Arrest of Cell Cycle by Avian Reovirus p17 through Its Interaction with Bub3
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Viruses
2.2. Antibodies and Reagents
2.3. Constructions of Recombinant Plasmids
2.4. RNA Extraction and RT-qPCR
2.5. Bub3 Knockdown by RNAi
2.6. Yeast Two-Hybrid Screen and Colony Filter Assay
2.7. Western Blot
2.8. Co-Immunoprecipitation (Co-IP) Assay
2.9. Confocal Laser Scanning Microscopy Assays
2.10. Cell-Cycle Analysis
2.11. Measurement of ARV Growth in Vero-E6 Cells
2.12. Statistical Analysis
3. Results
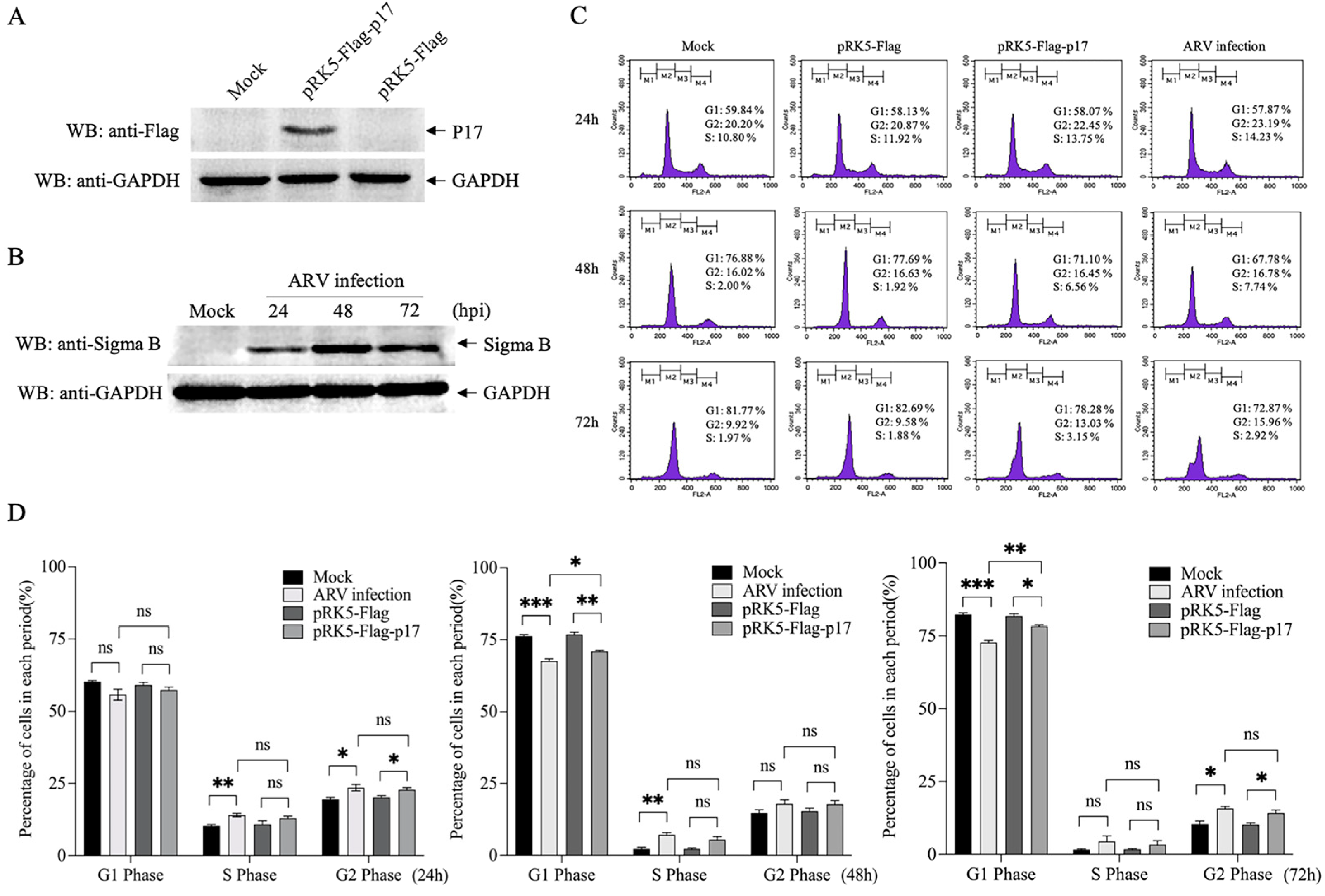
3.1. Ectopic Expression of ARV p17 Induces Cell-Cycle Arrest in Vero-E6 Cells
3.2. Identification of Cellular Proteins Interacting with p17
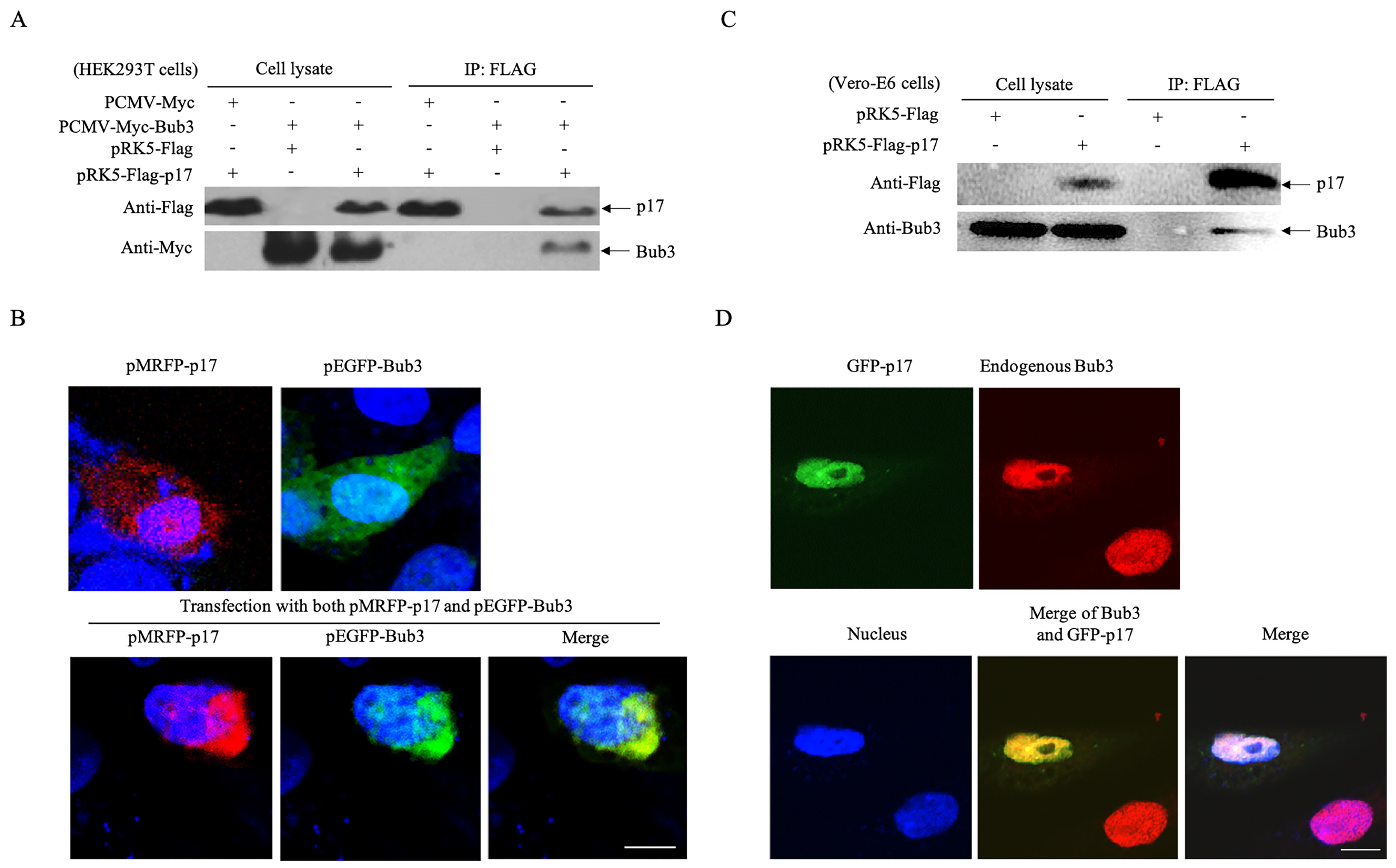
3.3. p17 Interacts with The Cellular Protein Bub3
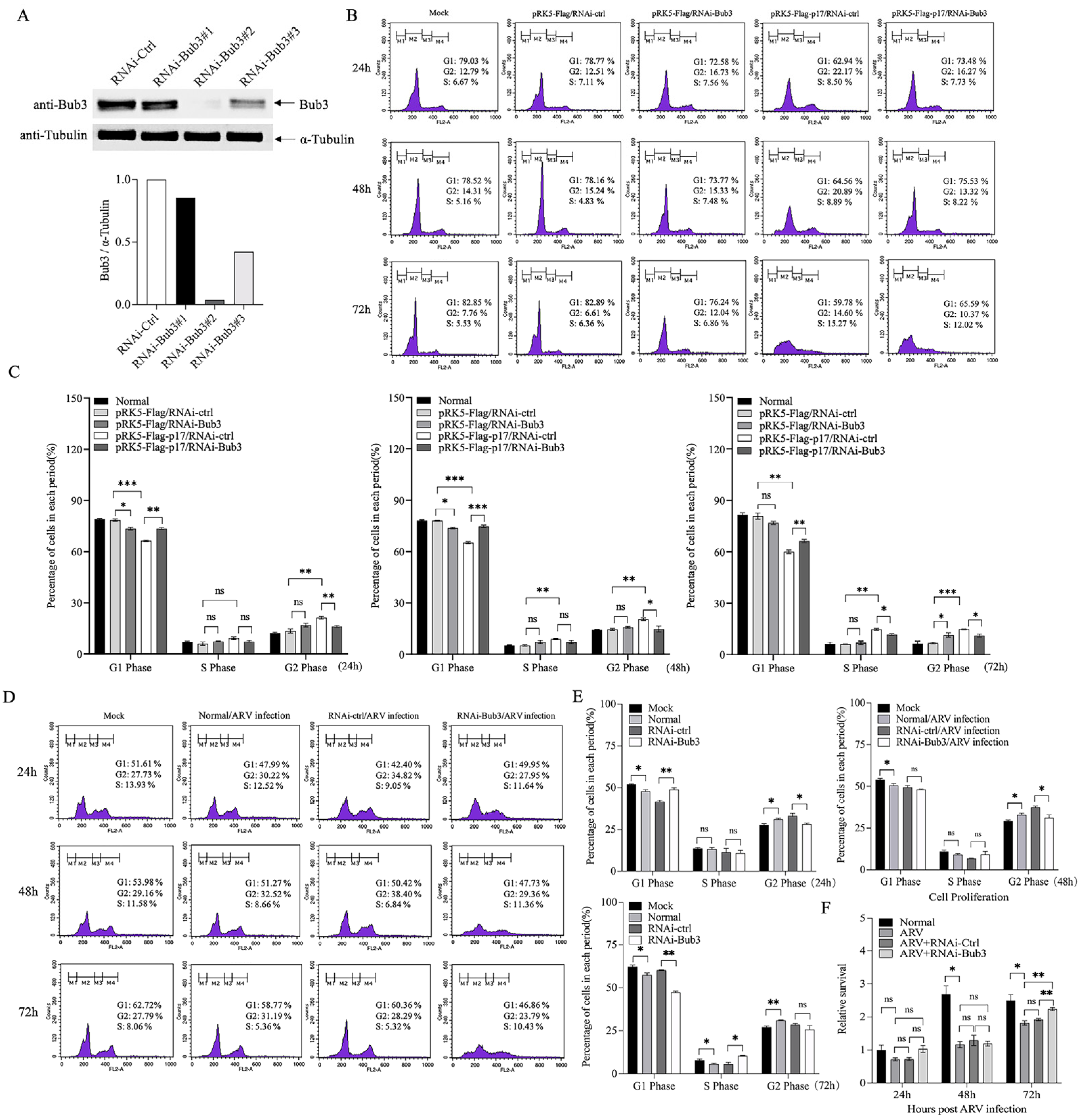
3.4. Bub3 Silence Alleviated Cell-Cycle Arrest Induced by ARV in Vero-E6 cells
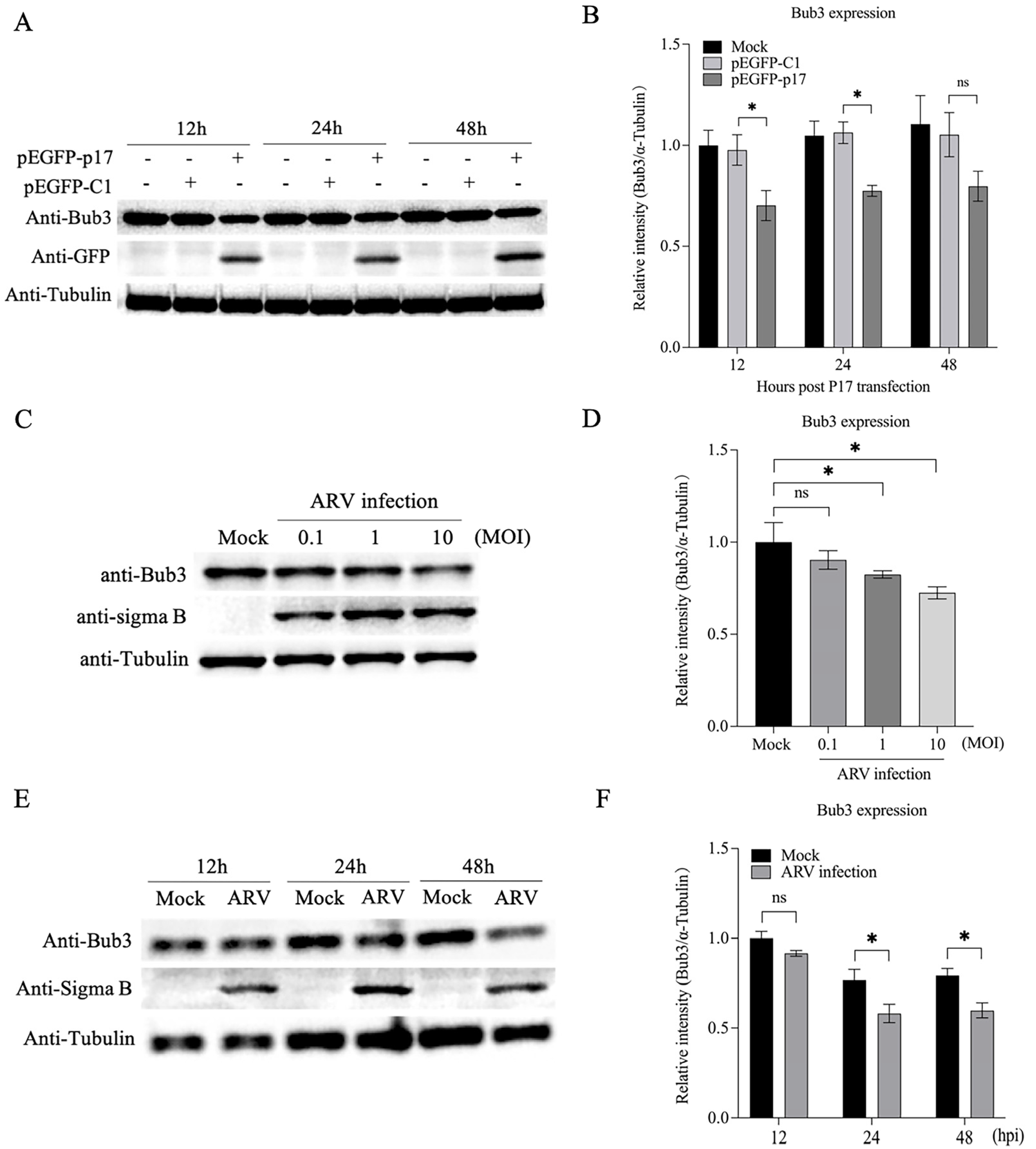
3.5. Both p17 Overexpression and ARV Infection Decreased Expression of Bub3
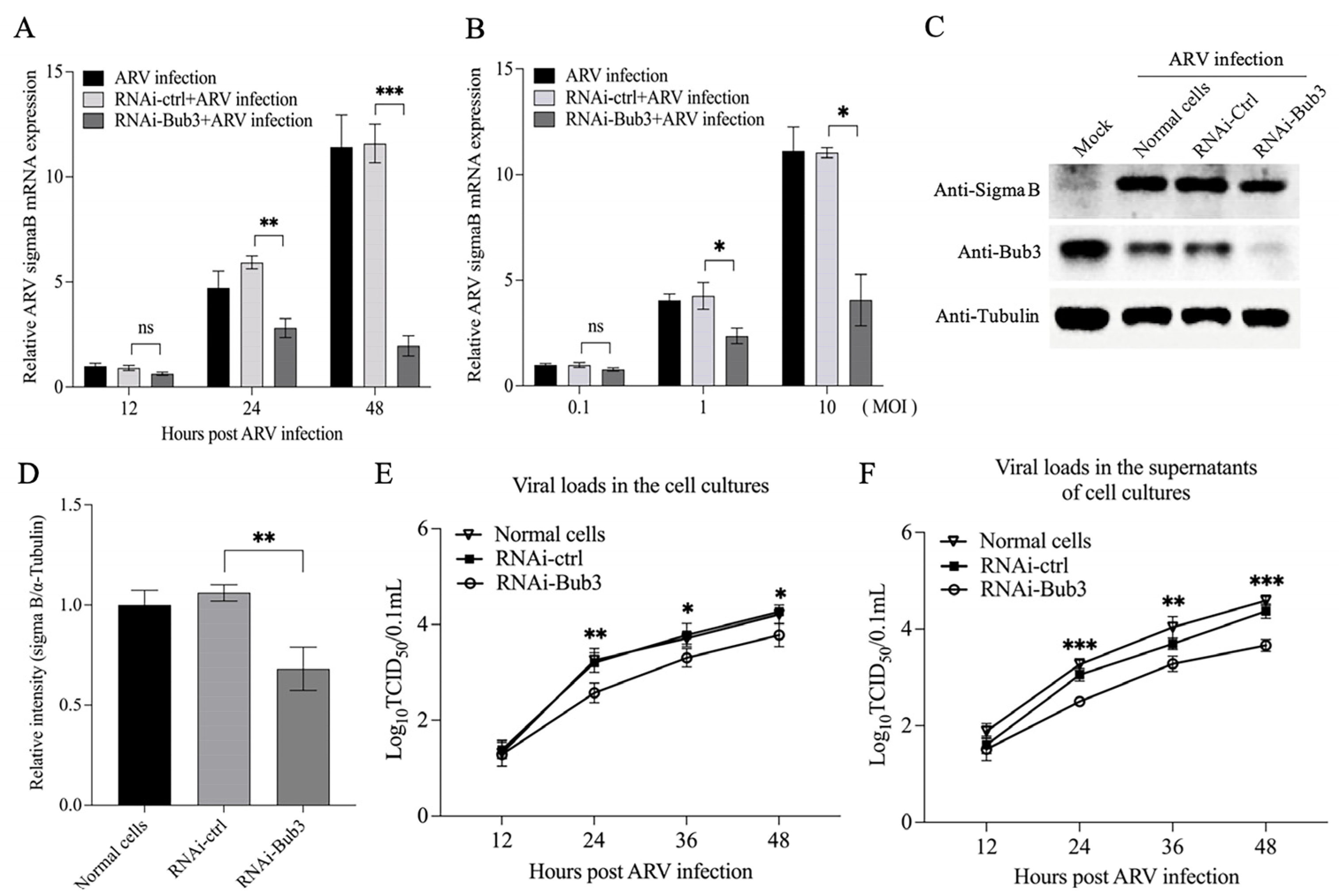
3.6. Knockdown of Bub3 Suppressed ARV Growth
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, H.J.; Guy, J.S.; Vaillancourt, J.P. Poult enteritis complex. Rev. Sci. Tech. 2000, 19, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Otto, P.; Liebler-Tenorio, E.M.; Elschner, M.; Reetz, J.; Löhren, U.; Diller, R. Detection of rotaviruses and intestinal lesions in broiler chicks from flocks with runting and stunting syndrome (RSS). Avian Dis. 2006, 50, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Page, R.K.; Fletcher, O.J.; Rowland, G.N.; Gaudry, D.; Villegas, P. Malabsorption syndrome in broiler chickens. Avian Dis. 1982, 26, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Sellers, H.; Linneman, E.; Icard, A.H.; Mundt, E. A purified recombinant baculovirus expressed capsid protein of a new astrovirus provides partial protection to runting-stunting syndrome in chickens. Vaccine 2010, 28, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.A.; Reece, R.L. Infectious stunting syndrome of chickens in Great Britain: Intestinal ultrastructural pathology. Avian Pathol. 1990, 19, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.A.; Davis, J.F.; McNulty, M.S.; Brown, J.; Player, E.C. Enteritis (so-called runting stunting syndrome) in Georgia broiler chicks. Avian Dis. 1993, 37, 451–458. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Davis, J.F.; Player, E.C. Reovirus-associated enteritis in Georgia broiler chicks. Avian Dis. 1993, 37, 229–233. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.S.; Allan, G.M.; Connor, T.J.; McFerran, J.B.; McCracken, R.M. An entero-like virus associated with the runting syndrome in broiler chickens. Avian Pathol. 1984, 13, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Pass, D.A.; Robertson, M.D.; Wilcox, G.E. Runting syndrome in broiler chickens in Australia. Vet. Rec. 1982, 110, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Strother, K.O.; Kisary, J. Partial genome sequence analysis of parvoviruses associated with enteric disease in poultry. Avian Pathol. 2008, 37, 435–441. [Google Scholar] [CrossRef]
- Benavente, J.; Martínez-Costas, J. Avian reovirus: Structure and biology. Virus Res. 2007, 123, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Giambrone, J.J.; Nielsen, B.L. Molecular characterization of avian reoviruses using nested PCR and nucleotide sequence analysis. J. Virol. Methods 1997, 65, 159–167. [Google Scholar] [CrossRef]
- Martínez-Costas, J.; Grande, A.; Varela, R.; García-Martínez, C.; Benavente, J. Protein architecture of avian reovirus S1133 and identification of the cell attachment protein. J. Virol. 1997, 71, 59–64. [Google Scholar] [CrossRef]
- Broude, E.V.; Swift, M.E.; Vivo, C.; Chang, B.D.; Davis, B.M.; Kalurupalle, S.; Blagosklonny, M.V.; Roninson, I.B. p21(Waf1/Cip1/Sdi1) mediates retinoblastoma protein degradation. Oncogene 2007, 26, 6954–6958. [Google Scholar] [CrossRef]
- Huang, W.R.; Chiu, H.C.; Liao, T.L.; Chuang, K.P.; Shih, W.L.; Liu, H.J. Avian Reovirus Protein p17 Functions as a Nucleoporin Tpr Suppressor Leading to Activation of p53, p21 and PTEN and Inactivation of PI3K/AKT/mTOR and ERK Signaling Pathways. PLoS ONE 2015, 10, e0133699. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Lin, P.Y.; Lee, J.W.; Hsu, H.Y.; Shih, W.L. Retardation of cell growth by avian reovirus p17 through the activation of p53 pathway. Biochem. Biophys. Res. Commun. 2005, 336, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Bagga, S.; Bouchard, M.J. Cell cycle regulation during viral infection. Methods Mol. Biol. 2014, 1170, 165–227. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.V.; Brooks, G. The mammalian cell cycle: An overview. Methods Mol. Biol. 2005, 296, 113–153. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Lara-Gonzalez, P.; Westhorpe, F.G.; Taylor, S.S. The spindle assembly checkpoint. Curr. Biol. 2012, 22, R966–R980. [Google Scholar] [CrossRef]
- Li, X.; Nicklas, R.B. Mitotic forces control a cell-cycle checkpoint. Nature 1995, 373, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.L.; Cole, R.W.; Khodjakov, A.; Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995, 130, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A.; Salmon, E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007, 8, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Pines, J. Cubism and the cell cycle: The many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 2011, 12, 427–438. [Google Scholar] [CrossRef]
- Sacristan, C.; Kops, G.J. Joined at the hip: Kinetochores, microtubules, and spindle assembly checkpoint signaling. Trends Cell Biol. 2015, 25, 21–28. [Google Scholar] [CrossRef]
- Han, J.S.; Vitre, B.; Fachinetti, D.; Cleveland, D.W. Bimodal activation of BubR1 by Bub3 sustains mitotic checkpoint signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E4185–E4193. [Google Scholar] [CrossRef]
- Yang, Y.; Lacefield, S. Bub3 activation and inhibition of the APC/C. Cell Cycle 2016, 15, 1–2. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Tsuchiya, D.; Lacefield, S. Bub3 promotes Cdc20-dependent activation of the APC/C in S. cerevisiae. J. Cell Biol. 2015, 209, 519–527. [Google Scholar] [CrossRef]
- Nascimento, R.; Costa, H.; Parkhouse, R.M. Virus manipulation of cell cycle. Protoplasma 2012, 249, 519–528. [Google Scholar] [CrossRef]
- Chiu, H.C.; Huang, W.R.; Liao, T.L.; Wu, H.Y.; Munir, M.; Shih, W.L.; Liu, H.J. Suppression of Vimentin Phosphorylation by the Avian Reovirus p17 through Inhibition of CDK1 and Plk1 Impacting the G2/M Phase of the Cell Cycle. PLoS ONE 2016, 11, e0162356. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Wang, Y.; Li, X.; Cao, H.; Zheng, S. Development and identification of monoclonal antibodies (mcabs) against avian reovirus(arv) σb protein (in Chinese). Chin. J. Vet. Med. 2015, 51, 3–6. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Overlack, K.; Bange, T.; Weissmann, F.; Faesen, A.C.; Maffini, S.; Primorac, I.; Müller, F.; Peters, J.M.; Musacchio, A. BubR1 Promotes Bub3-Dependent APC/C Inhibition during Spindle Assembly Checkpoint Signaling. Curr. Biol. 2017, 27, 2915–2927.e2917. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Huang, W.R.; Liao, T.L.; Chi, P.I.; Nielsen, B.L.; Liu, J.H.; Liu, H.J. Mechanistic insights into avian reovirus p17-modulated suppression of cell cycle CDK-cyclin complexes and enhancement of p53 and cyclin H interaction. J. Biol. Chem. 2018, 293, 12542–12562. [Google Scholar] [CrossRef]
- Cairo, G.; MacKenzie, A.M.; Lacefield, S. Differential requirement for Bub1 and Bub3 in regulation of meiotic versus mitotic chromosome segregation. J. Cell Biol. 2020, 219, e201909136. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.S.; Sampaio, P.; Williams, B.; Goldberg, M.; Sunkel, C.E. The Drosophila Bub3 protein is required for the mitotic checkpoint and for normal accumulation of cyclins during G2 and early stages of mitosis. J. Cell Sci. 2005, 118, 187–198. [Google Scholar] [CrossRef]
- Proudfoot, K.G.; Anderson, S.J.; Dave, S.; Bunning, A.R.; Sinha Roy, P.; Bera, A.; Gupta, M.L., Jr. Checkpoint Proteins Bub1 and Bub3 Delay Anaphase Onset in Response to Low Tension Independent of Microtubule-Kinetochore Detachment. Cell Rep. 2019, 27, 416–428.e414. [Google Scholar] [CrossRef]
- Grabsch, H.; Takeno, S.; Parsons, W.J.; Pomjanski, N.; Boecking, A.; Gabbert, H.E.; Mueller, W. Overexpression of the mitotic checkpoint genes BUB1, BUBR1, and BUB3 in gastric cancer--association with tumour cell proliferation. J. Pathol. 2003, 200, 16–22. [Google Scholar] [CrossRef]
- Morais da Silva, S.; Moutinho-Santos, T.; Sunkel, C.E. A tumor suppressor role of the Bub3 spindle checkpoint protein after apoptosis inhibition. J. Cell Biol. 2013, 201, 385–393. [Google Scholar] [CrossRef]
- Tange, Y.; Niwa, O. Schizosaccharomyces pombe Bub3 Is Dispensable for Mitotic Arrest Following Perturbed Spindle Formation. Genetics 2008, 179, 785–792. [Google Scholar] [CrossRef]
- Vermeulen, K.; Berneman, Z.N.; Van Bockstaele, D.R. Cell cycle and apoptosis. Cell Prolif. 2003, 36, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Costas, C.; Martínez-Costas, J.; Bodelón, G.; Benavente, J. The second open reading frame of the avian reovirus S1 gene encodes a transcription-dependent and CRM1-independent nucleocytoplasmic shuttling protein. J. Virol. 2005, 79, 2141–2150. [Google Scholar] [CrossRef] [PubMed][Green Version]
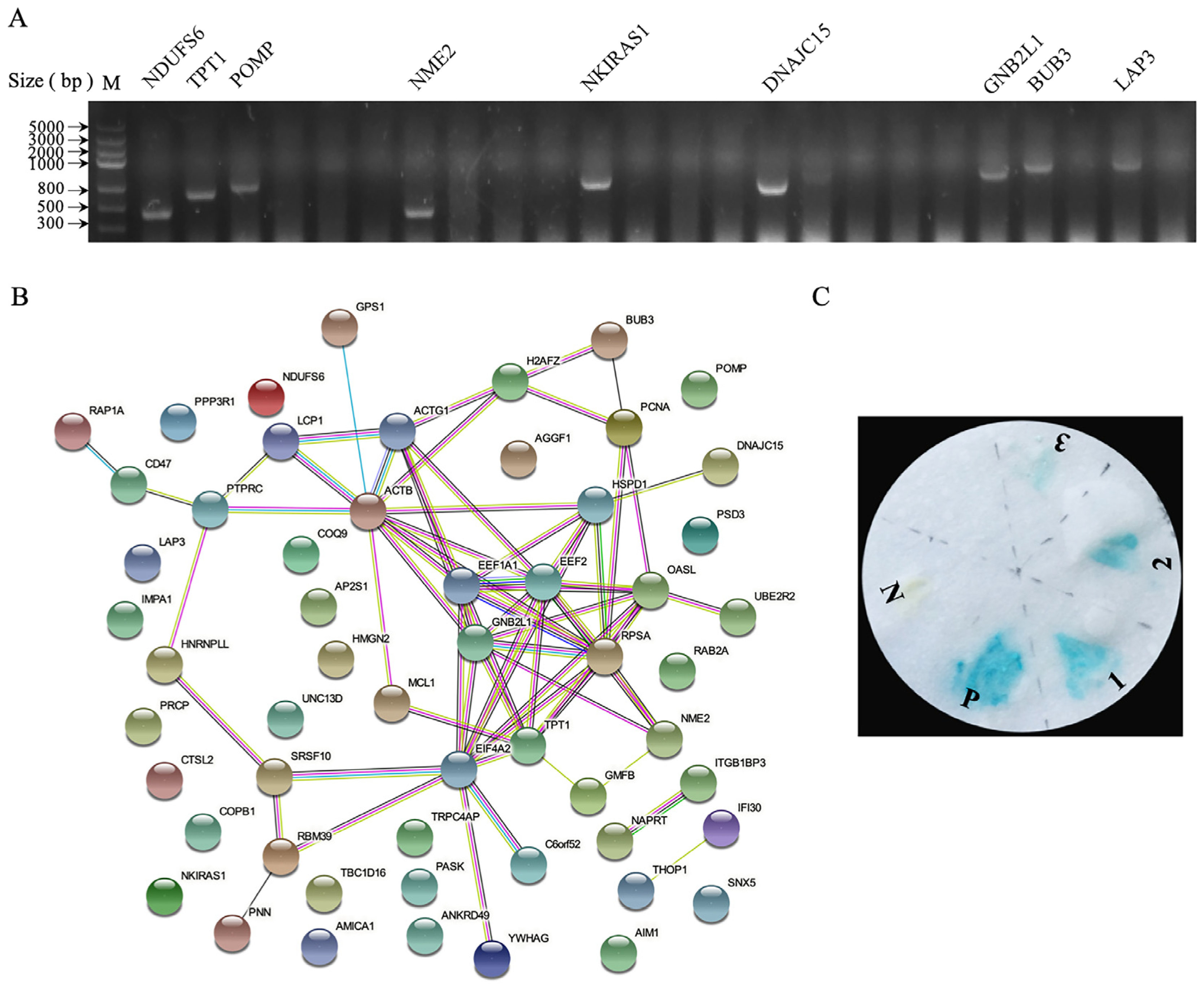
| Protein Name | No. GenBank | Function |
|---|---|---|
| Induced myeloid leukemia cell differentiation protein (MCL1) | XP_040508245.1 | Regulation of cell differentiation |
| Leucine aminopeptidase 3 (LAP3) | NP_001026507.1 | Peptidase activity |
| Tumor protein, translationally-controlled 1 (TPT1) | NP_990729.1 | Regulation of intrinsic apoptotic signaling pathway in response to DNA damage |
| NF-kappa-B inhibitor-interacting Ras-like protein 1 (NKIRAS1) | XP_046794149.1 | Regulation of I-kappaB kinase/NF-kappaB signaling |
| Eukaryotic translation elongation factor 1 alpha 1 (eEF1A1) | NP_001308445.1 | Translation elongation factor activity, GTPase activity |
| Guanine nucleotide-binding protein beta subunit2-like 1 (GNB2L1) | BAJ53010.1 | RNA binding, regulation of protein kinase activity |
| Eukaryotic translation elongation factor 2 (eEF2) | NP_990699.2 | Regulation of cell growth |
| Mitotic checkpoint protein (Bub3) | NP_001006506.1 | Ubiquitin binding, regulation of mitotic cell cycle |
| Eukaryotic initiation factor 4A-II (EIF4A2) | NP_989880.1 | Translation initiation factor activity, RNA helicase activity |
| 2′-5′-oligoadenylate synthase-like protein (OASL) | XP_046782048.1 | Regulation of viral genome replication and RIG-I pathway |
| Ubiquitin-conjugating enzyme E2 R2 (UBE2R2) | NP_001026582.2 | Ubiquitin-conjugating enzyme activity |
| Heat shock protein family D member 1 (HSPD1) | NP_001012934.1 | Ubiquitin protein ligase binding |
| Nucleoside diphosphate kinase (NME2) | NP_990378.1 | Regulation of apoptotic process |
| Serine/arginine-rich splicing factor 10 (SRSF10) | XP_015153161.1 | Regulation of mRNA splicing, cytosolic transport |
| DnaJ homolog subfamily C member 15 (DNAJC15) | NP_001264705.2 | ATPase activator activity, regulation of mitochondrial electron transport |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Fu, M.; Chen, X.; Zhao, Y.; Gao, L.; Cao, H.; Li, X.; Zheng, S.J.; Wang, Y. Arrest of Cell Cycle by Avian Reovirus p17 through Its Interaction with Bub3. Viruses 2022, 14, 2385. https://doi.org/10.3390/v14112385
Tang J, Fu M, Chen X, Zhao Y, Gao L, Cao H, Li X, Zheng SJ, Wang Y. Arrest of Cell Cycle by Avian Reovirus p17 through Its Interaction with Bub3. Viruses. 2022; 14(11):2385. https://doi.org/10.3390/v14112385
Chicago/Turabian StyleTang, Junyu, Mengjiao Fu, Xiang Chen, Yimeng Zhao, Li Gao, Hong Cao, Xiaoqi Li, Shijun J. Zheng, and Yongqiang Wang. 2022. "Arrest of Cell Cycle by Avian Reovirus p17 through Its Interaction with Bub3" Viruses 14, no. 11: 2385. https://doi.org/10.3390/v14112385
APA StyleTang, J., Fu, M., Chen, X., Zhao, Y., Gao, L., Cao, H., Li, X., Zheng, S. J., & Wang, Y. (2022). Arrest of Cell Cycle by Avian Reovirus p17 through Its Interaction with Bub3. Viruses, 14(11), 2385. https://doi.org/10.3390/v14112385






