKAWGKSYFVRAAK | 328 | NA | |||
| NS1913-929 | GKSYFVRAAKTNNSFVV | 98 | NA | |||
| NS1920-939 | AAKTNNSFVVDGDTLKECPL | 82 | NA | |||
| NS1930-949 | DGDTLKECPLKHRAWNSFLV | 160 | NA | |||
| NS1940-958 | KHRAWNSFLVEDHGFGVFH | 194 | NA | |||
| NS1976-995 | AVIGTAVKGKEAVHSDLGYW | 102 | NA | |||
| NS11106-1125 | CCRECTMPPLSFRAKDGCWY | 88 | NA | |||
| NS21230-1248 | KVRPALLVSFIFRANWTPR | 202 | CD8 | VSFIFRAN | Kb | 92 |
| VSFIFRANW | Kb | 348 | ||||
| NS21283-1320 | LAIRAMVVPRTDNITLAILA | 90 | NA | |||
| NS21322-1341 | TCGGFMLLSLKGKGSVKKNL | 152 | NA | |||
| NS21332-1351 | KGKGSVKKNLPFVMALGLTA | 48 | CD4 | |||
| NS21352-1371 | VRLVDPINVVGLLLLTRSGK | 208 | NA | |||
| NS21467-1486 | REIILKVVLMTICGMNPIAI | 130 | CD8 | VLMTICGM | Db | 123 |
| CGMNPIAI | Db | 676 | ||||
| NS21477-1496 | TICGMNPIAIPFAAGAWYVY | 134 | NA | |||
| NS21487-1506 | PFAAGAWYVYVKTGKRSGAL | 224 | NA | |||
| NS31650-1667 | GLYGNGVVIKNGSYVSAI | 291 | CD8 | VVIKNGSYV | Db | 332 |
| NGSYVSAI | Db | 236 | ||||
| NS31716-1734 | KTRLRTVILAPTRVVAAEM | 108 | NA | |||
| NS31754-1773 | HSGTEIVDLMCHATFTSRLL | 162 | CD4 | |||
| NS31764-1783 | CHATFTSRLLQPIRVPNYNL | 164 | CD4 | |||
| NS31791-1809 | FTDPSSIAARGYISTRVEM | 128 | CD8 | SSIAARGYI | Db | 436 |
| NS31855-1874 | TDHSGKTVWFVPSVRNGNEI | 111 | CD8/CD4 | PSVRNGNEI | Kb | 46 |
| SVRNGNEI | Db | 39 | ||||
| NS31936-1955 | ILDGERVILAGPMPVTHASA | 514 | NA | |||
| NS32092-2111 | LKPRWMDARVCSDHAALKSF | 259 | CD4 | |||
| NS42130-2149 | GTLPGHMTERFQEAIDNLAV | 259 | CD8/CD4 | FQEAIDNL | Db | 812 |
| FQEAIDNLAV | Db | 56 | ||||
| NS42158-2177 | RPYKAAAAQLPETLETIMLL | 88 | CD8/CD4 | QLPETLETI | Db | 58 |
| NS42168-2187 | PETLETIMLLGLLGTVSLGI | 112 | NA | |||
| NS42178-2194 | GLLGTVSLGIFFVLMRNKGI | 76 | CD8/CD4 | VSLGIFFVLM | Kb | 178 |
| NS42275-2293 | LERTKSDLSHLMGRREEGA | 144 | NA | |||
| NS42284-2303 | HLMGRREEGATIGFSMDIDL | 124 | NA | |||
| NS42092-2116 | TIGFSMDIDLRPASAWAIYA | 180 | NA | |||
| NS42294-2313 | RPASAWAIYAALTTFITPAV | 236 | NA | |||
| NS42349-2367 | MGKGMPFYAWDFGVPLLMI | 84 | CD8 | YAWDFGVPL | Kb | 120 |
| YAWDFGVPLL | Kb | 150 | ||||
| NS42358-2376 | WDFGVPLLMIGCYSQLTPL | 106 | CD4 | |||
| NS42475-2492 | LWEGSPNKYWNSSTATSL | 180 | CD4 | |||
| NS42483-2502 | YWNSSTATSLCNIFRGSYLA | 263 | CD8/CD4 | CNIFRGSYL | Kb | 236 |
| NS42493-2512 | CNIFRGSYLAGASLIYTVTR | 77 | CD4 | |||
| NS52503-2520 | GASLIYTVTRNAGLVKRR | 82 | NA | |||
| NS52519-2536 | RRGGGTGETLGEKWKARL | 286 | NA | |||
| NS52527-2545 | TLGEKWKARLNQMSALEFY | 108 | NA | |||
| NS52546-2525 | SYKKSGITEVCREEARRALK | 96 | NA | |||
| NS52566-2585 | DGVATGGHAVSRGSAKLRWL | 98 | NA | |||
| NS52605-2623 | GGWSYYAATIRKVQEVKGY | 76 | CD8 | WSYYAATI | Kb | 308 |
| NS52667-2685 | IGESSSSPEVEEARTLRVL | 81 | CD8/CD4 | EVEEARTL | Db | 168 |
| NS52722-2741 | YGGGLVRVPLSRNSTHEMYW | 40 | CD8 | |||
| NS52732-2751 | SRNSTHEMYWVSGAKSNTIK | 96 | CD8/CD4 | |||
| NS52823-2842 | TWAYHGSYEAPTQGSASSLI | 338 | CD8/CD4 | |||
| NS52833-2851 | PTQGSASSLINGVVRLLSK | 654 | CD8 | SSLINGVVRL | Db | 382 |
| NS52842-2859 | INGVVRLLSKPWDVVTGV | 58 | CD8/CD4 | |||
| NS52850-2868 | SKPWDVVTGVTGIAMTDTT | 84 | CD4 | |||
| NS52955-2973 | LVDKEREHHLRGECQSCVY | 142 | CD8 | |||
| NS52991-3010 | GSRAIWYMWLGARFLEFEAL | 152 | CD8 | RAIWYMWL | Kb | |
| GSRAIWYM | Db | |||||
| NS53064-3083 | SRFDLENEALITNQMEKGHR | 88 | NA | |||
| NS53093-3112 | TYQNKVVKVLRPAEKGKTVM | 88 | CD4 | |||
| NS53216-3235 | WKPSTGWDNWEEVPFCSHHF | 54 | CD4 | TGWDNWEEV | Db | 40 |
| NS53311-3330 | PTGRTTWSIHGKGEWMTTED | 142 | CD8/CD4 |
| Name | Peptides Sequence | SCFs/106 | CD4/CD8 | Epitopes Sequence | MHC | SCFs/106 |
|---|---|---|---|---|---|---|
| C1-19 | MKNPKKKSGGFRIVNMLKR | 68 | CD4 | |||
| C10-27 | GFRIVNMLKRGVARVSPF | 138 | NA | |||
| E283-302 | LLIAPAYSIRCIGVSNRDFV | 104 | NA | AYSIRCIGV | Kd | 124 |
| E293-311 | CIGVSNRDFVEGMSGGTWV | 64 | NA | |||
| E340-359 | VSNMAEVRSYCYEASISDMA | 72 | CD4 | SYCYEASI | Kd | 524 |
| CYEASISDM | Kd | 108 | ||||
| E350-369 | CYEASISDMASDSRCPTQGE | 104 | CD8 | |||
| E380-398 | YVCKRTLVDRGWGNGCGLF | 46 | NA | |||
| E429-448 | IMLSVHGSQHSGMIVNDTGH | 208 | CD4/CD8 | |||
| E477-496 | GLDCEPRTGLDFSDLYYLTM | 122 | NA | |||
| E496-515 | MNNKHWLVHKEWFHDIPLPW | 44 | NA | |||
| E584-600 | KMDKLRLKGVSYSLCTAAF | 40 | NA | SYSLCTAA | Kd | 84 |
| E640-659 | QTLTPVGRLITANPVITEST | 72 | CD4 | |||
| E754-770 | SQILIGTLLMWLGLNTK | 96 | CD4 | |||
| NS1940-958 | KHRAWNSFLVEDHGFGVFH | 56 | CD4/CD8 | |||
| NS1996-1015 | IESEKNDTWRLKRAHLIEMK | 76 | CD4/CD8 | |||
| NS11006-1025 | LKRAHLIEMKTCEWPKSHTL | 52 | CD4 | |||
| NS11054-1071 | YRTQMKGPWHSEELEIRF | 1000 | CD8 | KGPWHSEEL | Dd | 376 |
| GYRTQMKGPW | Kd | 174 | ||||
| NS21239-1256 | FIFRANWTPRESMLLALA | 72 | CD4 | |||
| NS21247-1266 | PRESMLLALASCLLQTAISA | 64 | CD8 | |||
| NS21342-1360 | PFVMALGLTAVRLVDPINVV | 60 | CD4/CD8 | |||
| NS21371-1390 | KRSWPPSEVLTAVGLICALA | 108 | CD4 | |||
| NS21410-1428 | LIVSYVVSGKSVDMYIERA | 176 | CD4 | |||
| NS21457-1476 | SLVEDDGPPMREIILKVVLM | 60 | CD4 | |||
| NS21477-1496 | TICGMNPIAIPFAAGAWYVY | 184 | CD4 | |||
| NS31544-1561 | QEGVFHTMWHVTKGSALR | 52 | CD4 | |||
| NS31602-1621 | VPPGERARNIQTLPGIFKTK | 79 | NA | |||
| NS31640-1659 | PILDKCGRVIGLYGNGVVIK | 49 | NA | LYGNGVVI | Kd | 80 |
| NS31791-1809 | FTDPSSIAARGYISTRVEM | 372 | CD4/CD8 | GYISTRVEM | Kd | 174 |
| NS31800-1819 | RGYISTRVEMGEAAAIFMTA | 240 | CD8 | |||
| NS32002-2020 | QDGLIASLYRPEADKVAAI | 208 | CD8 | LYRPEADKV | Kd | 158 |
| NS42112-2129 | KEFAAGKRGAAFGVMEAL | 153 | NA | |||
| NS42120-2139 | GAAFGVMEALGTLPGHMTER | 168 | CD4/CD8 | |||
| NS42304-2323 | RPASAWAIYAALTTFITPAV | 360 | CD4/CD8 | IYAALTTFI | Kd | 128 |
| NS42349-2367 | MGKGMPFYAWDFGVPLLMI | 924 | CD8 | KGMPFYAWDF | Dd | 564 |
| FYAWDFGVPLL | Kd | 230 | ||||
| NS42387-2406 | AHYMYLIPGLQAAAARAAQK | 963 | CD4/CD8 | LIPGLQAAAARAAQK | H2-I | 168 |
| NS42405-2423 | QKRTAAGIMKNPVVDGIVV | 78 | NA | |||
| NS42475-2492 | LWEGSPNKYWNSSTATSL | 1178 | CD4/CD8 | GSPNKYWNSSTATSL | H2-I | 534 |
| NS52638-2657 | SYGWNIVRLKSGVDVFHMAA | 1000 | CD4/CD8 | SYGWNIVRL | Kd | 66 |
| NS53272-3291 | ETACLAKSYAQMWQLLYFHR | 124 | CD4/CD8 | SYAQMWQLL | Kd | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xiao, W.; Zhao, M.; Zhao, Y.; Zhang, Y.; Lu, D.; Lu, S.; Zhang, Q.; Peng, W.; Shu, L.; et al. The CD8+ and CD4+ T Cell Immunogen Atlas of Zika Virus Reveals E, NS1 and NS4 Proteins as the Vaccine Targets. Viruses 2022, 14, 2332. https://doi.org/10.3390/v14112332
Zhang H, Xiao W, Zhao M, Zhao Y, Zhang Y, Lu D, Lu S, Zhang Q, Peng W, Shu L, et al. The CD8+ and CD4+ T Cell Immunogen Atlas of Zika Virus Reveals E, NS1 and NS4 Proteins as the Vaccine Targets. Viruses. 2022; 14(11):2332. https://doi.org/10.3390/v14112332
Chicago/Turabian StyleZhang, Hangjie, Wenling Xiao, Min Zhao, Yingze Zhao, Yongli Zhang, Dan Lu, Shuangshuang Lu, Qingxu Zhang, Weiyu Peng, Liumei Shu, and et al. 2022. "The CD8+ and CD4+ T Cell Immunogen Atlas of Zika Virus Reveals E, NS1 and NS4 Proteins as the Vaccine Targets" Viruses 14, no. 11: 2332. https://doi.org/10.3390/v14112332
APA StyleZhang, H., Xiao, W., Zhao, M., Zhao, Y., Zhang, Y., Lu, D., Lu, S., Zhang, Q., Peng, W., Shu, L., Zhang, J., Liu, S., Zong, K., Wang, P., Ye, B., Li, S., Tan, S., Zhang, F., Zhou, J., ... Liu, W. J. (2022). The CD8+ and CD4+ T Cell Immunogen Atlas of Zika Virus Reveals E, NS1 and NS4 Proteins as the Vaccine Targets. Viruses, 14(11), 2332. https://doi.org/10.3390/v14112332






