Abstract
Coronavirus-19 (COVID-19), preliminarily a respiratory virus, can affect multiple organs, including the heart. Myocarditis is a well-known complication among COVID-19 infections, with limited large-scale studies evaluating outcomes associated with COVID-19-related Myocarditis. We used the National Inpatient Sample (NIS) database to compare COVID-19 patients with and without Myocarditis. A total of 1,659,040 patients were included in the study: COVID-19 with Myocarditis (n = 6,455, 0.4%) and COVID-19 without Myocarditis (n = 1,652,585, 99.6%). The primary outcome was in-hospital mortality. Secondary outcomes included mechanical ventilation, vasopressor use, sudden cardiac arrest, cardiogenic shock, acute kidney injury requiring hemodialysis, length of stay, health care utilization costs, and disposition. We conducted a secondary analysis with propensity matching to confirm results obtained by traditional multivariate analysis. COVID-19 patients with Myocarditis had significantly higher in-hospital mortality compared to COVID-19 patients without Myocarditis (30.5% vs. 13.1%, adjusted OR: 3 [95% CI 2.1–4.2], p < 0.001). This cohort also had significantly increased cardiogenic shock, acute kidney injury requiring hemodialysis, sudden cardiac death, required more mechanical ventilation and vasopressor support and higher hospitalization cost. Vaccination and more research for treatment strategies will be critical for reducing worse outcomes in patients with COVID-19-related Myocarditis.
1. Introduction
The Severe Acute Respiratory Virus Syndrome Coronavirus 2 (SARS-CoV-2) has caused worldwide pandemic affecting millions of people across the globe since it was first detected in Wuhan, China in December 2019 [1,2]. Though it is preliminarily a respiratory virus, it affects multiple organ systems including the cardiovascular system [3]. Cardiac complications can affect 20–30% of COVID-19 patients and lead to worse morbidity and mortality [4,5]. Cardiovascular complications can include myocarditis, acute myocardial infarction, heart failure, and arrhythmias [3]. Though myocarditis is associated with worse outcomes in COVID-19 patients [6], there are limited large-scale studies which evaluate outcomes associated with COVID-19-related myocarditis.
Myocarditis association with COVID-19 infection is well established by multiple studies [6,7,8]. Myocarditis refers to inflammation of cardiac muscles caused by infectious and non-infectious etiologies [9,10,11]) with viral etiologies being most common in the United States and other developed countries [11]. It can present with focal or diffuse myocardial involvement and can be characterized as an acute, subacute, or chronic disease process [11,12]. Its symptoms and signs are highly variable and can include generalized fatigue, chest pain, sinus tachycardia, new onset congestive heart failure, arrhythmia, cardiogenic shock, and death [11,12,13]. It is frequently associated with elevated troponin, C-reactive protein, and erythrocyte sedimentation rate (ESR) [12]. Echocardiogram is part of the standard evaluation and can show reduced ejection fraction [12]. While endomyocardial biopsy is the gold standard for myocarditis diagnosis, Cardiac MRI is the gold standard for non-invasively assessing ventricular volumes, cardiac mass, and ejection fraction [12].
The pathophysiology of COVID-19-related myocarditis is hypothesized to involve a combination of immune-mediated damage and direct cytotoxic effects of the virus in myocardium [14]. Angiotensinogen-converting enzyme 2 (ACE2) receptor is found in multiple organ systems including cardiac tissue [14]. It is postulated to play a role in facilitating the entry of the virus into the cell by binding to the virus’s spike protein and causing direct cytotoxic effects [14].
Our study’s objective is to assess outcomes between COVID-19 patients with and without myocarditis utilizing data from National Inpatient Sample (NIS). The primary outcome was in-hospital mortality. Secondary outcomes included mechanical ventilation, vasopressor use, sudden cardiac arrest, cardiogenic shock, acute kidney injury (AKI) requiring hemodialysis (HD), length of stay (LOS), health care utilization costs, and disposition.
2. Materials and Methods
This retrospective study utilized NIS Healthcare Cost Utilization Project [HCUP] sponsored by the Agency for Healthcare and Research and Quality [AHRQ] database, which is an all-payer database that approximates a 20% stratified sample of discharges from US community hospitals [15]. In this analysis, we used the 2020 NIS data set, which included hospitalization from 1 January 2020 to 31 December 2020, and was made available to the public in October 2022.
All patients 18 years of age and older admitted to the hospital with myocarditis and COVID-19 infection were included in this study. International classification of diseases 10th-clinical modification (ICD-10-CM) codes were used to retrieve patient samples with comorbid conditions, and ICD-10 procedure codes were used to identify inpatient procedures. A detailed code summary is provided in Table S1. Patients who were under the age of 18 years or were transferred out of the hospital were excluded from this study.
2.1. Covariates
The NIS database contains data regarding in-hospital outcomes, procedures, and other discharge-related information. Variables were divided into patient-related, hospital-related, and indicators of illness severity as below:
- a.
- Patient: age, race, sex, comorbidities, insurance status, mean income in patient’s zip code, and disposition.
- b.
- Hospital: location, teaching status, bed size, and region.
- c.
- Illness severity: length of stay, mortality, hospitalization cost, Elixhauser comorbidity score [16], in-hospital complications, mechanical ventilation, circulatory support, and vasopressor use.
2.2. Study Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included mechanical ventilation, vasopressor use, sudden cardiac arrest, cardiogenic shock, acute kidney injury requiring hemodialysis, length of stay, health care utilization costs, and disposition.
2.3. Statistical Methods
Descriptive statistics were used to summarize the continuous and categorical variables. Continuous variables were summarized as mean ± SD; categorical data as the number and percentage. Univariate analyses for between-group comparisons used the Rao-Scott Chi-square test for categorical variables (e.g., sex and risk factors) and weighted simple linear regression for continuous variables (e.g., age). On an unmatched sample, univariate regression was used to identify independent variables (p ≤ 0.2), which were utilized to build a multivariate regression model. As our control group (COVID-positive without myocarditis) had a significantly higher sample than the test group (COVID-positive with myocarditis), we conducted a secondary analysis with propensity matching (PSM) to confirm results obtained by traditional multivariate analysis. Baseline demographics (age, race, sex, income status, insurance status) were matched using 1:1 nearest neighbor propensity score with 0.05 caliper width. On matched cohorts, a secondary multivariate regression model was built as described above. All analysis was performed using Stata 90 software version 17.0 (Stata Corporation, College Station, TX, USA). p values of less than 0.05 were considered statistically significant.
3. Results
3.1. Demographics and Baseline Comorbidities
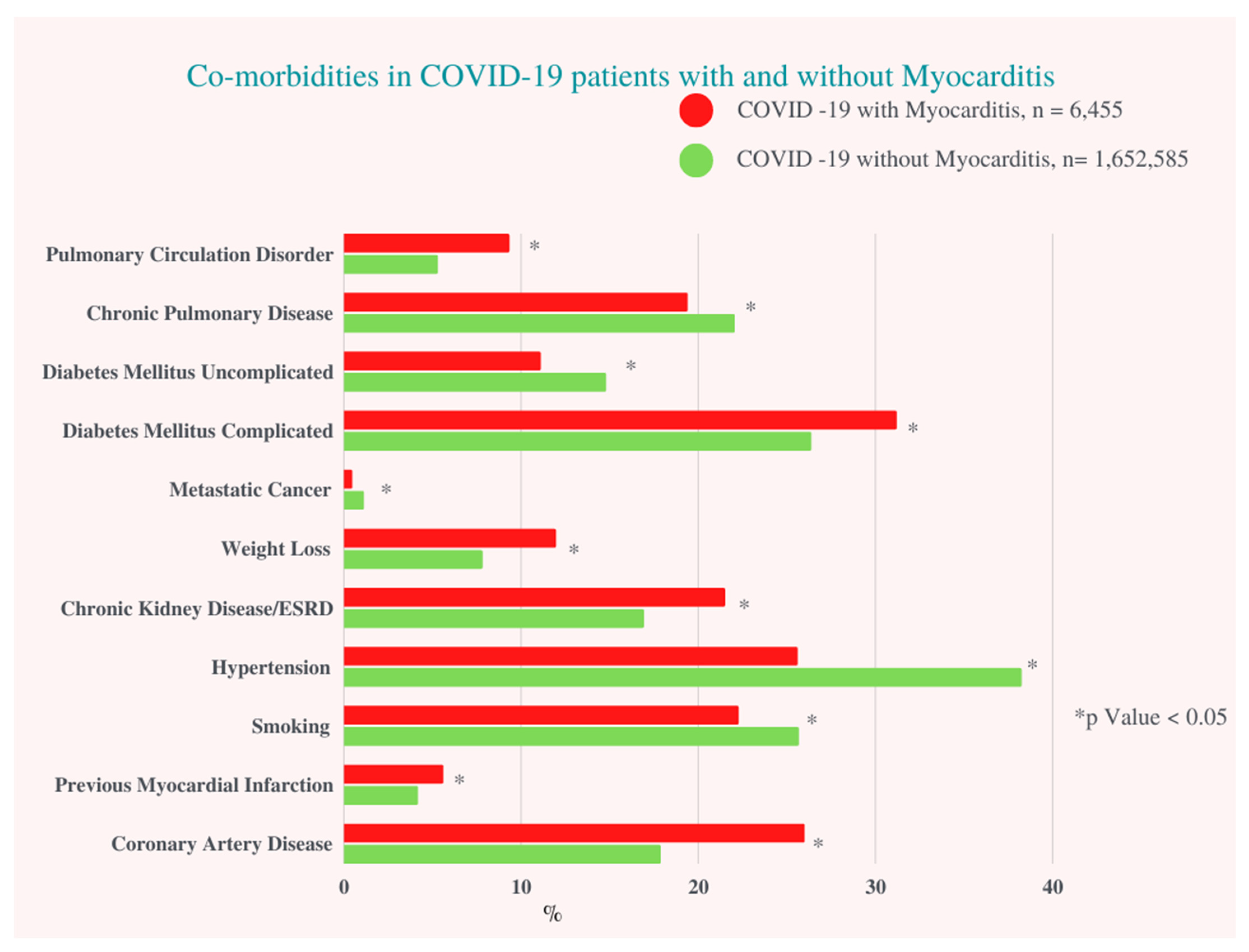
In our study population, a total of 1,659,040 hospitalized COVID-19 patients between 1 January and 31 December 2020, and 6,455 (0.4%) patients diagnosed with COVID-19 infection with concomitant myocarditis were included. COVID-19 infection with myocarditis was more prevalent in males (61.4% vs. 52.0%, p < 0.001), had a greater proportion of Hispanics (23.2% vs. 21.5%, p = 0.02), Asians (4.2% vs. 3.2%, p = 0.02), African Americans (20.8% vs. 19.1%, p = 0.02), and Native Americans (1.4% vs. 1.0%, p = 0.02), and were more likely to have a household income above $80,000 (19.0% vs. 16.5%, p < 0.005) when compared to COVID-19 patients without myocarditis (Figure 1) (Table 1).
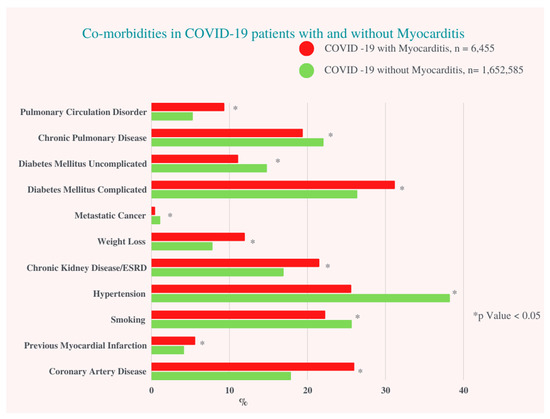
Figure 1.
Co-morbidities in COVID-19 Patients with and without Myocarditis. ESRD: End Stage Renal Artery Disease.

Table 1.
COVID-19 and Myocarditis Unmatched Patient-level Characteristics.
Patients with COVID-19-associated myocarditis had more complicated diabetes mellitus (DM) (31.1% vs. 26.3%, p < 0.001), chronic kidney disease (CKD)/end stage renal disease (ESRD) (21.5% vs. 16.9%, p < 0.001), underlying coronary artery disease (CAD) (26% vs. 17.9%, p < 0.001) and history of past myocardial infarction (MI) (5.6% vs. 4.2%, p = 0.01). There was no significant difference regarding smoking status, history of previous percutaneous coronary intervention (PCI), alcohol use, or obesity between the two groups (Table S2).
There was some variability in the geographic distribution of the patients and most patients with COVID-19 infection with and without myocarditis were seen at urban teaching hospitals (77.5% and 71.5%, respectively, p < 0.001). Moreover, both cohorts had a higher proportion of Medicare beneficiaries (53.5% vs. 53.2%, p = 0.466). Table 1 outlines the baseline characteristics of the study cohort.
3.2. In-Hospital Mortality
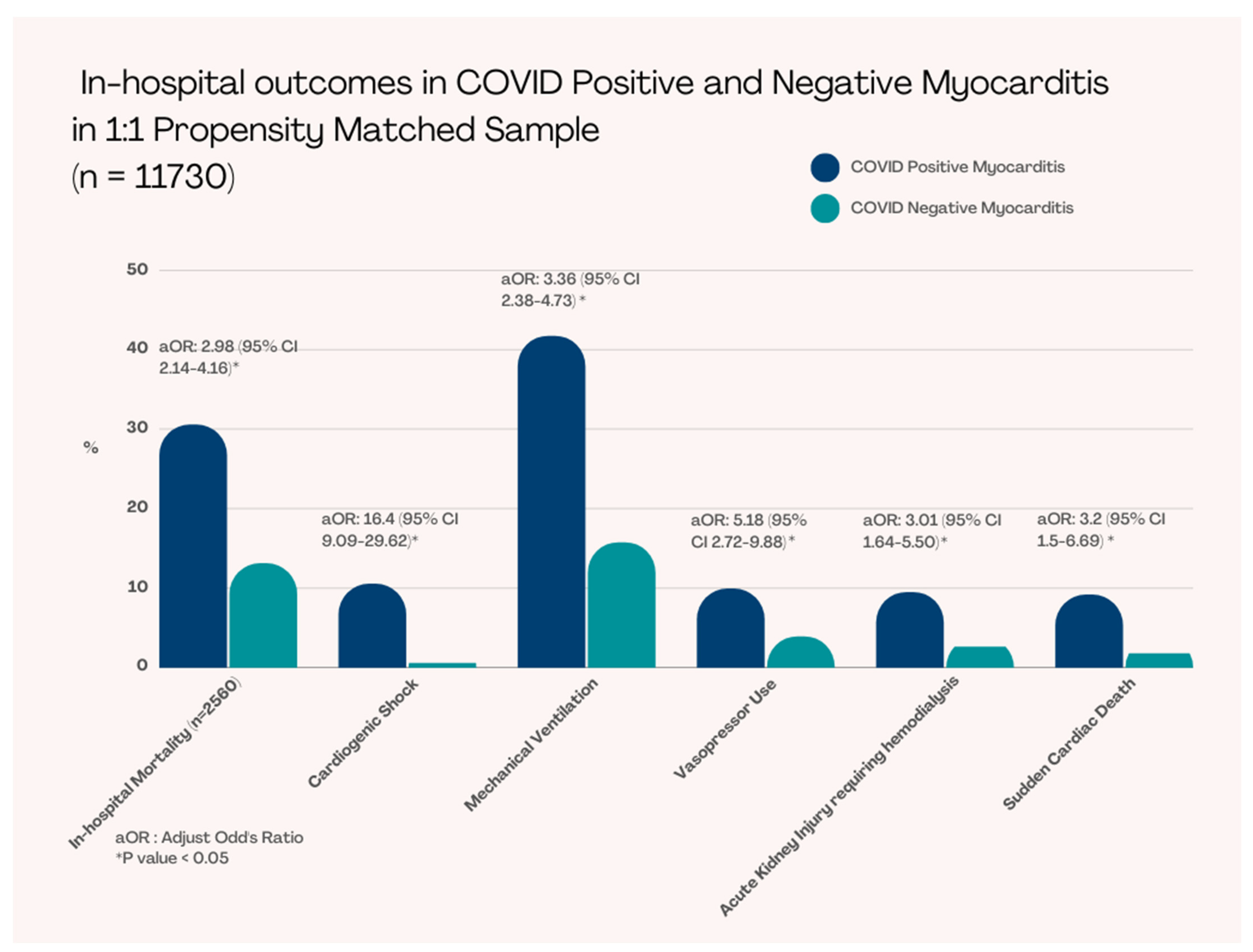
Propensity matching was performed regarding patient age, sex, race, income, and insurance status (Table 2). After PSM, we had a total of 5,864 patients in each group (with myocarditis and without myocarditis) (Table 2). The in-hospital mortality was significantly higher in COVID-19 patients with myocarditis compared to without myocarditis COVID-19 patients (30.5% vs 13.1%, adjusted OR [aOR]: 3 [95% CI 2.1–4.2], p < 0.001) (Figure 2) (Table 3).

Table 2.
COVID-19 Propensity 1:1 Matched patient-level Characteristics 2.
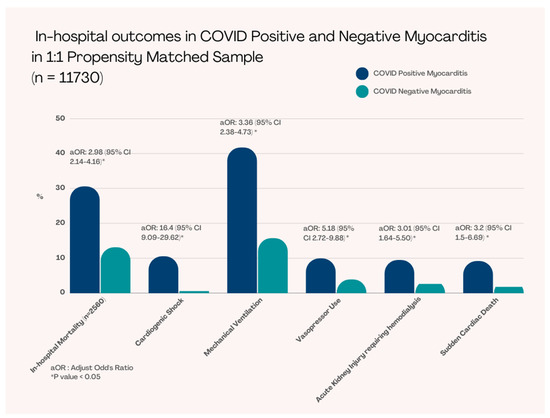
Figure 2.
In-hospital Outcomes in COVID-19 Positive and Negative Myocarditis in 1:1 Propensity Matched Sample.

Table 3.
In-hospital Outcomes for 1:1 PS Matched Sample.
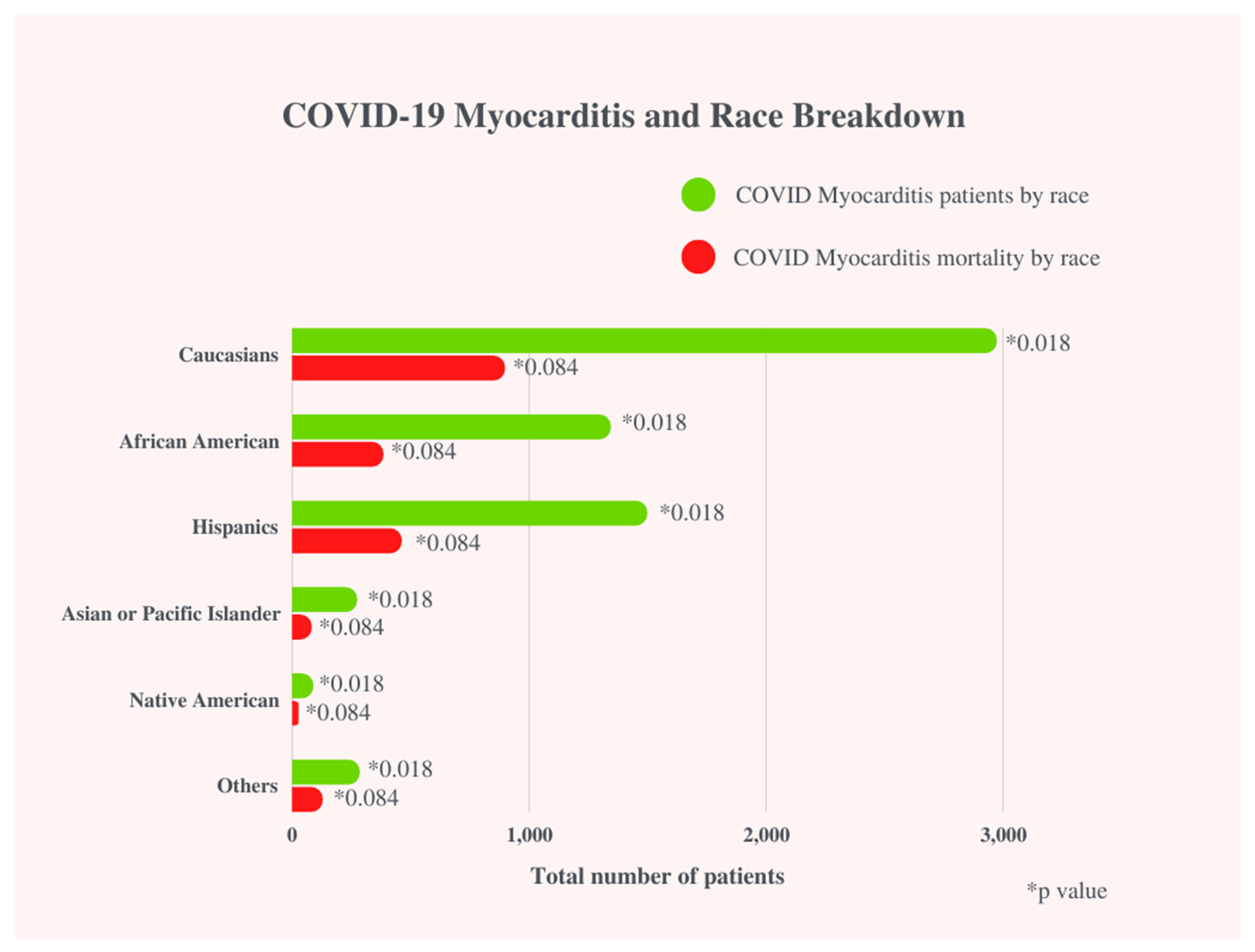
Mortality was further divided, evaluating gender, race, and age. Mortality in COVID-19 patients with myocarditis was significantly higher among younger patients: years ≥ 18–29 (1.5% vs. 0.6%, p < 0.001), years 30–49 (7.3% vs. 4.9%, p < 0.001, years 50–69 (39.1% vs. 30.4%, p < 0.001) as compared to COVID-19 patients without myocarditis. Mortality for patients with COVID-19 without myocarditis was significantly higher among older age patients: years ≥ 70 (64.1% vs. 52.0%, p < 0.001) when compared to COVID-19 patients with myocarditis. (Figure 3)
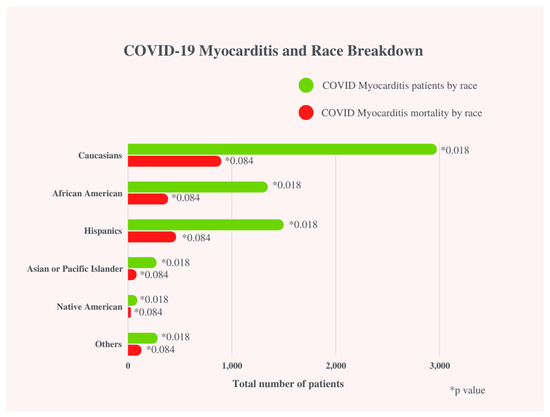
Figure 3.
COVID-19 Myocarditis and Race Breakdown.
3.3. Mortality Predictors in COVID Myocarditis
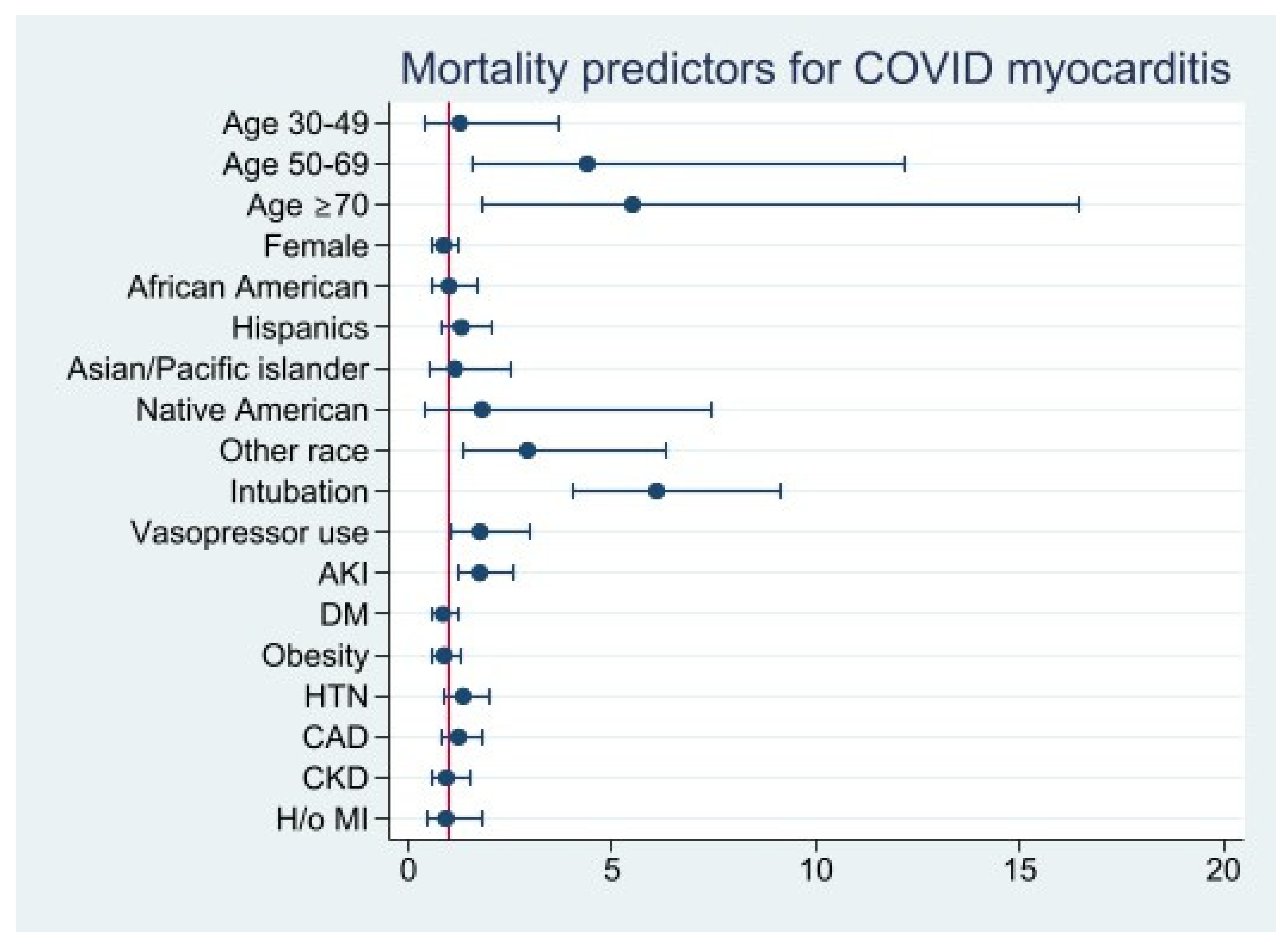
Patients over the age of 50 had a significantly increased risk of mortality (age 50–69, aOR: 4.4 [95% CI 1.6–12.2], p = 0.005; age ≥ 70, aOR: 5.5 [95% CI 1.8–16.5], p = 0.002). Higher mortality was observed in patients requiring mechanical ventilation (aOR: 6.1 [95% CI 4.1–9.1], p < 0.001), vasopressor use (aOR: 1.8 [95% CI 1.0-3.0], p = 0.03), and developing AKI (aOR: 1.7 [95% CI 1.2–2.6], p = 0.004). Underlying cardiovascular comorbidities (i.e., CAD, hypertension, DM, CKD, history of previous MI, and obesity) were not statistically significant predictors of mortality in COVID-19 myocarditis patients (Figure 4) (Table S3).
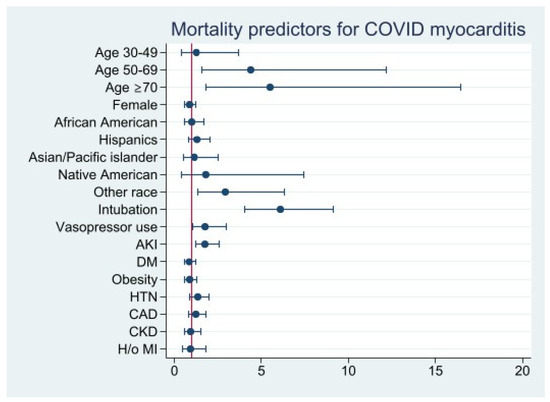
Figure 4.
Mortality Predictors for COVID 19 Myocarditis.
3.4. In-Hospital Complications
Patients who presented with COVID-19 with myocarditis required more mechanical ventilation (41.7% vs. 15.8%, aOR: 3.4 [95% CI 2.4–4.7], p < 0.001), higher vasopressors use (9.9% vs. 3.9%, aOR: 5.2 [95% 2.7–9.9], p < 0.001), and experienced more cardiogenic shock (10.5% vs. 0.6%, aOR: 16 [95% CI 9.1–29.6], p < 0.001); however, did not require significant mechanical circulatory support (2.6% vs. 0.5%, aOR: 2.5 [95% CI 0.5–11.4], p = 0.29). These patients also had statistically significant higher rates of acute kidney injury requiring hemodialysis (9.5% vs. 2.6%, aOR: 3.0 [95% CI 1.6–5.5], p < 0.001) and sudden cardiac arrest (9.1% vs. 1.8%, aOR: 3.2 [95% CI 1.5–6.7], p = 0.002) (Table 3).
3.5. In-Hospital Quality Measures and Disposition
Patients with COVID-19 and myocarditis had an increased mean length of stay (11.6 days vs. 8.4 days, adjusted length of stay of 1.9 days, p = 0.007) which was not significantly higher than COVID-19 patients without myocarditis. Patients with COVID-19 and myocarditis had higher mean total hospitalization cost (173,226 USD vs. 72,072 USD, adjusted total cost 61,153 USD, p < 0.001). Of those patients who survived, no significant difference was noticed in the disposition from the hospital. Patients in the COVID-19 with myocarditis cohort were able to be discharged to home (51.2% vs. 51.6%, p = 0.01) and required skilled nursing or long-term acute care compared to the COVID-19 without myocarditis group (28.3% vs. 24.8%, p = 0.01) (Table 3).
4. Discussion
In this retrospective analysis, we identified 1,659,040 patients hospitalized between 1 January 2020 and 31 December 2020 with a diagnosis of COVID-19 infection, out of which 6455 (Prevalence–0.4%) were diagnosed with COVID-19 infection and myocarditis. Our cohort included 1,652,585 COVID-19 patients without myocarditis during this same study duration. Major findings of this study include (1) COVID-19 patients with myocarditis had significantly higher in-hospital mortality compared to COVID-19 patients without myocarditis; (2) Patients in the COVID-19 and myocarditis cohort had significantly increased cardiogenic shock, sudden cardiac death, acute kidney injury requiring hemodialysis, and required more mechanical ventilation and vasopressor support; (3) In-hospital mortality was higher among younger age patients with COVID-19 with myocarditis and older age (≥70 years) patients with COVID-19 without myocarditis; (4) Underlying cardiovascular comorbidities in COVID-19 patients with myocarditis were not predictors of increased mortality.
The prevalence of COVID-19-related myocarditis was between 0.1% to 0.4% in prior literature, which was similar to our prevalence data of 0.4% [6,7,8]. To our knowledge, our study is the largest study comparing outcomes in hospitalized patients with COVID-19 infection with and without myocarditis.
In Boehmer et al. and Sawalha et al., patients with COVID-19-related myocarditis were predominantly males (59.3% and 58%, respectively) [7,17]. These studies were consistent with our study findings. This increased incidence in males can be explained as men are more likely to undergo cardiac remodeling, leading to development of myocardial fibrosis compared to women [18]. This is a result of testosterone and its pro-inflammatory effect, which increases myocardial inflammation, whereas estrogen (17-beta-estradiol) is protective and decreases inflammation [19]. Although in our study males are more predisposed to having COVID-19 and myocarditis than females, we found that gender was not a predictor of mortality.
In Ammirati et al., 7.8% of Hispanics and 15.7% of African Americans were affected COVID-19-associated myocarditis patients [12]. Our study suggests Hispanics, Asians, African Americans, and Native Americans are more likely to have COVID-19-related myocarditis but there was no significant difference in mortality when compared to COVID-19 without myocarditis patients. We did not find any large adult studies investigating race as a risk factor for mortality in COVID-19-induced myocarditis. Further studies are necessary to assess race as a risk factor for mortality in COVID-19-related myocarditis in adult patients. The disparities in these groups across various studies can be explained by socio-economic factors (i.e., medical debt and distrust, lack of access to appropriate healthcare, differences in care from white counterparts, and language barriers) that already predispose these populations to disadvantages [20,21].
In our study, patients with COVID-19 and myocarditis had significantly higher in-hospital mortality at 30.5%, which was similar to mortality of 27% to 30.0% reported by Ho et al. and Rubens et al. [6,22]. The association between myocarditis and mortality was evident after propensity matching of the groups. This high mortality can partly be the result of lack of effective and available COVID-19 therapies and no available vaccinations during the early pandemic. When mortality breakdown was performed, which analyzed gender, race, and age, it was found that although gender and race are significant factors in terms of contracting COVID-19-related myocarditis, these factors do not contribute significance toward in-hospital mortality, as discussed above. This differs from age; it was found that mortality was significant among younger age patients (under 70 years) within the COVID-19 and myocarditis cohort in comparison with COVID-19 without myocarditis. This was consistent with a reported age range of 45–70 and an average age of 52.3 years for patients with mortality from myocarditis in the literature [23,24]. Younger patients are more predisposed to mortality due to a robust immune system, leading to a cytokine and inflammatory storm and resulting in complications like that of myocarditis [23]. Moreover, mortality was significant in older age patients (≥70 years) within the non-myocarditis COVID-19 cohort, which correlated with the literature [25]. In Kang et al., elderly patients 70 years and older with COVID-19 infection had mortality of 10.9%–26.6% (varied based on geography) and was believed to be a result of aging immunity, increasing number of comorbidities, and impact of congregate housing [25].
Per our data, patients with COVID-19-associated Myocarditis were more likely to have complicated Diabetes mellitus, Chronic kidney disease/End stage renal disease, and have a history of coronary artery disease or myocardial infarction; however, having these underlying cardiovascular comorbidities was not determined to be a predictor of mortality in COVID-19 myocarditis. A multicenter case series by Laganà et al. noted a higher prevalence of cardiac diseases and risk factors in their COVID-19 myocarditis cohort but did not find significance in how it related to mortality and complications [26]. COVID-19-related myocarditis mortality is likely due to the burden of the inflammatory disease, independent of cardiovascular risk factors [27]. However, other studies suggest the opposite in that even the presence of one comorbidity increased the risk of mortality [6,7,8,28]. Prospective studies would be beneficial for clarification. Underlying mechanisms related to an increased rate of acute kidney injury in patients with myocarditis are unclear. Still, they may be related to decreased perfusion in the setting of new onset congestive heart failure, vasopressor use and shock [29,30].
Our cohort of COVID-19 patients with myocarditis had significantly increased cardiogenic shock, mechanical ventilation and vasopressor requirements, acute kidney injury requiring hemodialysis, and sudden cardiac death compared to patients with COVID-19 without myocarditis. This was consistent with a meta-analysis of 2,389 patients by Santoso et al., which suggested similar outcomes in that cardiac injury was associated with higher mortality (65%, RR: 8.0 [95% CI 5.1–12.3], p < 0.001), higher need for intensive care unit (ICU)-level care (79%, RR: 7.9 [95% CI 1.5–41.8], p = 0.01), and severe COVID-19 disease (RR: 13.8 [95% CI 5.5–34.5], p < 0.001) [31]. That study indicated severity of myocarditis correlated with troponin elevation and inflammatory markers and thus, explains why these patients have worse outcomes than non-myocarditis COVID-19 patients. Additionally, reliance on cardiac enzymes, electrocardiograms (EKGs), and clinical suspicion (irrespective of cardiac history) when echocardiograms and Cardiac MRIs are not available for confirmatory diagnosis or when use of these modalities was delayed due to the early pandemic isolation effect can explain poor outcomes, too [22,28,32]. One of the limitations of our data is it did not include lab values and imaging since NIS diagnoses are based on ICD-10 codes. History, exam, and laboratory data pertaining to myocarditis can be nonspecific and thus, recognizing myocarditis and treating patients early and aggressively are pertinent to improving morbidity and mortality [33].
Patients with co-diagnosis of COVID-19 and myocarditis in our study had significantly higher mean total hospitalization cost and longer length of stay than non-myocarditis COVID-19 patients. This is not surprising given patients with COVID-19 and myocarditis required higher levels of care, including stays in ICUs and life support measures, as discussed above.
Management of COVID-19-related myocarditis is primarily supportive while treating the underlying COVID-19 infection. Steroids, antivirals, and comfort medications are typically given to COVID-19 patients with benefit but it remains unclear if these measures have any benefit directed toward myocarditis specifically [34,35,36]. Per Taggarsi, further insight is needed regarding the effect of remdesivir specifically on mitigating adverse cardiovascular events [37]. Further management can include treatment of complications and tertiary comorbidities that develop from the secondary myocarditis, such as heart failure, which should be treated based on current standards of care with goal-directed medical therapy (GDMT) [38,39].
In regard to vaccination against COVID-19 with m-RNA vaccines, there have been concerns attributed to myocarditis as a potential side effect. In a review article by Bozkurt et al., 61 cases of myocarditis were reported out of >300 million administered vaccines (< 0.00002%) [40]. In another review article by Verma et al., a direct causal relationship between vaccination and myocarditis could not be definitively established due to lack of tissue testing for antibodies [41]. Moreover, a study by Patone et al. noted that risk of extra myocarditis events in the month after COVID-19 vaccination to be significantly lower compared to risk of myocarditis after COVID-19 infection (1–10 events per million persons vs. 40 extra events per million) [42]. Based on these studies, the benefits of vaccination (i.e., decreased severity of COVID-19 disease, hospitalization, mortality, and risk of myocarditis) outweigh risks associated with vaccination [43]. As suggested by Bozkurt et al., a collaborative registry of myocarditis secondary to COVID-19 vaccination and secondary to COVID-19 disease with collected data on patient demographics, clinical presentation, and biomarkers (troponin, EKG, echocardiogram, Cardiac MRI) in conjunction with a paired registry of blood and cardiac tissue samples would be of value to answer many of these remaining questions [40].
Immune responses, including by pathogenic T-cells and inflammatory monocytes, may play a significant role in tissue damage (including myocardium) seen in COVID-19 infection [44,45]. Multi-omic profiling is an emerging approach to understanding immune responses associated with COVID-19 infection and disease severity. Su et al. identified a significant shift between mild and moderate disease in inflammatory signalling in plasma multi-omic analysis of 139 COVID-19 patients studied before the introduction of vaccination [46]. Increased inflammatory signals reflecting stress environments were noted with loss in metabolic resources and a drop in xenobiotic metabolism and postulated to influence the immune response in COVID-19 patients [46]. Sue et al. noted that disease severity is associated with non-monotonic evolution of CD8+ cell phenotypic composition (with increase in naive clusters and decrease in activated effector T cells noted in severe disease), non-monotonic changes of CD8+ T cells’ polyfunctionality and dysfunctional monocyte subpopulation. They also noted the transition from mild to moderate disease was accompanied by the expansion of CD8+ clones and two distinct CD4+ T cell phenotypes. These results were further supported by a study by Filblin et al., who studied the association of plasma proteomics with the role of immune cells and disease severity in 306 COVID-19 patients and 78 symptomatic controls over time [45]. They found an association between severe COVID-19 disease with heterogeneous plasma proteomic response. This study suggested the predominant role of viral infection and pre-existing comorbidities with advanced age rather than immune-mediated process in the underlying pathology of COVID-19 infection. Significant expansion of naive B cell and antibody-secreting cells in moderate to severe COVID-19 disease noted in this study may play a role in dysregulated humoral immunity response [45]. This study provided further evidence that plasma proteomes from circulating immune cells ( monocytes, plasmablasts, CD8+ T and NK cells) can play a direct role in tissue damage in multiple organs, including the heart [45]. Lee et al. in study of 198 COVID-19 patients with single cell multi-nomics and analysis of plasma metabolite found that increasing disease severity correlated with division of monocytes into two metabolically and functionally distinct subsets, metabolically hyperactive CD8+ T cell subpopulation, marked metabolic heterogeneity with CD4+ cells and metabolically dominant NK cell subpopulation [47]. This study further suggested metabolic reprogramming of immune cells with COVID-19 infection. These studies show that multi-omic profiling can be very promising to help undercover molecular underpinnings of myocarditis in COVID-19 patients. We were unable to perform multi-omic profiling due to limitations of the data obtained from the National Inpatient sample database. Future studies which evaluate multi-omic profiling of patients with COVID-19 and myocarditis will be very valuable to understand the pathophysiology of this association.
COVID-19 has affected millions of people across the globe. Even though effective vaccination and treatment strategies have helped to reduce acute COVID-19 severity, its long-term effects four weeks after acute infection in the form of long COVID are increasingly being recognized [48]. In the study done by Puntmann et al. in patients with mild COVID infection, upto 57% of participants continued to have persistent cardiac symptoms with a median follow-up of 329 days (IQR 274–383 days) [49]. Myocarditis as part of long COVID has been reported in multiple studies [49,50,51,52,53]. In a large study by Patone et al. with people 16 years and older, an extra 40 myocarditis events per million were noted 1–28 days after a positive SARS-COV-2 test [42], while another study indicated that males aged 12–17 were likely to have 450 cases per million infections within three months of COVID-19 infection [52]. Mechanisms underlying potential long COVID and myocarditis need to be better understood. Su et al. performed a deep multi-omic longitudinal study to evaluate PASC (post-acute sequelae of COVID-19) anticipating biological factors [46]. One of the limitations of our study based on the 2020 NIS dataset is that we could not study long COVID given the ICD-10 code for long COVID was introduced in 2021 [54]. Prospective studies utilizing multi-omic analysis can enhance understanding of relationship and pathophysiology of long COVID and myocarditis.
The NIS database has provided new information that can be used for the awareness of COVID-19-related myocarditis. It highlights the importance of stricter inpatient monitoring and cardiology outpatient follow-up for patients who develop cardiac complications. With this data, there can be further investigations into development of directed therapies for COVID-19-related myocarditis in order to improve mortality and the aforementioned outcomes. This data also allows for the promotion and emphasis of vaccinations.
5. Limitations
Our study data was collected from the NIS which may introduce selection bias. Most patients with COVID-19 and myocarditis were diagnosed at urban teaching hospitals which have more resources like MRI compared to smaller hospitals. It may result in less final diagnosis of myocarditis at smaller hospitals despite the initial clinical suspicion. Diagnosis of myocarditis was based on ICD-10 codes as NIS data lacks lab values and imaging and may make it prone to errors. Our larger sample size helps to reduce the effects of these errors. The study cohort also included primarily non-vaccinated individuals as the FDA first approved COVID vaccination under EUA on 11 December 2020. It is possible COVID-19 vaccination may alter outcome in myocarditis between vaccinated and unvaccinated individuals.
6. Conclusions
Our study found that myocarditis in COVID-19 patients was associated with worse outcomes both in terms of morbidity and mortality, including increased acute kidney injury needing hemodialysis, cardiogenic shock with vasopressor support, mechanical ventilation and sudden cardiac death. Vaccination may play a pivotal role in reducing the incidence of COVID-19-related myocarditis. Increased risk of worse outcomes coupled with limited treatment options highlights the need for further research for effective vaccination and treatment strategies to improve outcomes in COVID-19-positive patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14122791/s1, Table S1: ICD-10 Codes; Table S2: Baseline co-morbidities of unmatched sample; Table S3: Mortality predictors for COVID-19 Myocarditis
Author Contributions
Conceptualization, A.B.S. and R.S.; data curation, K.G., H.S., A.B. and A.B.S.; writing—original draft preparation, A.B.S., M.G.D., K.G., P.C., R.S.; writing—review and editing, K.G., A.F., P.C., D.D., R.S. and A.B.S.; supervision, A.B.S., S.R.A. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from the National Inpatient Sample database, US.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Rampal, L.; Liew, B.S. Coronavirus disease (COVID-19) pandemic. Med. J. Malays. 2020, 75, 95–97. [Google Scholar]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Cizgici, A.Y.; Zencirkiran Agus, H.; Yildiz, M. COVID-19 myopericarditis: It should be kept in mind in today’s conditions. Am. J. Emerg. Med. 2020, 38, 1547.e5–1547.e6. [Google Scholar] [CrossRef]
- Rubens, M.; Ramamoorthy, V.; Saxena, A.; Zevallos, J.C.; Ruiz-Pelaez, J.G.; Ahmed, M.A.; Zhang, Z.; McGranaghan, P.; Veledar, E.; Jimenez, J.; et al. Hospital Outcomes Among COVID-19 Hospitalizations With Myocarditis from the California State Inpatient Database. Am. J. Cardiol. 2022, 183, 109–114. [Google Scholar] [CrossRef]
- Boehmer, T.K.; Kompaniyets, L.; Lavery, A.M.; Hsu, J.; Ko, J.Y.; Yusuf, H.; Romano, S.D.; Gundlapalli, A.V.; Oster, M.E.; Harris, A.M. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data—United States, March 2020-January 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef]
- Annie, F.H.; Embrey, S.; Alkhaimy, H.; Elashery, A.R.; Nanjundappa, A. Association between myocarditis and mortality in covid-19 patients in a large registry. J. Am. Coll. Cardiol. 2021, 77, 3037. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Mason, J.W.; O’Connell, J.B.; Herskowitz, A.; Rose, N.R.; McManus, B.M.; Billingham, M.E.; Moon, T.E. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 1995, 333, 269–275. [Google Scholar] [CrossRef]
- Kang, M.; Chippa, V.; An, J. Viral Myocarditis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef] [PubMed]
- Dec, G.W.; Palacios, I.F.; Fallon, J.T.; Aretz, H.T.; Mills, J.; Lee, D.C.; Johnson, R.A. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N. Engl. J. Med. 1985, 312, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- NIS Database Documentation. Available online: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp (accessed on 25 November 2022).
- Stagg, V. ELIXHAUSER: Stata Module to Calculate Elixhauser Index of Comorbidity. 2015. Available online: https://econpapers.repec.org/software/bocbocode/s458077.htm. (accessed on 15 October 2022).
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cocker, M.S.; Abdel-Aty, H.; Strohm, O.; Friedrich, M.G. Age and gender effects on the extent of myocardial involvement in acute myocarditis: A cardiovascular magnetic resonance study. Heart 2009, 95, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Brandt, J.E.; Kim, E.; Bucek, A.; Bedja, D.; Abston, E.D.; Shin, J.; Gabrielson, K.L.; Mitzner, W.; Fairweather, D. Testosterone and interleukin-1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1726–H1736. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, F.J.; Anderson, N.W. Trends in Health Equity in the United States by Race/Ethnicity, Sex, and Income, 1993–2017. JAMA Netw. Open 2019, 2, e196386. [Google Scholar] [CrossRef]
- Raifman, M.A.; Raifman, J.R. Disparities in the Population at Risk of Severe Illness From COVID-19 by Race/Ethnicity and Income. Am. J. Prev. Med. 2020, 59, 137–139. [Google Scholar] [CrossRef]
- Ho, J.S.; Sia, C.-H.; Chan, M.Y.; Lin, W.; Wong, R.C. Coronavirus-induced myocarditis: A meta-summary of cases. Heart Lung 2020, 49, 681–685. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Jung, S.I. Age-Related Morbidity and Mortality among Patients with COVID-19. Infect. Chemother. 2020, 52, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Laganà, N.; Cei, M.; Evangelista, I.; Cerutti, S.; Colombo, A.; Conte, L.; Mormina, E.; Rotiroti, G.; Versace, A.G.; Porta, C.; et al. Suspected myocarditis in patients with COVID-19: A multicenter case series. Medicine 2021, 100, e24552. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Haussner, W.; DeRosa, A.P.; Haussner, D.; Tran, J.; Torres-Lavoro, J.; Kamler, J.; Shah, K. COVID-19 associated myocarditis: A systematic review. Am. J. Emerg. Med. 2022, 51, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.; Yokoyama, S.; Shinada, T.; Kobayashi, N.; Shirakabe, A.; Tomita, K.; Kitamura, M.; Kurihara, O.; Takahashi, Y. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur. J. Heart Fail. 2010, 12, 32–37. [Google Scholar] [CrossRef]
- Singh, S.; Kanwar, A.; Sundaragiri, P.R.; Cheungpasitporn, W.; Truesdell, A.G.; Rab, S.T.; Singh, M.; Vallabhajosyula, S. Acute kidney injury in cardiogenic shock: An updated narrative review. J. Cardiovasc. Dev. Dis. 2021, 8, 88. [Google Scholar] [CrossRef]
- Santoso, A.; Pranata, R.; Wibowo, A.; Al-Farabi, M.J.; Huang, I.; Antariksa, B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am. J. Emerg. Med. 2021, 44, 352–357. [Google Scholar] [CrossRef]
- Ma, K.-L.; Liu, Z.-H.; Cao, C.; Liu, M.-K.; Liao, J.; Zou, J.-B.; Kong, L.-X.; Wan, K.-Q.; Zhang, J.; Wang, Q.-B.; et al. COVID-19 Myocarditis and Severity Factors: An Adult Cohort Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Priyadarshni, S.; Westra, J.; Kuo, Y.-F.; Baillargeon, J.G.; Khalife, W.; Raji, M. COVID-19 Infection and Incidence of Myocarditis: A Multi-Site Population-Based Propensity Score-Matched Analysis. Cureus 2022, 14, e21879. [Google Scholar] [CrossRef]
- Närhi, F.; Moonesinghe, S.R.; Shenkin, S.D.; Drake, T.M.; Mulholland, R.H.; Donegan, C.; Dunning, J.; Fairfield, C.J.; Girvan, M.; Hardwick, H.E.; et al. ISARIC4C investigators Implementation of corticosteroids in treatment of COVID-19 in the ISARIC WHO Clinical Characterisation Protocol UK: Prospective, cohort study. Lancet Digit. Health 2022, 4, e220–e234. [Google Scholar] [CrossRef] [PubMed]
- Kariyanna, P.T.; Sutarjono, B.; Grewal, E.; Singh, K.P.; Aurora, L.; Smith, L.; Chandrakumar, H.P.; Jayarangaiah, A.; Goldman, S.A.; Salifu, M.O.; et al. A Systematic Review of COVID-19 and Myocarditis. AJMCR 2020, 8, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Agdamag, A.C.C.; Edmiston, J.B.; Charpentier, V.; Chowdhury, M.; Fraser, M.; Maharaj, V.R.; Francis, G.S.; Alexy, T. Update on COVID-19 Myocarditis. Medicina 2020, 56, 678. [Google Scholar] [CrossRef] [PubMed]
- Taggarsi, D.A. Is It Time to Revisit Remdesivir Use for Severe COVID-19? Indian J. Crit. Care Med. 2022, 26, 983–984. [Google Scholar] [CrossRef]
- Mehra, M.R.; Desai, S.S.; Kuy, S.; Henry, T.D.; Patel, A.N. Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. N. Engl. J. Med. 2020, 382, e102. [Google Scholar] [CrossRef]
- Kuster, G.M.; Pfister, O.; Burkard, T.; Zhou, Q.; Twerenbold, R.; Haaf, P.; Widmer, A.F.; Osswald, S. SARS-CoV2: Should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020, 41, 1801–1803. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after Covid-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Jain, V.K.; Iyengar, K.P.; Ish, P. Elucidating causes of COVID-19 infection and related deaths after vaccination. Diabetes Metab. Syndr. 2021, 15, 102212. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.R.; Mehta, A.; Schneider, A.M.; Kays, K.R.; Guess, J.R.; Gentili, M.; Fenyves, B.G.; Charland, N.C.; Gonye, A.L.K.; Gushterova, I.; et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep. Med. 2021, 2, 100287. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Su, Y.; Baloni, P.; Chen, D.; Pavlovitch-Bedzyk, A.J.; Yuan, D.; Duvvuri, V.R.; Ng, R.H.; Choi, J.; Xie, J.; et al. Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19. Nat. Biotechnol. 2022, 40, 110–120. [Google Scholar] [CrossRef]
- Long COVID or Post-COVID Conditions. CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 4 October 2022).
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Eiros, R.; Barreiro-Pérez, M.; Martín-García, A.; Almeida, J.; Villacorta, E.; Pérez-Pons, A.; Merchán, S.; Torres-Valle, A.; Sánchez-Pablo, C.; González-Calle, D.; et al. En representación de los investigadores CCC (cardiac COVID-19 healthcare workers) [Pericardial and myocardial involvement after SARS-CoV-2 infection: A cross-sectional descriptive study in healthcare workers]. Rev. Esp. Cardiol. 2022, 75, 735–747. [Google Scholar] [CrossRef]
- Szarpak, L.; Pruc, M.; Filipiak, K.J.; Popieluch, J.; Bielski, A.; Jaguszewski, M.J.; Gilis-Malinowska, N.; Chirico, F.; Rafique, Z.; Peacock, F.W. Myocarditis: A complication of COVID-19 and long-COVID-19 syndrome as a serious threat in modern cardiology. Cardiol. J. 2022, 29, 178–179. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Bull-Otterson, L.; Boehmer, T.K.; Baca, S.; Alvarez, P.; Hong, K.; Hsu, J.; Harris, A.M.; Gundlapalli, A.V.; Saydah, S. Post-COVID-19 Symptoms and Conditions Among Children and Adolescents—United States, 1 March 2020–31 January 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 993–999. [Google Scholar] [CrossRef]
- Bajaj, R.; Sinclair, H.C.; Patel, K.; Low, B.; Pericao, A.; Manisty, C.; Guttmann, O.; Zemrak, F.; Miller, O.; Longhi, P.; et al. Delayed-onset myocarditis following COVID-19. Lancet Respir. Med. 2021, 9, e32–e34. [Google Scholar] [CrossRef]
- CDC. New ICD-10-CM code for the 2019 Novel Coronavirus (COVID-19). 3 December 2020. Available online: https://www.cdc.gov/nchs/data/icd/Announcement-New-ICD-code-for-coronavirus-19-508.pdf (accessed on 27 November 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).