Isolation and Identification of a New Isolate of Anguillid Herpesvirus 1 from Farmed American Eels (Anguilla rostrata) in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Virus Detection
2.3. Cell Culture
2.4. Virus Isolation and Purification
2.5. Morphology Observation of Viruses
2.6. Experimental Infection and Histopathological Observation
2.7. Viral Genome Sequencing and Bioinformatic Analysis
2.8. Phylogenetic Analysis
3. Results
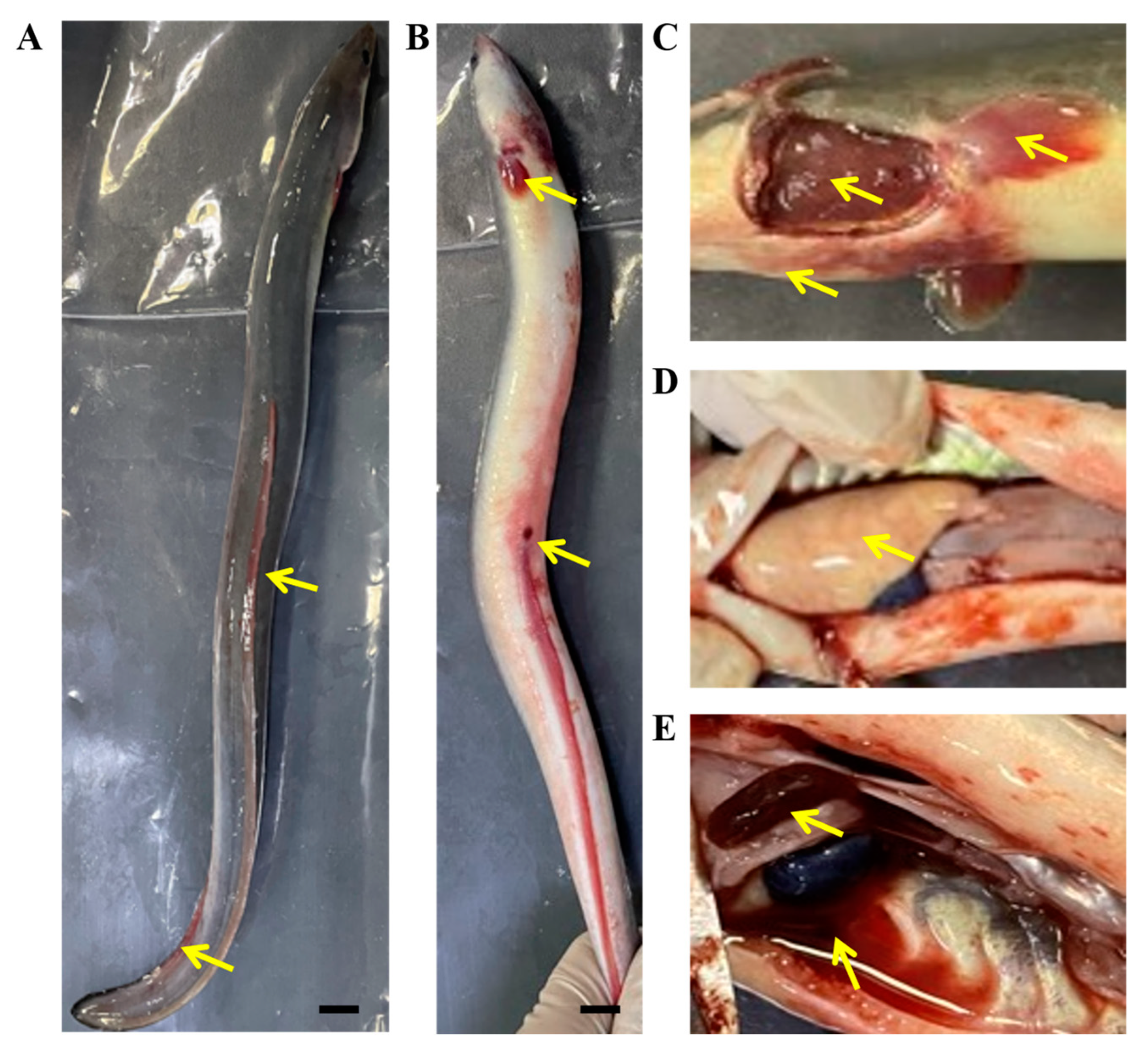
3.1. Clinical Symptoms and Virus Detection
3.2. Virus Isolation
3.3. Pathogenicity of AngHV-1-FC
3.4. Identification of AngHV-1-FC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, Q.; Fan, H.; Lin, Y.; Cai, L. Current situation and analysis of eel industry standard system in China. In Proceedings of the symposium of the 17th China Standardization Forum, Fuzhou, China, 19 November 2020; pp. 762–772. [Google Scholar]
- Jakob, E.; Neuhaus, H.; Steinhagen, D.; Luckhardt, B.; Hanel, R. Monitoring of Herpesvirus anguillae (HVA) infections in European eel, Anguilla anguilla (L.), in northern Germany. J. Fish Dis. 2009, 32, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Haenen, O.L.M.; Dijkstra, S.G.; Van Tulden, P.W.; Davidse, A.; Van Nieuwstadt, A.P.; Wagenaar, F.; Wellenberg, G.J. Herpesvirus anguillae (HVA) isolations from disease outbreaks in cultured European eel, Anguilla anguilla in The Netherlands since 1996. Bull. Eur. Assoc. Fish Pat. 2002, 22, 247–257. [Google Scholar]
- van Ginneken, V.; Haenen, O.; Coldenhoff, K.; Willemze, R.; Antonissen, E.; van Tulden, P.; Dijkstra, S.; Wagenaar, F.; van den Thillart, G. Presence of eel viruses in eel species from various geographic regions. Bull. Eur. Assoc. Fish Pat. 2004, 24, 268–271. [Google Scholar]
- Ge, J.; Yang, J.; Gong, H.; Lin, T. Isolation and identification of a herpesvirus from cultured European eels Anguilla anguilla in China. J. Fish. China 2014, 38, 1579–1583. [Google Scholar] [CrossRef]
- Békési, L.; Horváth, I.; Kovacs-Gayer, E.; Csaba, G. Demonstration of herpesvirus like particles in skin lesions of European eel (Anguilla anguilla). J. Appl. Ichthyol. 1986, 2, 190–192. [Google Scholar] [CrossRef]
- Sano, M.; Fukuda, H.; Sano, T. Isolation and characterization of a new herpesvirus from eel. Pathol. Mar. Sci. 1990, 15, 15–31. [Google Scholar] [CrossRef]
- Kempter, J.; Hofsoe, P.; Panicz, R.; Bergmann, S.M. First detection of Anguillid herpesvirus 1 (AngHV1) in European eel (Anguilla anguilla) and imported American eel (Anguilla rostrata) in Poland. Bull. Eur. Assoc. Fish Pat. 2014, 34, 87–94. [Google Scholar]
- Zhuo, Y. Herpesvirus isolation and identification of the cultured eel with ‘Red Liver Disease’. Fujian J. Agric. Sci. 2015, 30, 117–120. [Google Scholar] [CrossRef]
- Panicz, R.; Eljasik, P.; Nguyen, T.T.; Vo Thi, K.T.; Hoang, D.V. First detection of Herpesvirus anguillae (AngHV-1) associated with mortalities in farmed giant mottled eel (Anguilla marmorata) in Vietnam. J. Fish Dis. 2021, 44, 847–852. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Yang, J.; Song, T.; Ge, J. Pathogenicity of Anguillid herpesvirus to Anguilla anguilla. J. Fish. China 2021, 45, 940–947. [Google Scholar] [CrossRef]
- Hangalapura, B.N.; Zwart, R.; Engelsma, M.Y.; Haenen, O.L. Pathogenesis of Herpesvirus anguillae (HVA) in juvenile European eel Anguilla anguilla after infection by bath immersion. Dis. Aquat. Organ. 2007, 78, 13–22. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwstadt, A.P.; Dijkstra, S.G.; Haenen, O.L.M. Persistence of herpesvirus of eel Herpesvirus anguillae in farmed European eel Anguilla anguilla. Dis. Aquat. Organ. 2001, 45, 103–107. [Google Scholar] [CrossRef] [PubMed]
- van Beurden, S.J.; Bossers, A.; Voorbergen-Laarman, M.H.; Haenen, O.L.; Peters, S.; Abma-Henkens, M.H.C.; Peeters, B.P.H.; Rottier, P.J.M.; Engelsma, M.Y. Complete genome sequence and taxonomic position of Anguillid herpesvirus 1. J. Gen. Virol. 2010, 91, 880–887. [Google Scholar] [CrossRef] [PubMed]
- van Beurden, S.J.; Gatherer, D.; Kerr, K.; Galbraith, J.; Herzyk, P.; Peeters, B.P.H.; Rottier, P.J.M.; Engelsma, M.Y.; Davison, A.J. Anguillid herpesvirus 1 transcriptome. J. Virol. 2012, 86, 10150–10161. [Google Scholar] [CrossRef]
- Wen, C.M.; Liu, P.C.; Nan, F.H. Complete genome sequence of herpesvirus anguillae strain HVA980811 isolated in Chiayi, Taiwan. Genome Announc. 2017, 5, e01677-16. [Google Scholar] [CrossRef]
- Donohoe, O.; Zhang, H.; Delrez, N.; Gao, Y.; Suárez, N.M.; Davison, A.J.; Vanderplasschen, A. Genomes of Anguillid herpesvirus 1 strains reveal evolutionary disparities and low genetic diversity in the Genus Cyprinivirus. Microorganisms 2021, 9, 998. [Google Scholar] [CrossRef]
- Rijsewijk, F.; Pritz-Verschuren, S.; Kerkhof, S.; Botter, A.; Willemsen, M.; van Nieuwstadt, T.; Haenen, O. Development of a polymerase chain reaction for the detection of Anguillid herpesvirus DNA in eels based on the herpesvirus DNA polymerase gene. J. Virol. Methods 2005, 124, 87–94. [Google Scholar] [CrossRef]
- Li, M.; Wu, B.; Lin, N.; Wang, X.; Jiang, X.; Lin, G.; Fan, H. The established kidney cell line from european eel Anguilla anguilla and its susceptibility to Anguillid herpesvirus. Acta Hydrobiol. Sin. 2020, 44, 237–244. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuo, X.; Cheng, J.; Wang, X.; Liang, K.; Chen, X. PU.1 regulates cathepsin S expression in large yellow croaker (Larimichthys crocea) macrophages. Front. Immunol. 2022, 12, 819029. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Chen, X.; Lin, K.; Wang, X. Outbreaks of an iridovirus disease in maricultured large yellow croaker, Larimichthys crocea (Richardson), China. J. Fish Dis. 2003, 26, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, S.; Ren, Q.; He, T.; Chen, X. An outbreak of visceral white nodules disease caused by Pseudomonas plecoglossicida at a water temperature of 12 °C in cultured large yellow croaker (Larimichthys crocea) in China. J. Fish Dis. 2020, 43, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P. MetaSPAdes: A new versatile de novo metagenomics assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Treangen, T.J.; Pop, M. Bambus 2: Scaffolding metagenomes. Bioinformatics 2011, 27, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Glöckner, F.O.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bandín, I.; Souto, S.; Cutrín, J.M.; López-Vázquez, C.; Olveira, J.G.; Esteve, C.; Alcaide, E.; Dopazo, C.P. Presence of viruses in wild eels Anguilla anguilla L., from the Albufera Lake (Spain). J. Fish Dis. 2014, 37, 597–607. [Google Scholar] [CrossRef]
- Zhuo, Y. Ultramorphological evidence of the coinfection of herpesvirus and PPLO in cultured eel. J. Chin. Electron Microsc. Soc. 2014, 2, 168–171. [Google Scholar] [CrossRef]
- Waltzek, T.B.; Kelley, G.O.; Alfaro, M.E.; Kurobe, T.; Davison, A.J.; Hedrick, R.P. Phylogenetic relationships in the family Alloherpesviridae. Dis. Aquat. Organ. 2009, 84, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Miyazaki, T. Characterization and pathogenicity of a herpesvirus isolated from cutaneous lesion in Japanese eel, Anguilla japonica. Fish Pathol. 1997, 32, 89–95. [Google Scholar] [CrossRef][Green Version]
- Chakrabarty, A.M. Nucleoside diphosphate kinase: Role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol. Microbiol. 1998, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Jeudy, S.; Claverie, J.M.; Abergel, C. The nucleoside diphosphate kinase from mimivirus: A peculiar affinity for deoxypyrimidine nucleotides. J. Bioenerg. Biomembr. 2006, 38, 247–254. [Google Scholar] [CrossRef]
- Nguyen, S.; Jovcevski, B.; Pukala, T.L.; Bruning, J.B. Nucleoside selectivity of Aspergillus fumigatus nucleoside-diphosphate kinase. FEBS J. 2021, 288, 2398–2417. [Google Scholar] [CrossRef]






| Isolates | ANIb (%) |
|---|---|
| AngHV-1-FJ | 99.44 |
| AngHV-1-CVI | 99.44 |
| AngHV-1-HVA | 99.43 |
| AngHV-1-DK1 | 99.46 |
| AngHV-1-DK2 | 99.28 |
| AngHV-1-DK3 | 99.36 |
| AngHV-1-DK4 | 99.55 |
| AngHV-1-UK | 99.41 |
| AngHV-1-TW | 99.28 |
| CyHV-1-NG-J1 | 62.36 |
| CyHV-2-ST-J1 | 66.17 |
| CyHV-3-KHV-U | 63.39 |
| FC | CVI | DK1 | FJ | HVA | TW | DK4 | UK | DK3 | |
|---|---|---|---|---|---|---|---|---|---|
| CVI | 1.37 × 10−3 | ||||||||
| DK1 | 1.35 × 10−3 | 3.64 × 10−5 | |||||||
| FJ | 1.54 × 10−3 | 4.05 × 10−6 | 3.24 × 10−5 | ||||||
| HVA | 1.18 × 10−3 | 3.20 × 10−4 | 3.24 × 10−4 | 3.40 × 10−4 | |||||
| TW | 1.68 × 10−3 | 6.73 × 10−4 | 6.69 × 10−4 | 9.16 × 10−4 | 5.18 × 10−4 | ||||
| DK4 | 1.09 × 10−3 | 6.17 × 10−4 | 6.14 × 10−4 | 6.21 × 10−4 | 4.34 × 10−4 | 7.96 × 10−4 | |||
| UK | 1.40 × 10−3 | 9.93 × 10−4 | 9.97 × 10−4 | 1.03 × 10−3 | 8.70 × 10−4 | 1.18 × 10−3 | 8.95 × 10−4 | ||
| DK3 | 1.24 × 10−3 | 1.07 × 10−3 | 1.06 × 10−3 | 1.14 × 10−3 | 9.24 × 10−4 | 1.29 × 10−3 | 9.08 × 10−4 | 1.11 × 10−3 | |
| DK2 | 1.28 × 10−3 | 1.22 × 10−3 | 1.22 × 10−3 | 1.28 × 10−3 | 1.13 × 10−3 | 1.50 × 10−3 | 1.11 × 10−3 | 1.30 × 10−3 | 7.80 × 10−4 |
| Mean value | 1.35 × 10−3 # | 8.31 × 10−4 * | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Zhang, Z.; He, T.; Li, M.; Zhuo, Y.; Yang, X.; Fan, H.; Chen, X. Isolation and Identification of a New Isolate of Anguillid Herpesvirus 1 from Farmed American Eels (Anguilla rostrata) in China. Viruses 2022, 14, 2722. https://doi.org/10.3390/v14122722
Guo R, Zhang Z, He T, Li M, Zhuo Y, Yang X, Fan H, Chen X. Isolation and Identification of a New Isolate of Anguillid Herpesvirus 1 from Farmed American Eels (Anguilla rostrata) in China. Viruses. 2022; 14(12):2722. https://doi.org/10.3390/v14122722
Chicago/Turabian StyleGuo, Rui, Zheng Zhang, Tianliang He, Miaomiao Li, Yuchen Zhuo, Xiaoqiang Yang, Haiping Fan, and Xinhua Chen. 2022. "Isolation and Identification of a New Isolate of Anguillid Herpesvirus 1 from Farmed American Eels (Anguilla rostrata) in China" Viruses 14, no. 12: 2722. https://doi.org/10.3390/v14122722
APA StyleGuo, R., Zhang, Z., He, T., Li, M., Zhuo, Y., Yang, X., Fan, H., & Chen, X. (2022). Isolation and Identification of a New Isolate of Anguillid Herpesvirus 1 from Farmed American Eels (Anguilla rostrata) in China. Viruses, 14(12), 2722. https://doi.org/10.3390/v14122722






