Tick-Borne Encephalitis Virus Prevalence in Sheep, Wild Boar and Ticks in Belgium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. Sheep Sera
2.1.2. Wild Boar Sera
2.2. Ethics Statement
2.3. Serological Analysis
2.4. Tick Collection
2.5. Tick Pooling and Homogenization, RNA Extraction, and qPCR Analysis
2.6. Statistical Analysis and Mapping
3. Results
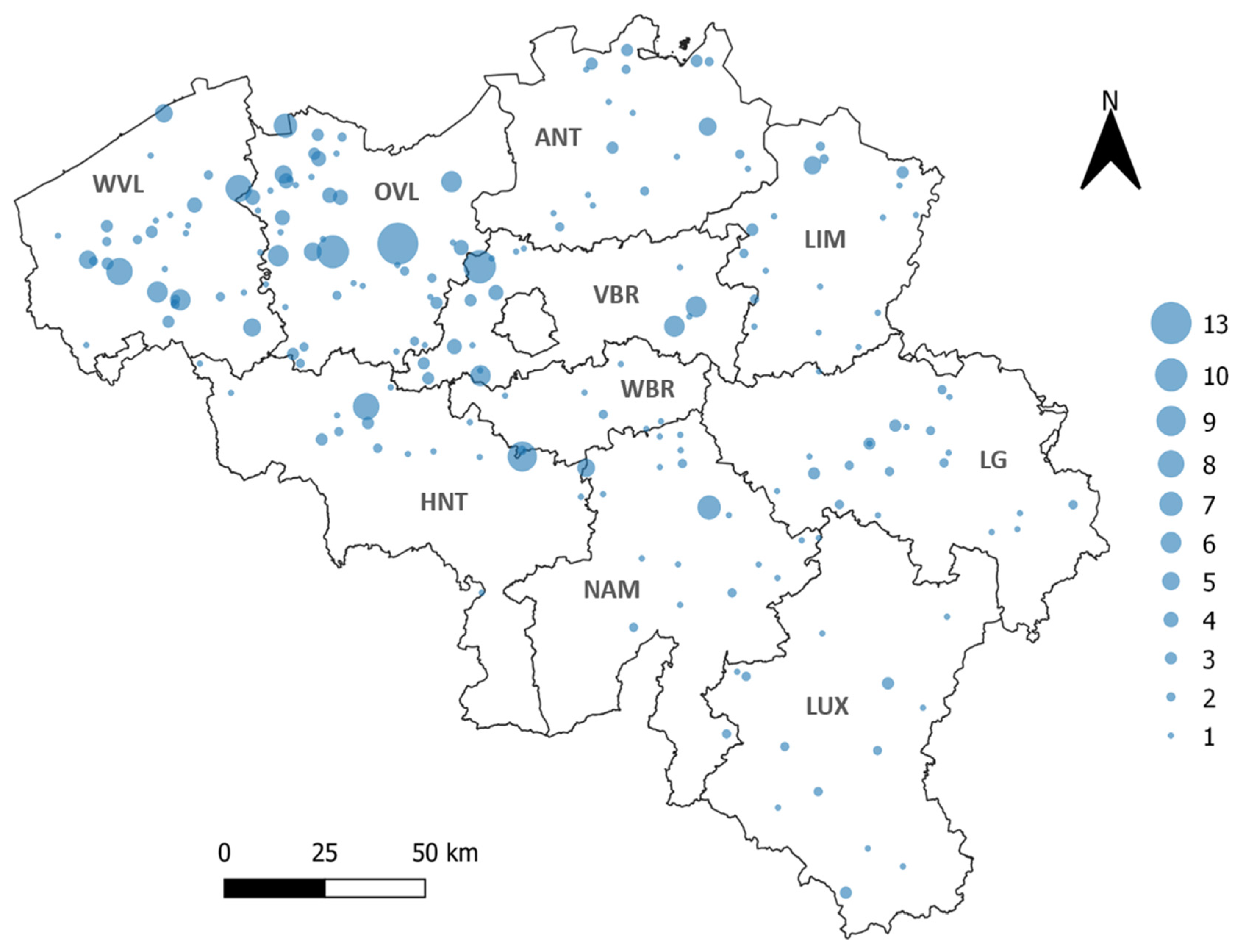
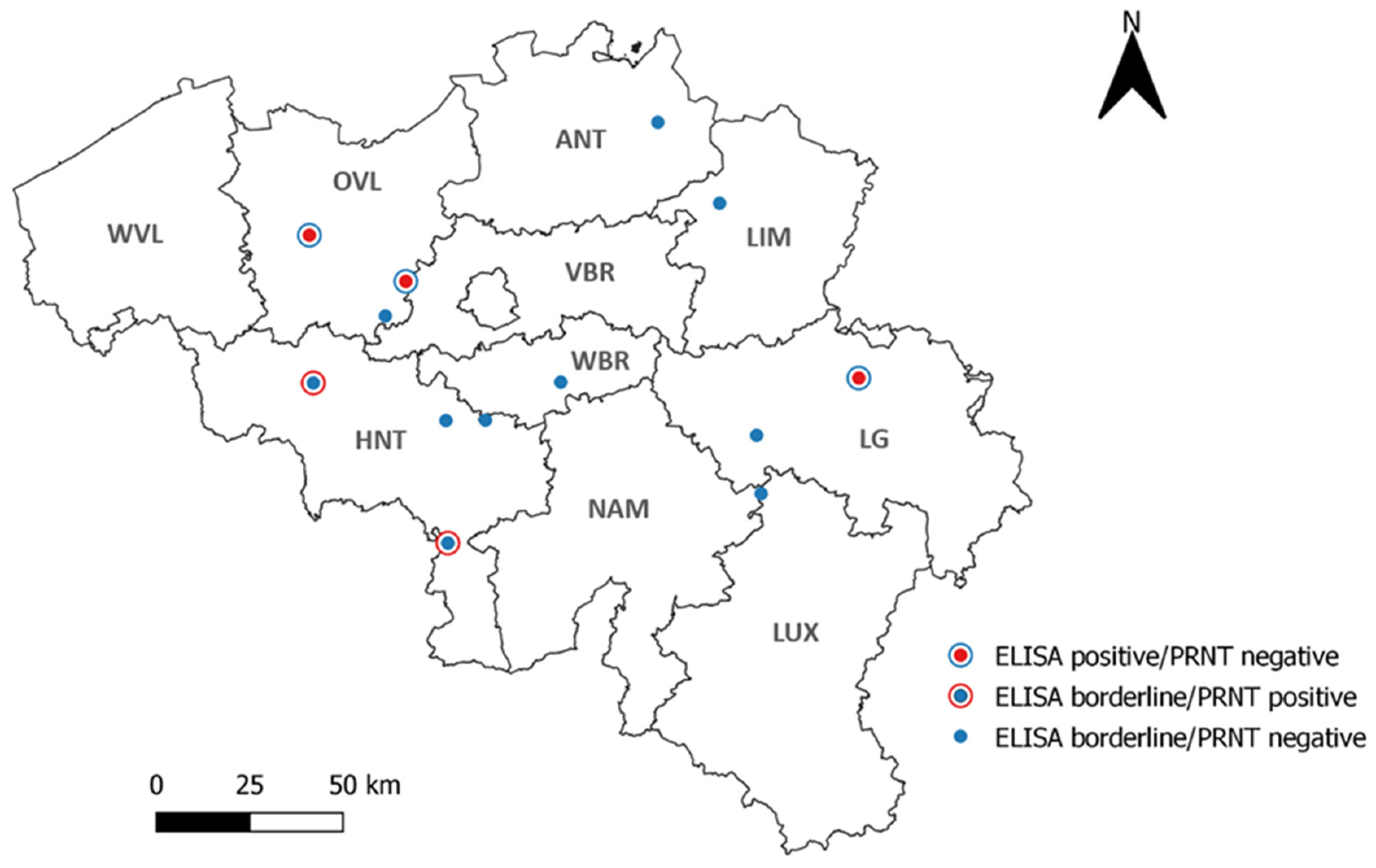
3.1. TBEV-Specific Antibody Detection in Sheep
3.2. TBEV-Specific Antibody Detection in Wild Boar
3.3. TBEV Detection in Ticks
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kollaritsch, H.; Chmelík, V.; Dontsenko, I.; Grzeszczuk, A.; Kondrusik, M.; Usonis, V.; Lakos, A. The Current Perspective on Tick-Borne Encephalitis Awareness and Prevention in Six Central and Eastern European Countries: Report from a Meeting of Experts Convened to Discuss TBE in Their Region. Vaccine 2011, 29, 4556–4564. [Google Scholar] [CrossRef] [PubMed]
- Amicizia, D.; Domnich, A.; Panatto, D.; Lai, P.L.; Cristina, M.L.; Avio, U.; Gasparini, R. Epidemiology of Tick-Borne Encephalitis (TBE) in Europe and Its Prevention by Available Vaccines. Hum. Vaccines Immunother. 2013, 9, 1163–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieille, N.; Klaus, C.; Hoffmann, D.; Péter, O.; Voordouw, M.J. Goats as Sentinel Hosts for the Detection of Tick-Borne Encephalitis Risk Areas in the Canton of Valais, Switzerland. BMC Vet. Res. 2017, 13, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Shang, G.; Lu, S.; Yang, J.; Xu, J. A New Subtype of Eastern Tick-Borne Encephalitis Virus Discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Kozlova, I.V.; Demina, T.V.; Tkachev, S.E.; Doroshchenko, E.K.; Lisak, O.V.; Verkhozina, M.M.; Karan, L.S.; Dzhioev, Y.P.; Paramonov, A.I.; Suntsova, O.V.; et al. Characteristics of the Baikal Subtype of Tick-Borne Encephalitis Virus Circulating in Eastern Siberia. Acta Biomed. Sci. 2018, 3, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses 2019, 11, 669. [Google Scholar] [CrossRef] [Green Version]
- Tonteri, E.; Kipar, A.; Voutilainen, L.; Vene, S.; Vaheri, A.; Vapalahti, O.; Lundkvist, Å. The Three Subtypes of Tick-Borne Encephalitis Virus Induce Encephalitis in a Natural Host, the Bank Vole (Myodes glareolus). PLoS ONE 2013, 8, 15–19. [Google Scholar] [CrossRef]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-Borne Encephalitis in Europe and Russia: Review of Pathogenesis, Clinical Features, Therapy, and Vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef]
- Grešíková, M.; Sekeyová, M.; Stúpalová, S.; Necas, S. Sheep Milk-Borne Epidemic of Tick-Borne Encephalitis in Slovakia. Intervirology 1975, 5, 57–61. [Google Scholar] [CrossRef]
- Caini, S.; Szomor, K.; Ferenczi, E.; Székelyné Gáspár, A.; Csohán, A.; Krisztalovics, K.; Molnár, Z.; Horváth, J.K. Tick-Borne Encephalitis Transmitted by Unpasteurised Cow Milk in Western Hungary, September to October 2011. Eurosurveillance 2012, 17, 20128. [Google Scholar] [CrossRef]
- Kohl, I.; Kozuch, O.; Eleckova, E.; Labuda, M.; Zaludko, J. Family Outbreak of Alimentary Tick-Borne Encephalitis in Slovakia Associated with a Natural Focus of Infection. Eur. J. Epidemiol. 1996, 12, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, H.; Aberle, S.W.; Stiasny, K.; Werner, P.; Mischak, A.; Zainer, B.; Netzer, M.; Koppi, S.; Bechter, E.; Heinz, F.X. Tick-Borne Encephalitis from Eating Goat Cheese in a Mountain Region of Austria. Emerg. Infect. Dis. 2009, 15, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.; Ferenczi, E.; Szeles, K.; Stefanoff, P.; Gut, W.; Szomor, K.N.; Takacs, M.; Berencsi, G. Tick-Borne Encephalitis Outbreak in Hungary Due to Consumption of Raw Goat Milk. J. Virol. Methods 2010, 163, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Ličková, M.; Fumačová Havlíková, S.; Sláviková, M.; Klempa, B. Alimentary Infections by Tick-Borne Encephalitis Virus. Viruses 2022, 14, 56. [Google Scholar] [CrossRef]
- Ilic, M.; Barbic, L.; Bogdanic, M.; Tabain, I.; Savic, V.; Kosanovic Licina, M.L.; Kaic, B.; Jungic, A.; Vucelja, M.; Angelov, V.; et al. Tick-Borne Encephalitis Outbreak Following Raw Goat Milk Consumption in a New Micro-Location, Croatia, June 2019. Ticks Tick. Borne. Dis. 2020, 11, 101513. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Bournez, L.; Moraes, R.A.; Marine, D.; Galon, C.; Vorimore, F.; Cochin, M.; Nougairède, A.; Hennechart-Collette, C.; Perelle, S.; et al. A One-Health Approach to Investigating an Outbreak of Alimentary Tick-Borne Encephalitis in a Non-Endemic Area in France (Ain, Eastern France): A Longitudinal Serological Study in Livestock, Detection in Ticks, and the First Tick-Borne Encephalitis Virus. Front. Microbiol. 2022, 13, 863725. [Google Scholar] [CrossRef] [PubMed]
- Labuda, M.; Jones, L.D.; Williams, T.; Danielova, V.; Nuttall, P.A. Efficient Transmission of Tick-Borne Encephalitis Virus Between Cofeeding Ticks. J. Med. Entomol. 1993, 30, 295–299. [Google Scholar] [CrossRef]
- Brinkley, C.; Nolskog, P.; Golovljova, I.; Bergstro, T. Tick-Borne Encephalitis Virus Natural Foci Emerge in Western Sweden. Int. J. Med. Microbiol. 2008, 298, 73–80. [Google Scholar] [CrossRef]
- Gaumann, R.; Muhlemann, K.; Strasser, M.; Beuret, C.M. High-Throughput Procedure for Tick Surveys of Tick-Borne Encephalitis Virus and Its Application in a National Surveillance Study in Switzerland. Appl. Environ. Microbiol. 2010, 76, 4241–4249. [Google Scholar] [CrossRef] [Green Version]
- Kupča, A.M.; Essbauer, S.; Zoeller, G.; de Mendonça, P.G.; Brey, R.; Rinder, M.; Pfister, K.; Spiegel, M.; Doerrbecker, B.; Pfeffer, M.; et al. Isolation and Molecular Characterization of a Tick-Borne Encephalitis Virus Strain from a New Tick-Borne Encephalitis Focus with Severe Cases in Bavaria, Germany. Ticks Tick. Borne. Dis. 2010, 1, 44–51. [Google Scholar] [CrossRef]
- Patel, R.; Singh, R.; Gupta, B.; Rai, A.; Dubey, S.; Singh Dhakad, B.M.; Divya Soni, D. Tick Borne Viral Zoonotic Diseases: A Review. J. Entomol. Zool. Stud. 2020, 8, 2034–2042. [Google Scholar]
- European Center for Disease Prvention and Control. Tick-Borne Encephalitis. In Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/tick-borne-encephalitis-annual-epidemiological-report-2019 (accessed on 19 October 2022).
- Kaiser, R. Tick-Borne Encephalitis. Infect. Dis. Clin. N. Am. 2008, 22, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P.; Lotric-furlan, S.; Strle, F. What Tick-Borne Encephalitis May Look like: Clinical Signs and Symptoms. Travel Med. Infect. Dis. 2010, 8, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.; Vapalhati, O. Tick-Borne Encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Host Immunogenetics in Tick-Borne Encephalitis Virus Infection—The CCR5 Crossroad. Ticks Tick Borne Dis. 2019, 10, 729–741. [Google Scholar] [CrossRef]
- Böhm, B.; Schade, B.; Bauer, B.; Hoffmann, B.; Hoffmann, D.; Ziegler, U.; Beer, M.; Klaus, C.; Weissenböck, H.; Böttcher, J. Tick-Borne Encephalitis in a Naturally Infected Sheep. BMC Vet. Res. 2017, 13, 267. [Google Scholar] [CrossRef] [Green Version]
- Süss, J.; Dobler, G.; Zoller, G.; Essbauer, S.; Pfeffer, M.; Klaus, C.; Liebler-Tenorio, E.M.; Gelpi, E.; Stark, B.; Hotzel, H. Genetic Characterisation of a Tick-Borne Encephalitis Virus Isolated from the Brain of a Naturally Exposed Monkey (Macaca sylvanus). Int. J. Med. Microbiol. 2008, 298, 295–300. [Google Scholar] [CrossRef]
- Weissenböck, H.; Suchy, A.; Holzmann, H. Tick-Borne Encephalitis in Dogs: Neuropathological Findings and Distribution of Antigen. Acta Neuropathol. 1998, 95, 361–366. [Google Scholar] [CrossRef]
- Klaus, C.; Beer, M.; Saier, R.; Schau, U.; Moog, U.; Hoffmann, B.; Diller, R.; Süss, J. Goats and Sheep as Sentinels for Tick-Borne Encephalitis (TBE) Virus—Epidemiological Studies in Areas Endemic and Non-Endemic for TBE Virus in Germany. Ticks Tick. Borne. Dis. 2012, 3, 27–37. [Google Scholar] [CrossRef]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe—A Review on the Prevalence in Domestic Animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef]
- Casati Pagani, S.; Frigerio Malossa, S.; Klaus, C.; Hoffmann, D.; Beretta, O.; Bomio-pacciorini, N.; Lazzaro, M.; Merlani, G.; Ackermann, R.; Beuret, C. Ticks and Tick-Borne Diseases First Detection of TBE Virus in Ticks and Sero-Reactivity in Goats in a Non-Endemic Region in the Southern Part of Switzerland (Canton of Ticino). Ticks Tick Borne Dis. 2019, 10, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Frimmel, S.; Löbermann, M.; Feldhusen, F.; Seelmann, M.; Stiasny, K.; Süss, J.; Reisinger, E.C. Detection of Tick-Borne Encephalitis Virus Antibodies in Sera of Sheep and Goats in Mecklenburg-Western Pomerania (North-Eastern Germany). Ticks Tick Borne Dis. 2019, 10, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Hoffmann, B.; Moog, U.; Schau, U.; Beer, M.; Süss, J. Can Goats Be Used as Sentinels for Tick-Borne Encephalitis (TBE) in Non- Endemic Areas? Experimental Studies and Epizootiological Observations. Berl. Munch. Tierarztl. Wochenschr. 2010, 12, 441–445. [Google Scholar] [CrossRef]
- Bournez, L.; Umhang, G.; Faure, E.; Boucher, J.M.; Boué, F.; Jourdain, E.; Sarasa, M.; Llorente, F.; Jiménez-Clavero, M.A.; Moutailler, S.; et al. Exposure of Wild Ungulates to the Usutu and Tick-Borne Encephalitis Viruses in France in 2009–2014: Evidence of Undetected Flavivirus Circulation a Decade Ago. Viruses 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwaiger, M.; Cassinotti, P. Development of a Quantitative Real-Time RT-PCR Assay with Internal Control for the Laboratory Detection of Tick Borne Encephalitis Virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef]
- Roelandt, S.; Heyman, P.; De Filette, M.; Vene, S.; Van Der Stede, Y.; Caij, A.B.; Tavernier, P.; Dobly, A.; De Bosschere, H.; Vyt, P.; et al. Tick-Borne Encephalitis Virus Seropositive Dog Detected in Belgium: Screening of the Canine Population as Sentinels for Public Health. Vector-Borne Zoonotic Dis. 2011, 11, 1371–1376. [Google Scholar] [CrossRef]
- Roelandt, S.; Suin, V.; Van der Stede, Y.; Lamoral, S.; Marche, S.; Tignon, M.; Saiz, J.C.; Escribano-Romero, E.; Casaer, J.; Brochier, B.; et al. First TBEV Serological Screening in Flemish Wild Boar. Infect. Ecol. Epidemiol. 2016, 6, 4–7. [Google Scholar] [CrossRef]
- Roelandt, S.; Suin, V.; Riocreux, F.; Lamoral, S.; Van Der Heyden, S.; Van Der Stede, Y.; Lambrecht, B.; Caij, B.; Brochier, B.; Roels, S.; et al. Autochthonous Tick-Borne Encephalitis Virus-Seropositive Cattle in Belgium: A Risk-Based Targeted Serological Survey. Vector-Borne Zoonotic Dis. 2014, 14, 640–647. [Google Scholar] [CrossRef]
- Tavernier, P.; Sys, S.U.; De Clercq, K.; De Leeuw, I.; Caij, A.B.; De Baere, M.; De Regge, N.; Fretin, D.; Roupie, V.; Govaerts, M.; et al. Serologic Screening for 13 Infectious Agents in Roe Deer (Capreolus capreolus) in Flanders. Infect. Ecol. Epidemiol. 2015, 5, 29862. [Google Scholar] [CrossRef]
- Roelandt, S.; Suin, V.; Van Gucht, S.; Van Der Stede, Y.; Roels, S. Comparative Tick-Borne Encephalitis (Virus) Surveillance in Belgium 2009-2015: Experiences with Diagnostic Tests, Sentinel Species and Surveillance Designs. J. Zoonotic Dis. Public Health 2017, 1, 4. [Google Scholar]
- Stoefs, A.; Heyndrickx, L.; de Winter, J.; Coeckelbergh, E.; Willekens, B.; Alonso-Jiménez, A.; Tuttino, A.M.; Geerts, Y.; Ariën, K.K.; van Esbroeck, M. Autochthonous Cases of Tick-Borne Encephalitis, Belgium, 2020. Emerg. Infect. Dis. 2021, 27, 217–2182. [Google Scholar] [CrossRef] [PubMed]
- Michiels, R.; Van Mael, E.; Quinet, C.; Welby, S.; Cay, A.B.; De Regge, N. Seroprevalence and Risk Factors Related to Small Ruminant Lentivirus Infections in Belgian Sheep and Goats. Prev. Vet. Med. 2018, 151, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rutten, A.; Cox, K.; Scheppers, T.; Vanden Broecke, B.; Leirs, H.; Casaer, J. Analysing the Recolonisation of a Highly Fragmented Landscape by Wild Boar Using a Landscape Genetic Approach. Wildl. Biol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Holzmann, H.; Kundi, M.; Stiasny, K.; Clement, J.; McKenna, P.; Kunz, C.; Heinz, F.X. Correlation between ELISA, Hemagglutination Inhibition, and Neutralization Tests after Vaccination against Tick-Borne Encephalitis. J. Med. Virol. 1996, 48, 102–107. [Google Scholar] [CrossRef]
- Kollaritsch, H.; Paulke-korinek, M.; Holzmann, H.; Hombach, J.; Bjorvatn, B.; Barrett, A. Vaccines and Vaccination against Tick-Borne Encephalitis. Expert Rev. Vaccines 2012, 11, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Mel, R.; Marchi, A.; Mancuso, S.; Russino, F.; Da, G.; Papa, N.; Bertiato, G.; Fiorentini, C.; Grazia Ciufolini, M. Humoral Immunity and Correlation between ELISA, Hemagglutination Inhibition, and Neutralization Tests after Vaccination against Tick-Borne Encephalitis Virus in Children. J. Virol. Methods 2006, 134, 136–139. [Google Scholar] [CrossRef]
- Weissbach, F.H.; Hirsch, H. Comparison of Two Commercial Tick-Borne Encephalitis Virus IgG Enzyme-Linked Immunosorbent Assays. Clin. Vaccine Immunol. 2015, 22, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Van Der Stede, Y.; Cox, E.; Verdonck, F.; Vancaeneghem, S.; Goddeeris, B.M. Reduced Faecal Excretion of F4 + -E. coli by the Intramuscular Immunisation of Suckling Piglets by the Addition Of 1a,25-dihydroxyvitamin D3 or CpG-oligodeoxynucleotides. Vaccine 2003, 21, 1023–1032. [Google Scholar] [CrossRef]
- Vandekerkhove, K. Integration of Nature Protection in Forest Policy in Flanders (Belgium): INTEGRATE Country Report; EFICENT-OEF: Freiburg, Switzerland, 2013; pp. 1–65. [Google Scholar]
- Hillyard, P.D. Ticks of North-West Europe; Backhyus Publishers: London, UK, 1996. [Google Scholar]
- Deviatkin, A.A.; Kholodilov, I.S.; Vakulenko, Y.A.; Karganova, G.G.; Lukashev, A.N. Tick-Borne Encephalitis Virus: An Emerging Ancient Zoonosis? Viruses 2020, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Holding, M.; Dowall, S.D.; Medlock, J.M.; Carter, D.P.; Pullan, S.T.; Lewis, J.; Vipond, R.; Rocchi, M.S.; Baylis, M.; Hewson, R. Tick-Borne Encephalitis Virus, United Kingdom. Emerg. Infect. Dis. 2020, 26, 90–96. [Google Scholar] [CrossRef]
- Agergaard, C.N.; Rosenstierne, M.W.; Bødker, R.; Rasmussen, M.; Andersen, P.H.S.; Fomsgaard, A. New Tick-Borne Encephalitis Virus Hot Spot in Northern Zealand, Denmark, October 2019. Eurosurveillance 2019, 24, 1900639. [Google Scholar] [CrossRef] [PubMed]
- Kreusch, T.M.; Holding, M.; Hewson, R.; Harder, T.; Medlock, J.M.; Hansford, K.M.; Dowall, S.; Semper, A.; Brooks, T.; Walsh, A.; et al. A Probable Case of Tick-Borne Encephalitis (TBE) Acquired in England, July 2019. Eurosurveillance 2019, 24, 1900679. [Google Scholar] [CrossRef] [PubMed]
- Süss, J. Tick-Borne Encephalitis 2010: Epidemiology, Risk Areas, and Virus Strains in Europe and Asia-An Overview. Ticks Tick Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Hoffmann, B.; Beer, M.; Müller, W.; Stark, B.; Bader, W.; Stiasny, K.; Heinz, F.X.; Süss, J. Seroprevalence of Tick-Borne Encephalitis (TBE) in Naturally Exposed Monkeys (Macaca sylvanus) and Sheep and Prevalence of TBE Virus in Ticks in a TBE Endemic Area in Germany. Ticks Tick Borne Dis. 2010, 1, 141–144. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Epidemiological Situation of Tick-Borne Encephalitis in the European Union and European Free Trade Association Countries; ECDC: Stockholm, Sweden, 2012; Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/TBE-in-EU-EFTA.pdf (accessed on 28 September 2022).
- Carpi, G.; Cagnacci, F.; Neteler, M.; Rizzoli, A. Tick Infestation on Roe Deer in Relation to Geographic and Remotely Sensed Climatic Variables in a Tick-Borne Encephalitis Endemic Area. Epidemiol. Infect. 2008, 136, 1416–1424. [Google Scholar] [CrossRef]
- Gerth, H.J.; Grimshandl, D.; Stage, B.; Döller, G.; Kunz, C. Roe Deer as Sentinels for Endemicity of Tick-Borne Encephalitis Virus. Epidemiol. Infect. 1995, 115, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Haemig, P.D.; Sjöstedt De Luna, S.; Grafström, A.; Lithner, S.; Lundkvist, Å.; Waldenström, J.; Kindberg, J.; Stedt, J.; Olsén, B. Forecasting Risk of Tick-Borne Encephalitis (TBE): Using Data from Wildlife and Climate to Predict next Year’s Number of Human Victims. Scand. J. Infect. Dis. 2011, 43, 366–372. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Špitalská, E.; et al. Ixodes Ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Palo, R.T. Tick-Borne Encephalitis Transmission Risk: Its Dependence on Host Population Dynamics and Climate Effects. Vector-Borne Zoonotic Dis. 2014, 14, 346–352. [Google Scholar] [CrossRef]
- Klaus, C.; Ziegler, U.; Hoffmann, D.; Press, F.; Fast, C.; Beer, M. Tick-Borne Encephalitis Virus (TBEV) Antibodies in Animal Sera—Occurrence in Goat Flocks in Germany, Longevity and Ability to Recall Immunological Information after More than Six Years. BMC Vet. Res. 2019, 15, 399. [Google Scholar] [CrossRef]
- Khamassi Khbou, M.; Romdhane, R.; Foughali, A.A.; Sassi, L.; Suin, V.; Rekik, M.; Benzarti, M. Presence of Antibodies against Tick-Borne Encephalitis Virus in Sheep in Tunisia, North Africa. BMC Vet. Res. 2020, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Salat, J.; Strakova, P.; Stefanik, M.; Slosarkova, S.; Ruzek, D. Sero-Epidemiology of Tick-Borne Encephalitis in Small Ruminants in the Czech Republic. Ticks Tick Borne Dis. 2022, 13, 101996. [Google Scholar] [CrossRef] [PubMed]
- Salat, J.; Mihalca, A.D.; Mihaiu, M.; Modrý, D.; Ruzek, D. Tick-Borne Encephalitis in Sheep, Romania. Emerg. Infect. Dis. 2017, 23, 2065–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Esbroeck, M.; Lernout, T.; Suin, V.; Van Gucht, S. TBE in Belgium. Chapter 12b; In The TBE Book, 5th ed.; Dobler, G., Erber, W., Bröker, M., Schmitt, H.J., Eds.; Global Health Press: Singapore, 2022. [Google Scholar] [CrossRef]
- Gómez Martínez, C. Role of Cervids and Wild Bor on the Presence of Tick-Borne Encephalitis Virus in Sweden. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2014. [Google Scholar]
- Cisak, E.; Wójcik-Fatla, A.; Sroka, J.; Zajac, V.; Bilska-Zajc, E.; Chmurzyńska, E.; Dutkiewicz, J. Prevalence of Tick-Borne Encephalitis Virus Antibodies in Domestic and Game Animals from Eastern Poland. Bull. Vet. Inst. Pulawy 2012, 56, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Garigliany, M.; Linden, A.; Gilliau, G.; Levy, E.; Sarlet, M.; Franssen, M.; Benzarti, E.; Derouaux, A.; Francis, F.; Desmecht, D. Usutu Virus, Belgium, 2016. Infect. Genet. Evol. 2017, 48, 116–119. [Google Scholar] [CrossRef]
- Rouffaer, L.O.; Steensels, M.; Verlinden, M.; Vervaeke, M.; Boonyarittichaikij, R.; Martel, A.; Lambrecht, B. Usutu Virus Epizootic and Plasmodium Coinfection in Eurasian Blackbirds (Turdus merula) in Flanders, Belgium. J. Wildl. Dis. 2018, 54, 859–862. [Google Scholar] [CrossRef]
- Topp, A.K.; Springer, A.; Dobler, G.; Bestehorn-Willmann, M.; Monazahian, M.; Strube, C. New and Confirmed Foci of Tick-Borne Encephalitis Virus (TBEV) in Northern Germany Determined by TBEV Detection in Ticks. Pathogens 2022, 11, 126. [Google Scholar] [CrossRef]
- Klemola, T.; Sormunen, J.J.; Mojzer, J.; Mäkelä, S.; Vesterinen, E.J. High Tick Abundance and Diversity of Tick-Borne Pathogens in a Finnish City. Urban Ecosyst. 2019, 22, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Rijks, J.M.; Montizaan, M.G.E.; Bakker, N.; de Vries, A.; Van Gucht, S.; Swaan, C.; van den Broek, J.; Gröne, A.; Sprong, H. Tick-Borne Encephalitis Virus Antibodies in Roe Deer, the Netherlands. Emerg. Infect. Dis. 2019, 25, 342–345. [Google Scholar] [CrossRef]
- Dekker, M.; Laverman, G.D.; de Vries, A.; Reimerink, J.; Geeraedts, F. Emergence of Tick-Borne Encephalitis (TBE) in the Netherlands. Ticks Tick. Borne. Dis. 2019, 10, 176–179. [Google Scholar] [CrossRef]
- Tack, W.; Madder, M.; Baeten, L.; De Frenne, P.; Verheyen, K. The Abundance of Ixodes Ricinus Ticks Depends on Tree Species Composition and Shrub Cover. Parasitology 2012, 139, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Tack, J. Wild Boar (Sus scrofa) Populations in Europe: A Scientific Review of Population Trends and Implications for Management; European Landowners’ Organization: Brussels, Switzerland, 2018; pp. 1–56. Available online: https://www.europeanlandowners.org/images/Wild_Boar_Report_2018/122193_WILD_BOAR_GB.pdf (accessed on 28 September 2022).
- Lernout, T.; De Regge, N.; Tersago, K.; Fonville, M.; Suin, V.; Sprong, H. Prevalence of Pathogens in Ticks Collected from Humans through Citizen Science in Belgium. Parasit. Vectors 2019, 12, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhoff, M.; Hagedorn, P.; Schulze, Y.; Hellenbrand, W.; Pfeffer, M.; Niedrig, M. Review: Sentinels of Tick-Borne Encephalitis Risk. Ticks Tick. Borne. Dis. 2015, 6, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.; Ulrich, K.; Ginsbach, P.; Öhme, R.; Hensley, O.B.; Falk, U.; Teinert, M.; Lenhard, T. Tick—Borne Encephalitis Virus (TBEV) Prevalence in Field—Collected Ticks (Ixodes ricinus) and Phylogenetic, Structural and Virulence Analysis in a TBE High—Risk Endemic Area in Southwestern Germany. Parasit. Vectors 2020, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Alfano, N.; Tagliapietra, V.; Rosso, F.; Ziegler, U.; Arnoldi, D.; Rizzoli, A. Tick-Borne Encephalitis Foci in Northeast Italy Revealed by Combined Virus Detection in Ticks, Serosurvey on Goats and Human Cases. Emerg. Microbes Infect. 2020, 9, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Bournez, L.; Umhang, G.; Moinet, M.; Richomme, C.; Demerson, J.M.; Caillot, C.; Devillers, E.; Boucher, J.M.; Hansmann, Y.; Boué, F.; et al. Tick-Borne Encephalitis Virus: Seasonal and Annual Variation of Epidemiological Parameters Related to Nymph-to-Larva Transmission and Exposure of Small Mammals. Pathogens 2020, 9, 518. [Google Scholar] [CrossRef]


| Province | Selected Samples for the Estimation of TBEV Seroprevalence |
|---|---|
| Antwerp | 37 |
| Limburg | 31 |
| East Flanders | 126 |
| West Flanders | 90 |
| Flemish Brabant | 52 |
| Flanders | 336 |
| Walloon Brabant | 10 |
| Hainaut | 44 |
| Namur | 32 |
| Liege | 35 |
| Luxembourg | 23 |
| Wallonia | 144 |
| Belgium | 480 |
| Province | Sampled | TBEV–PRNT Positive | Prevalence (%) |
|---|---|---|---|
| Antwerp | 325 | 28 | 8.62 (CI 95%: 6.03–12.17) |
| Limburg | 439 | 34 | 7.74 (CI 95%: 5.59–10.63) |
| Flemish Brabant | 67 | 15 | 22.39 (CI 95%: 14.06–33.71) |
| Flanders | 831 | 77 | 9.27 (CI 95%: 7.48–11.43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjadj, N.R.; Vervaeke, M.; Sohier, C.; Cargnel, M.; De Regge, N. Tick-Borne Encephalitis Virus Prevalence in Sheep, Wild Boar and Ticks in Belgium. Viruses 2022, 14, 2362. https://doi.org/10.3390/v14112362
Adjadj NR, Vervaeke M, Sohier C, Cargnel M, De Regge N. Tick-Borne Encephalitis Virus Prevalence in Sheep, Wild Boar and Ticks in Belgium. Viruses. 2022; 14(11):2362. https://doi.org/10.3390/v14112362
Chicago/Turabian StyleAdjadj, Nadjah Radia, Muriel Vervaeke, Charlotte Sohier, Mickaël Cargnel, and Nick De Regge. 2022. "Tick-Borne Encephalitis Virus Prevalence in Sheep, Wild Boar and Ticks in Belgium" Viruses 14, no. 11: 2362. https://doi.org/10.3390/v14112362






